Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Siva S. Panda and Version 2 by Dean Liu.
Natural products (NP) are one of the main sources of diverse pharmacologically active compounds. NPs and NP-scaffolds comprise a large portion of current-day pharmaceutical agents (over 70% of FDA-approved drugs).
- ursolic acid
- anticancer
- antitumor
- synthesis
1. Introduction
Cancer is the first or the second leading cause of death worldwide, accounting for 19.2 million new cases and 9.6 million deaths, in 2020 [1]. The most common causes of cancer death in 2020 were lung, colorectal, liver, stomach, and breast cancer. In addition, each year more than 40,000 children develop cancer [1]. In the United States, there will be an estimated 1.9 million new cancer cases and 609,360 cancer deaths in 2022 [2].
The cancer burden continues to grow globally, exerting tremendous physical, emotional, and financial strain on individuals, families, communities, and even the national health systems. Compared to developed counties, low- and middle-income countries have low survival rates because of the lack of accessible early detection, quality diagnosis, treatment, and survivorship care. The global economic burden of cancer is unknown, as accurate economic data are not available from many countries [1]. In the United States, the national patient economic burden associated with cancer care was USD 21.09 billion in 2019 [3].
Current treatments for malignant cancers include surgery, radiation, chemotherapy, adjuvant therapy, hormone therapy, and immunotherapy. Despite all these therapies and the tremendous progress, refinement, and entry of novel drugs, procedures, and treatment options, cancer continues to be a leading cause of death. The challenges to reducing cancer burden, and specifically for treatment failures, are associated with the development of drug resistance, disease progression, and dose-limiting systemic toxicities of potent drugs [4]. The much-heralded immunotherapy has low efficacy, with less than 20% of patients responding to the treatment. Novel effective and efficient pharmacological agents that act through unconventional mechanisms to enhance existing therapies or kill tumor cells resistant to other existing therapies are urgently needed.
Over four decades, bioactive compounds isolated from natural sources, such as plant and marine organisms, the natural products, have dominated the cancer prevention, treatment, and drug development areas. Natural products (NP) are one of the main sources of diverse pharmacologically active compounds [5][6][7][8][9][10][11][5,6,7,8,9,10,11]. NPs and NP-scaffolds comprise a large portion of current-day pharmaceutical agents (over 70% of FDA-approved drugs) [12][13][12,13]. At present, several native or modified NPs have attained the status of cancer therapeutics. These include irinotecan, vincristine, etoposide, and paclitaxel from plants, actinomycin D, and mitomycin C from bacteria, as well as marine-derived bleomycin.
2. Triterpenes as Bioactive NPs
Triterpenoids are compounds with a carbon skeleton based on six isoprene units, metabolites of isopentenyl pyrophosphate oligomers, representing the largest group of phytochemicals. These are a large and structurally diverse group of NPs having about 200 distinct skeletons, and more than 20,000 triterpenoids are isolated and identified from nature [14][15][14,15].
In the 1920s, for the first time, Ursolic acid (UA) was isolated and identified from epicuticular waxes of apples. UA is one of the most abundant and well-studied triterpenoids found in various cuticular waxes of edible fruits (apple, blueberry, cherry, cranberry, Japanese loquat, lemon, olive, orange, peach, pear, prune, quince, tangerine, and tembusu), leaves (coffee, elder, glossy privet, hawthorn, lavender, nerium, marjoram, olive, organum, rosemary, thyme, and whorled rosinweed), flowers (loquat, marigold), and bark (elder, olive, and silver birch) of medicinal plants [16][17][18][19][20][16,17,18,19,20]. UA is present in most edible plant products.
UA holds an important place among various triterpenoids because of its wide range of biological activities. UA is abundantly present in medicinal plants and shows many pharmacological activities including antiproliferative [21][22][23][21,22,23], antimicrobial [24][25][24,25], antiviral [26][27][26,27], antioxidant [28][29][30][28,29,30], and anti-inflammatory activities [30][31][30,31].
UA has been extensively applied in cancer treatment in Traditional Chinese Medicine (TCM) for several years. Over the last two decades, UA has been tested on several malignant cancers for both the prevention of cancer progression and as a treatment. Most studies are limited to its activities against energy metabolism, cell proliferation, and antioxidant activities [32][33][34][35][36][37][38][39][40][41][42][43][32,33,34,35,36,37,38,39,40,41,42,43]. These studies have established at least in vitro, that UA inhibits many activities of cancer cell growth, energy utilization, and tumor cell-induced inflammation as summarized in Figure 1 [44][45][46][47][48][49][50][51][52][53][44,45,46,47,48,49,50,51,52,53].
