Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joy Udensi | -- | 1041 | 2023-01-08 19:22:13 | | | |
| 2 | Jessie Wu | -5 word(s) | 1036 | 2023-01-09 04:48:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Udensi, J.; Loughman, J.; Loskutova, E.; Byrne, H.J. The Structural and Optical Properties of Carotenoid Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/39876 (accessed on 07 February 2026).
Udensi J, Loughman J, Loskutova E, Byrne HJ. The Structural and Optical Properties of Carotenoid Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/39876. Accessed February 07, 2026.
Udensi, Joy, James Loughman, Ekaterina Loskutova, Hugh J. Byrne. "The Structural and Optical Properties of Carotenoid Compounds" Encyclopedia, https://encyclopedia.pub/entry/39876 (accessed February 07, 2026).
Udensi, J., Loughman, J., Loskutova, E., & Byrne, H.J. (2023, January 08). The Structural and Optical Properties of Carotenoid Compounds. In Encyclopedia. https://encyclopedia.pub/entry/39876
Udensi, Joy, et al. "The Structural and Optical Properties of Carotenoid Compounds." Encyclopedia. Web. 08 January, 2023.
Copy Citation
Carotenoid compounds are ubiquitous in nature, providing the characteristic colouring of many algae, bacteria, fruits and vegetables. They are a critical component of the human diet and play a key role in human nutrition, health and disease.
carotenoids
lutein
beta carotene
zeaxanthin
xanthophyls
UV-vis
Raman
1. Introduction
Carotenoids encompass a wide range of fat-soluble pigmented compounds (Figure 1) and are the most widely occurring pigments in nature [1]. They are present across various parts of the ecosystem, including in plants, animals and even micro-organisms. Dietary sources in humans include carotenoid-rich fruits, vegetables, animal fat and other foods. Of the 42 dietary carotenoids, 14 are absorbed, circulated in the blood and deposited in tissue [2], where they play important roles in biological function, particularly as antioxidants and photoprotective agents [3]. Carotenoid consumption is associated with reduced risk of some cancers, including breast, oesophageal and lung [4][5][6][7], coronary heart disease [8][9], stroke [10][11], type 2 diabetes mellitus [12][13][14][15] and asthma in adults and children [16].
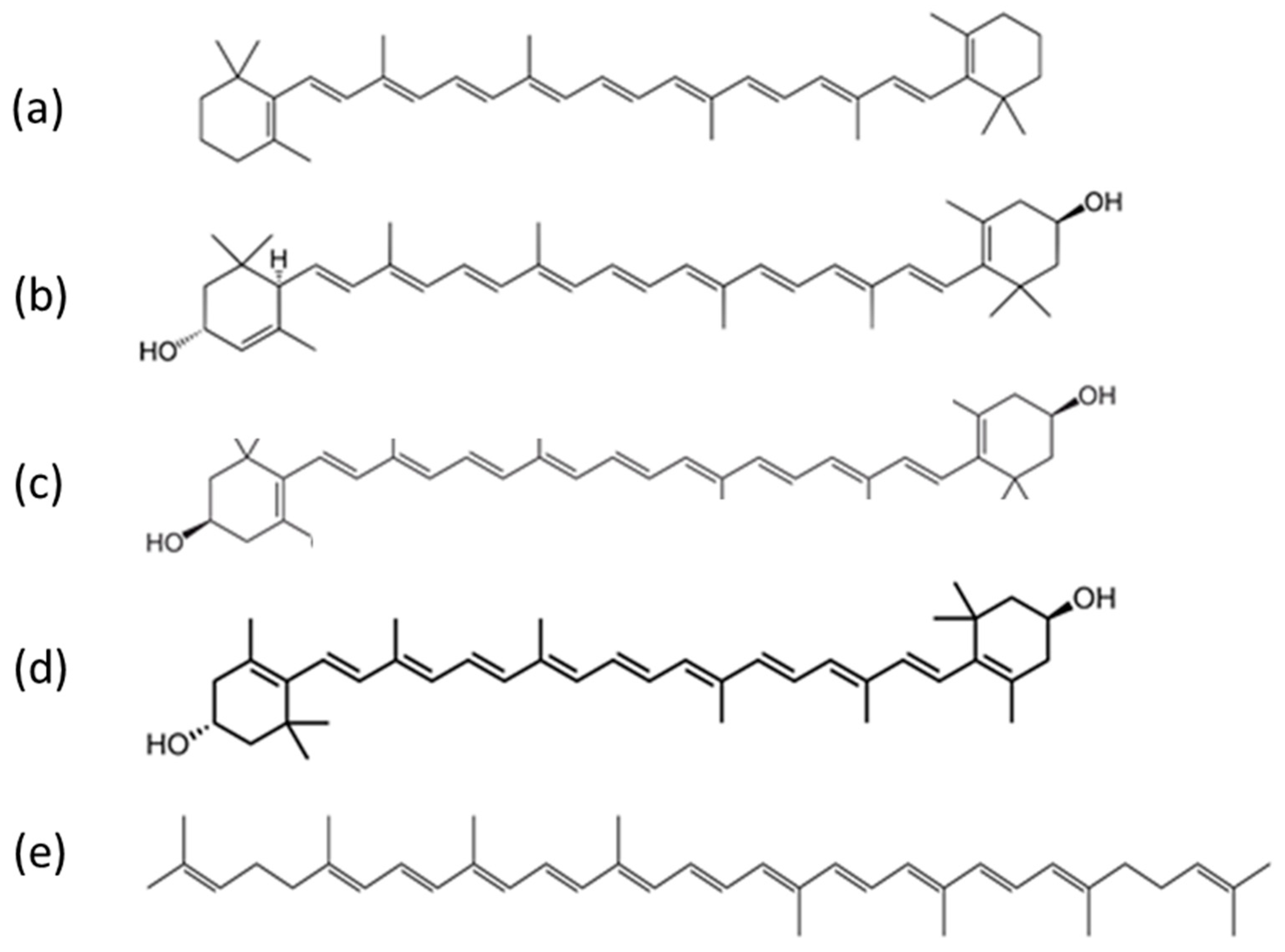
Figure 1. Examples of carotenoid compounds of relevance to human health: (a) beta carotene, (b) lutein, (c) zeaxanthin, (d) meso-zeaxanthin and (e) lycopene (public domain images reproduced from Wikimedia Commons).
2. Structure
Carotenoids are hydrocarbons made up of a basic polyene backbone structure, a hydrocarbon chain of 40 sp2 hybridised carbons, the structure of which is represented as alternating double and single bonds between the carbon atoms (Figure 1). The electrons are highly conjugated, giving rise to relatively broad molecular orbitals (MO) and low energy transitions in the visible region that produce their characteristically strong colouring [17][18]. Most naturally existing carotenoids have a trans configuration throughout their conjugated double bonds [19].
