Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Siva S. Panda | -- | 1067 | 2023-01-10 13:08:19 | | | |
| 2 | Dean Liu | Meta information modification | 1067 | 2023-01-11 02:46:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Panda, S.S.; Thangaraju, M.; Lokeshwar, B.L.; Panda, S. Triterpenes as Bioactive Natural Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/39969 (accessed on 07 February 2026).
Panda SS, Thangaraju M, Lokeshwar BL, Panda S. Triterpenes as Bioactive Natural Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/39969. Accessed February 07, 2026.
Panda, Siva S., Muthusamy Thangaraju, Bal L. Lokeshwar, Siva Panda. "Triterpenes as Bioactive Natural Products" Encyclopedia, https://encyclopedia.pub/entry/39969 (accessed February 07, 2026).
Panda, S.S., Thangaraju, M., Lokeshwar, B.L., & Panda, S. (2023, January 10). Triterpenes as Bioactive Natural Products. In Encyclopedia. https://encyclopedia.pub/entry/39969
Panda, Siva S., et al. "Triterpenes as Bioactive Natural Products." Encyclopedia. Web. 10 January, 2023.
Copy Citation
Natural products (NP) are one of the main sources of diverse pharmacologically active compounds. NPs and NP-scaffolds comprise a large portion of current-day pharmaceutical agents (over 70% of FDA-approved drugs).
ursolic acid
anticancer
antitumor
synthesis
1. Introduction
Cancer is the first or the second leading cause of death worldwide, accounting for 19.2 million new cases and 9.6 million deaths, in 2020 [1]. The most common causes of cancer death in 2020 were lung, colorectal, liver, stomach, and breast cancer. In addition, each year more than 40,000 children develop cancer [1]. In the United States, there will be an estimated 1.9 million new cancer cases and 609,360 cancer deaths in 2022 [2].
The cancer burden continues to grow globally, exerting tremendous physical, emotional, and financial strain on individuals, families, communities, and even the national health systems. Compared to developed counties, low- and middle-income countries have low survival rates because of the lack of accessible early detection, quality diagnosis, treatment, and survivorship care. The global economic burden of cancer is unknown, as accurate economic data are not available from many countries [1]. In the United States, the national patient economic burden associated with cancer care was USD 21.09 billion in 2019 [3].
Current treatments for malignant cancers include surgery, radiation, chemotherapy, adjuvant therapy, hormone therapy, and immunotherapy. Despite all these therapies and the tremendous progress, refinement, and entry of novel drugs, procedures, and treatment options, cancer continues to be a leading cause of death. The challenges to reducing cancer burden, and specifically for treatment failures, are associated with the development of drug resistance, disease progression, and dose-limiting systemic toxicities of potent drugs [4]. The much-heralded immunotherapy has low efficacy, with less than 20% of patients responding to the treatment. Novel effective and efficient pharmacological agents that act through unconventional mechanisms to enhance existing therapies or kill tumor cells resistant to other existing therapies are urgently needed.
Over four decades, bioactive compounds isolated from natural sources, such as plant and marine organisms, the natural products, have dominated the cancer prevention, treatment, and drug development areas. Natural products (NP) are one of the main sources of diverse pharmacologically active compounds [5][6][7][8][9][10][11]. NPs and NP-scaffolds comprise a large portion of current-day pharmaceutical agents (over 70% of FDA-approved drugs) [12][13]. At present, several native or modified NPs have attained the status of cancer therapeutics. These include irinotecan, vincristine, etoposide, and paclitaxel from plants, actinomycin D, and mitomycin C from bacteria, as well as marine-derived bleomycin.
2. Triterpenes as Bioactive NPs
Triterpenoids are compounds with a carbon skeleton based on six isoprene units, metabolites of isopentenyl pyrophosphate oligomers, representing the largest group of phytochemicals. These are a large and structurally diverse group of NPs having about 200 distinct skeletons, and more than 20,000 triterpenoids are isolated and identified from nature [14][15].