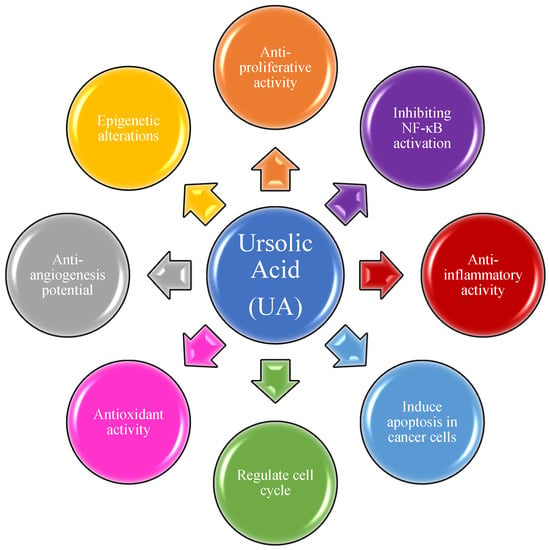
Figure 1. Ursolic acid and its molecular mechanism of action in cancer treatment.
Despite its versatile functions, UA has limitations. Most studies on UA are limited to cell cultures in vitro where UA has access to cells and it is not a concern. UA diluted in biocompatible solvents are added to cell cultures, and its activities on cells are determined by many analytical techniques. However, the availability of UA at the site of the tumors in an intact body is a significant concern. Like many natural, medicinal compounds, administration of UA parenterally is seldom used due to its poor solubility and potential toxicity of the solvents, such as Dimethyl Sulfoxide or Dimethyl Formamide. The oral route, via its addition to diets or drinking water, is of little use. The bioavailability of UA, in plasma, is limited to ≤500 nM [54]. Most in vitro studies have shown the inhibition of tumor cell proliferation in low micromolar concentrations. Studies using transgenic prostate cancer models have reported good tumor control within 12 weeks of treatment in well-established transgenic tumors via a solid diet at 1% w/w. The authors reported a ~60% reduction in prostate tumor volume following a 12-week observation, indicating the high efficacy of UA in the prevention of tumor progression in this model [55]. Studies in human volunteers were less promising, concerning UA as a potential therapeutic for cancer [18]. The mode of delivery of the compound by nano-formulations such as nanoliposomes has not shown much improvement in increased plasma levels [56]. In addition, the Phase I study of nanoliposome formulation showed no cumulative accumulation of UA in the body as performed by the multi-phase pharmacokinetics study. These studies suggest that while UA itself has promise as an anticancer therapeutic, structural modification for the UA molecule to enhance its solubility, absorption, low-protein binding, and longer tissue accumulation should significantly enhance its application in cancer patients.
Concerning the versatile properties of UA, rwesearchers focused on compiling the synthetic analogs of UA, which show potential anticancer properties, and analyzed their structure-activity relationship (SAR). UA (3-(β-hydroxy-urs-12-en-28-oic acid), 1) is a ubiquitous pentacyclic compound that possesses functional groups such as a carboxylic group at C28, β-hydroxy function at C3, and an alkene at C12–C13 (Figure 2). Considering UA as a lead molecule, researchers extensively modified and hybridized UA at these sites with the aim of enhancing its anticancer properties and overcoming the associated poor absorption and low bioavailability drawbacks. RWesearchers have categorized theour analysis based on modifications and/or alterations at different pharmacophoric sites.
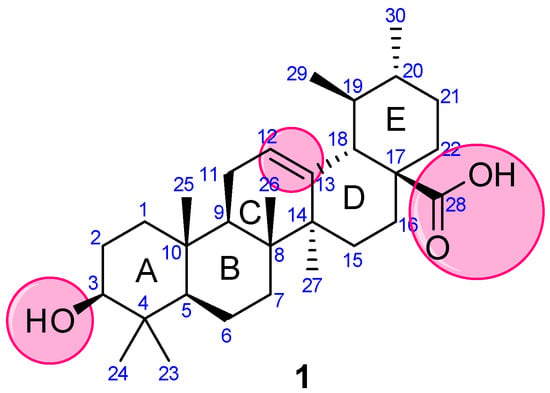
Figure 2. Structure of UA with highlighted pharmacophoric sites.