Carotenoids are broadly divided into two groups, carotenes, which contain only carbon and hydrogen atoms, e.g., alpha and beta carotenes, and a second group known as the xanthophylls; they contain oxygen atoms in addition to hydrogen and carbon atoms in their structures, e.g., lutein and zeaxanthin [20]. Both forms are poorly soluble in aqueous media and tend to aggregate in J (head to tail) or H (stacked) aggregates [21]. Xanthophylls contain at least one hydroxyl group and are, in general, more polar than carotenes. This has implications for how they aggregate [22][23][24][25] and are transported. In blood circulation, beta carotene and lycopene tend to be predominately localised in the low-density lipoproteins, while lutein and zeaxanthin are more evenly distributed among both low and high-density lipoproteins [26][27].
3. Optical Properties of Carotenoids
The absorption spectra of carotenoids are dominated by strong - * transition in the visible region of the spectrum, the wavelength positioning of which increases with increasing conjugation length [17][28]. For beta carotene in pure ethanol, the absorption maxima at 483 nm, 453 nm and 427 nm (Table 1) are, respectively, attributed to the 0–0, 0–1 and 0–2 transitions of the S0 (11Ag)-S2 (11Bu) vibrational manifold, transitions to the first S1 (21Ag) state being forbidden due to symmetry restrictions [18][24]. S2 state excitation rapidly decays by internal conversion to the S1 state, radiative relaxation from which is similarly symmetry forbidden, and thus carotenoids exhibit negligible fluorescence emission [29].
Table 1 highlights some important optical and chemical properties of dietary carotenoids, including their absorbance maximum, main Raman peak positions, conjugation length, number of hydroxyl groups and conjugated double bonds. Since the position of the longest wavelength absorption transition of carotenoids should normally be directly proportional to their conjugation length [28], zeaxanthin, which is the longer xanthophyll, should be shifted to the red when compared with lutein, especially if all other conditions, such as solvent type and temperature are kept constant for both carotenoids [28][30]. However, because of their lipophilic nature, carotenoids tend to aggregate in hydrophilic environments via weak intermolecular forces such as van der Waals interactions, hydrogen bonding and dipole forces [31]. This results in shifts in the absorption spectrum towards the red or blue region [21], depending on whether they form strongly (H) or weakly (J) coupled aggregates [31][32].