In the 1920s, for the first time, Ursolic acid (UA) was isolated and identified from epicuticular waxes of apples. UA is one of the most abundant and well-studied triterpenoids found in various cuticular waxes of edible fruits (apple, blueberry, cherry, cranberry, Japanese loquat, lemon, olive, orange, peach, pear, prune, quince, tangerine, and tembusu), leaves (coffee, elder, glossy privet, hawthorn, lavender, nerium, marjoram, olive, organum, rosemary, thyme, and whorled rosinweed), flowers (loquat, marigold), and bark (elder, olive, and silver birch) of medicinal plants [16][17][18][19][20]. UA is present in most edible plant products.
UA holds an important place among various triterpenoids because of its wide range of biological activities. UA is abundantly present in medicinal plants and shows many pharmacological activities including antiproliferative [21][22][23], antimicrobial [24][25], antiviral [26][27], antioxidant [28][29][30], and anti-inflammatory activities [30][31].
UA has been extensively applied in cancer treatment in Traditional Chinese Medicine (TCM) for several years. Over the last two decades, UA has been tested on several malignant cancers for both the prevention of cancer progression and as a treatment. Most studies are limited to its activities against energy metabolism, cell proliferation, and antioxidant activities [32][33][34][35][36][37][38][39][40][41][42][43]. These studies have established at least in vitro, that UA inhibits many activities of cancer cell growth, energy utilization, and tumor cell-induced inflammation as summarized in Figure 1 [44][45][46][47][48][49][50][51][52][53].
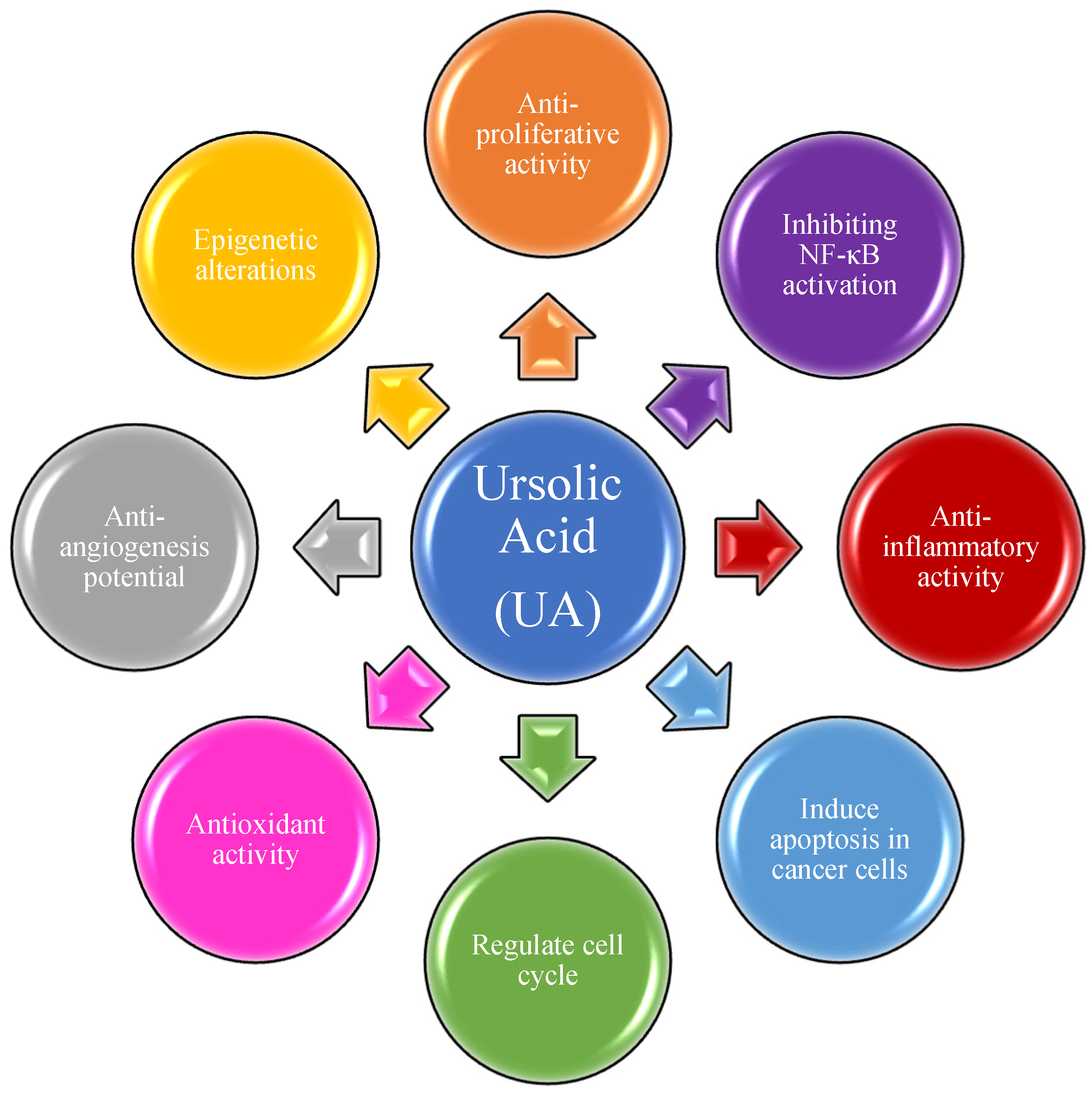
Figure 1. Ursolic acid and its molecular mechanism of action in cancer treatment.
Despite its versatile functions, UA has limitations. Most studies on UA are limited to cell cultures in vitro where UA has access to cells and it is not a concern. UA diluted in biocompatible solvents are added to cell cultures, and its activities on cells are determined by many analytical techniques. However, the availability of UA at the site of the tumors in an intact body is a significant concern. Like many natural, medicinal compounds, administration of UA parenterally is seldom used due to its poor solubility and potential toxicity of the solvents, such as Dimethyl Sulfoxide or Dimethyl Formamide. The oral route, via its addition to diets or drinking water, is of little use. The bioavailability of UA, in plasma, is limited to ≤500 nM [54]. Most in vitro studies have shown the inhibition of tumor cell proliferation in low micromolar concentrations. Studies using transgenic prostate cancer models have reported good tumor control within 12 weeks of treatment in well-established transgenic tumors via a solid diet at 1% w/w. The authors reported a ~60% reduction in prostate tumor volume following a 12-week observation, indicating the high efficacy of UA in the prevention of tumor progression in this model [55]. Studies in human volunteers were less promising, concerning UA as a potential therapeutic for cancer [18]. The mode of delivery of the compound by nano-formulations such as nanoliposomes has not shown much improvement in increased plasma levels [56]. In addition, the Phase I study of nanoliposome formulation showed no cumulative accumulation of UA in the body as performed by the multi-phase pharmacokinetics study. These studies suggest that while UA itself has promise as an anticancer therapeutic, structural modification for the UA molecule to enhance its solubility, absorption, low-protein binding, and longer tissue accumulation should significantly enhance its application in cancer patients.
Concerning the versatile properties of UA, researchers focused on compiling the synthetic analogs of UA, which show potential anticancer properties, and analyzed their structure-activity relationship (SAR). UA (3-(β-hydroxy-urs-12-en-28-oic acid), 1) is a ubiquitous pentacyclic compound that possesses functional groups such as a carboxylic group at C28, β-hydroxy function at C3, and an alkene at C12–C13 (Figure 2). Considering UA as a lead molecule, researchers extensively modified and hybridized UA at these sites with the aim of enhancing its anticancer properties and overcoming the associated poor absorption and low bioavailability drawbacks. Researchers have categorized the analysis based on modifications and/or alterations at different pharmacophoric sites.
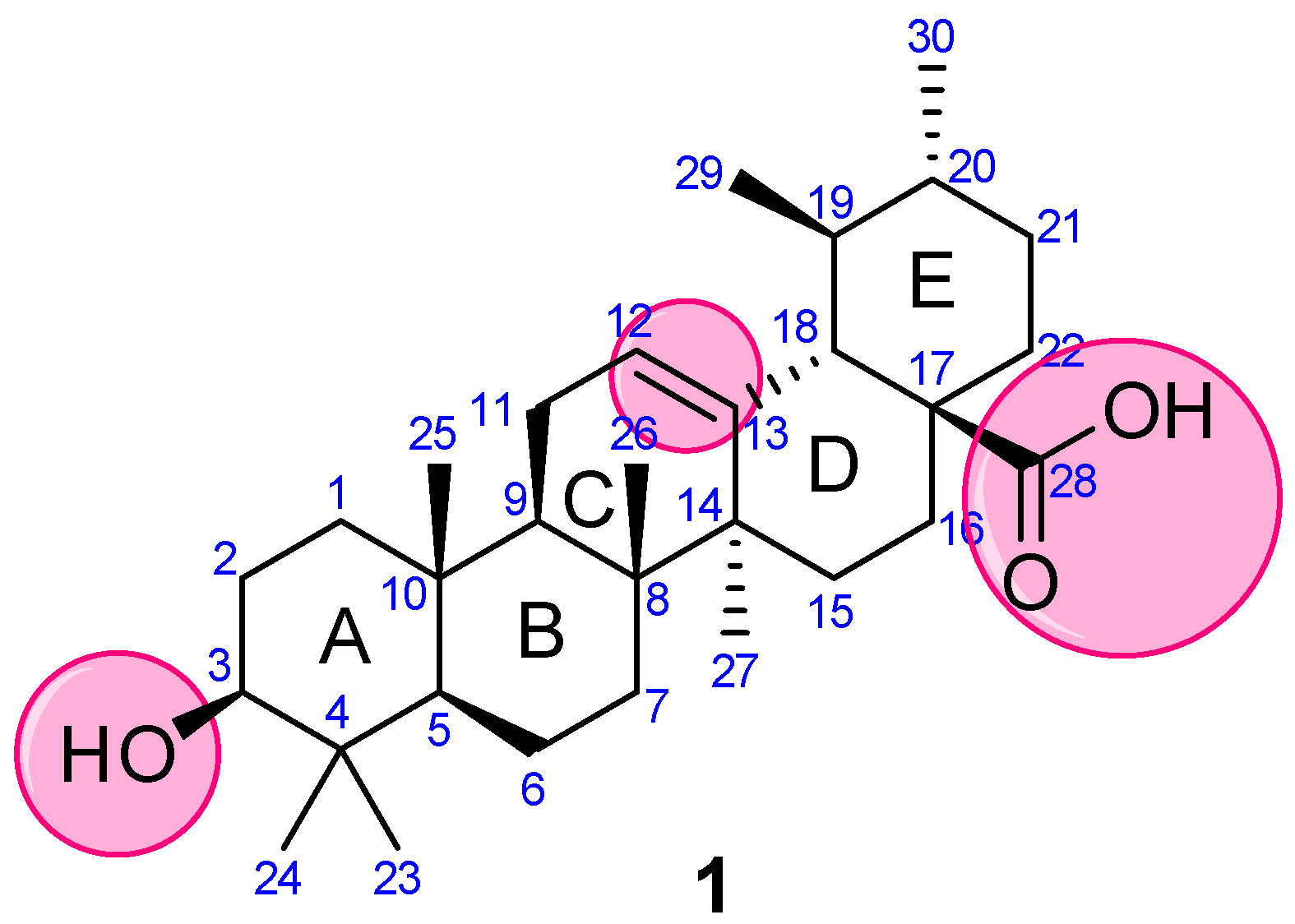
Figure 2. Structure of UA with highlighted pharmacophoric sites.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33.
- Yabroff, K.R.; Mariotto, A.M.; Tangka, F.; Zhao, J.; Islami, F.; Sung, H.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Ward, E.M. Annual Report to the Nation on the Status of Cancer, Part II: Patient Economic Burden Associated with Cancer Care. J. Natl. Cancer Inst. 2021, 113, 1670–1682.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int. J. Oncol. 2019, 54, 407–419.
- Pejin, B.; Jovanovi, K.K.; Mojovi, M.; Savi, A.G. New and Highly Potent Antitumor Natural Products from Marine-Derived Fungi: Covering the Period from 2003 to 2012. Curr. Top. Med. Chem. 2013, 13, 2745–2766.
- Sisodiya, P.S. Plant Derived Anticancer Agents: A Review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 293–308.
- Pejin, B.; Glumac, M.A. brief review of potent anti-CNS tumourics from marine sponges: Covering the period from 1994 to 2014. Nat. Prod. Res. 2018, 32, 375–384.
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310.
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59.
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699.
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335.
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176.
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369.
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996.
- Aksit, H.; Gozcu, S.; Altay, A. Isolation and cytotoxic activities of undescribed iridoid and xanthone glycosides from Centaurium erythraea Rafn. (Gentianaceae). Phytochemistry 2023, 205, 113484.
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751.
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248.
- Lopez-Hortas, L.; Perez-Larran, P.; Gonzalez-Munoz, M.J.; Falque, E.; Dominguez, H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018, 103, 130–149.
- Li, C.; Chen, W.; Zheng, L.; Zhang, B.; Yang, X.; Zhang, Q.; Wang, N.; Wang, Y.; Yang, J.; Sha, J. Ameliorative effect of ursolic acid on ochratoxin A-induced renal cytotoxicity mediated by Lonp1/Aco2/Hsp75. Toxicon 2019, 168, 141–146.
- Ozdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340.
- Kornel, A.; Nadile, M.; Tsiani, E. Evidence of the Beneficial Effects of Ursolic Acid against Lung Cancer. Molecules 2022, 27, 7466.
- Wang, L.; Yin, Q.; Liu, C.; Tang, Y.; Sun, C.; Zhuang, J. Nanoformulations of ursolic acid: A modern natural anticancer molecule. Front. Pharmacol. 2021, 12, 706121.
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-Planktonic and Anti-Biofilm Properties of Pentacyclic Triterpenes-Asiatic Acid and Ursolic Acid as Promising Antibacterial Future Pharmaceuticals. Biomolecules 2022, 12, 98.
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472.
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Negm, W.A.; Alexiou, A.; Batiha, G.E.S. Ursolic acid and SARS-CoV-2 infection: A new horizon and perspective. Inflammopharmacology 2022, 30, 1493–1501.
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891.
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and oleanolic acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599.
- Li, W.; Sun, K.; Hu, F.; Chen, L.; Zhang, X.; Wang, F.; Yan, B. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: A mini review. J. Biochem. Mol. Toxicol. 2021, 35, e22658.
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048.
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33.
- Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z.; Meng, Q. Advances in anti-inflammatory activity, mechanism and therapeutic application of ursolic acid. Mini-Revi. Med. Chem. 2022, 22, 423–437.
- Achiwa, Y.; Hasegawa, K.; Udagawa, Y.; Achiwa, Y.; Hasegawa, K.; Udagawa, Y. Effect of ursolic acid on MAPK in cyclin D1 signaling and RING-type E3 ligase (SCF E3s) in two endometrial cancer cell lines. Nutr. Cancer 2013, 65, 1026–1033.
- Prasad, S.; Yadav, V.R.; Sung, B.; Gupta, S.C.; Tyagi, A.K. Ursolic acid inhibits the growth of human pancreatic cancer and enhances the antitumor potential of gemcitabine in an orthotopic mouse model through suppression of the inflammatory microenvironment. Oncotarget 2016, 7, 13182.
- Huang, C.Y.; Lin, C.Y.; Tsai, C.W.; Yin, M.C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. Vitr. 2011, 25, 1274–1280.
- Kim, S.; Ryu, H.G.; Lee, J.; Shin, J.; Harikishore, A.; Jung, H.Y.; Kim, Y.S.; Lyu, H.N.; Oh, E.; Baek, N.I.; et al. Ursolic acid exerts anti-cancer activity by suppressing vaccinia-related kinase 1-mediated damage repair in lung cancer cells. Sci. Rep. 2015, 5, 14570.
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500.
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713–727.
- Gai, W.T.; Yu, D.P.; Wang, X.S.; Wang, P.T. Anti-cancer e_ect of ursolic acid activates apoptosis through ROCK/PTEN mediated mitochondrial translocation of cofilin-1 in prostate cancer. Oncol. Lett. 2016, 12, 2880–2885.
- Yang, L.; Liu, X.; Lu, Z.; Yuet, W.J.; Zhou, L.L.; Fung, K.P.; Wu, P.; Wu, S. Ursolic acid induces doxorubicin-resistant HepG2 cell death via the release of apoptosis-inducing factor. Cancer Lett. 2010, 298, 128–138.
- Zheng, Q.; Li, P.; Jin, F.; Yao, C.; Zhang, G.; Zang, T.; Ai, X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell. Signal. 2013, 25, 206–213.
- Xu, X.; Zhu, G.; Zhang, K.; Zhou, Y.; Li, X.; Xu, W.; Zhang, H.; Shao, Y.; Zhang, Z.; Sun, W. Cyclooxygenase-2 mediated synergistic effect of ursolic acid in combination with paclitaxel against human gastric carcinoma. Oncotarget 2017, 8, 92770–92777.
- Kim, E.S.; Moon, A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902.
- Prasad, S.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors. J. Biol. Chem. 2016, 291, 16924.
- Wang, C.; Lin, C.; Hua, C.; Jou, Y.; Liao, C.; Chang, Y.; Wan, L.; Huang, S.; Hour, M.; Lin, C. Cis-3-O-p-hydroxycinnamoyl ursolic acid induced ROS-dependent p53-mediated mitochondrial apoptosis in oral cancer cells. Biomol. Ther. 2019, 27, 54–62.
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid: Suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways. Nanoscale 2017, 9, 9428–9439.
- Kim, J.; Kim, Y.H.; Song, G.; Kim, D.; Jeong, Y.; Liu, K.; Chung, Y.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95.
- Zhang, R.; Li, Y.; Tian, D.; Liu, Y.; Nian, W.; Zou, X.; Chen, Q.; Zhou, L.; Deng, Z.; He, B. Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int. J. Oncol. 2016, 49, 1973–1982.
- Kim, S.; Jin, H.; Meng, R.Y.; Kim, D.Y.; Liu, Y.C.; Chai, O.H.; Park, B.H.; Kim, S. Activating hippo pathway via Rassf1 by ursolic acid suppresses the tumorigenesis of gastric cancer. Int. J. Mol. Sci. 2019, 20, 4709.
- Choi, W.; Hyung; Lee, I. A. The mechanism of action of ursolic acid as a potential anti-toxoplasmosis agent, and its immunomodulatory effects. Pathogens 2019, 8, 61.
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Kwang, S.A.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955.
- Wang, W.; Zhao, C.; Jou, D.; Lü, J.; Zhang, C.; Lin, L.; Lin, J. Ursolic acid inhibits the growth of colon cancer-initiating cells by targeting STAT3. Anticancer Res. 2013, 33, 4279–4284.
- Liu, T.; Ma, H.; Shi, W.; Duan, J.; Wang, Y.; Zhang, C.; Li, C.; Lin, J.; Li, S.; Lv, J.; et al. Inhibition of STAT3 signaling pathway by ursolic acid suppresses growth of hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 555–562.
- Xia, Y.; Wei, G.; Si, D.; Liu, C. Quantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 219–224.
- Shanmugam, N.K.N.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic Acid Inhibits the Initiation, Progression of Prostate Cancer and Prolongs the Survival of TRAMP Mice by Modulating Pro-Inflammatory Pathways. PLoS ONE 2012, 7, e32476.
- Zhu, Z.; Qian, Z.; Yan, Z.; Zhao, C.; Wang, H.; Ying, G. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int. J. Nanomed. 2012, 8, 129–136.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
11 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