Table 1. Summary of some optical and chemical properties of carotenoids.
| Carotenoid | Absorbance λ Max in Ethanol (nm) [25][33][34][35] | Main Raman Peaks Positions (cm−1) [36] | Conjugation Length (n) [30][37] | Hydroxyl Groups | Number of Conjugated Double Bonds [38][39][40] |
|---|---|---|---|---|---|
| Beta carotene | ~427, 453, 483 | ~1000, 1160, 1520 | 9.6 | none | 11 |
| Lycopene | ~447, 474, 504 | ~1000, 1160, 1520 | 11 | none | 11 |
| Lutein | ~424, 445, 472 | ~1000, 1160, 1520 | 9.3 | 2 | 10 |
| Zeaxanthin | ~424, 445, 472 | ~1000, 1160, 1520 | 9.6 | 2 | 11 |
The longest wavelength absorption maximum of beta carotene is shifted from ~476 nm in ethanol to ~515 nm in 1:1 ethanol–water solution due to J aggregation, while the 453 nm and 427 nm peaks are blue-shifted, attributed to H-aggregation [22][23][24][25]. Similar spectral shifting in a range of solvents has been documented depending on polarising efficiencies [41][42][43]. The UV-visible absorption spectrum of beta carotene in solution is also pressure dependent, with the longest wavelength absorption maximum shifting as far as ~580 nm in carbon disulphide solution at 0.96 GPa [44]. In H aggregates of zeaxanthin, prepared in ethanol:water mixtures, the UV-vis spectrum is dominated by a strong feature at 370 nm, while in the J aggregate, formed in 1:9 THF:water solutions, the longest wavelength absorption maximum is shifted from 485 nm to ~510 nm [45][46]. A similar red shift is seen in J aggregates of lutein [47].
Both beta carotene and the xanthophylls, lutein and zeaxanthin, when dissolved in hydrated organic solvents, can form either of the two kinds of aggregates depending on the nature of the solvent [32]. For instance, when incorporated into lipid bilayers, xanthophylls appear mostly in the monomeric form at concentrations below 0.5 mol%. With higher concentrations, they tend to form mostly H aggregates [32]. It was also recently shown that as a result of carotenoid–protein interactions, absorption changes consistent with J-type aggregation can occur [21][32]. The presence of two hydroxyl groups in a carotenoid (e.g., lutein and zeaxanthin) generally promotes the formation of the H-type of aggregates [21]. When the hydrogen-bond formation is intercepted, for example, by esterification or a lack of end-ring functional groups, J-type aggregates could be formed [21]. Additionally, zeaxanthin is less polar than lutein, making it easier to form H-type aggregates in water/ethanol mixtures than lutein [21][32].
In the bloodstream, carotenoids are predominantly associated with lipoproteins, which are bound by albumin [48][49][50]. In addition to the solubilising effect, facilitating transport around the body, the complexation has been shown to protect the electron-rich molecules from oxidation [51]. When complexed with bovine serum albumin (BSA), the longest wavelength absorption maximum of beta carotene has been observed at ~515 nm, indicating the presence of J aggregates [51][52]. A similar absorption profile was observed for zeaxanthin, while lutein showed a strong, blue-shifted absorption maximum, characteristic of H-aggregates [52]. In beta carotene-bound albumin nanoparticles, the absorption profile was seen to extend further to the red, towards 600 nm [53].
References
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 5th ed.; CRC Press: Abingdon, UK, 2017; ISBN 9781315372914.
- Yeum, K.-J.; Russell, R.M. Carotenoid Bioavailability And Bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504.
- Olson, J.A. Biological Actions of Carotenoids. J. Nutr. 1989, 119, 94–95.
- Lam, T.K.; Gallicchio, L.; Lindsley, K.; Shiels, M.; Hammond, E.; Tao, X.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G.; et al. Cruciferous Vegetable Consumption and Lung Cancer Risk: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184–195.
- Singh, P.N.; Fraser, G.E. Dietary Risk Factors for Colon Cancer in a Low-Risk Population. Am. J. Epidemiol. 1998, 148, 761–774.
- Romaguera, D.; Vergnaud, A.-C.; Peeters, P.H.; van Gils, C.H.; Chan, D.S.; Ferrari, P.; Romieu, I.; Jenab, M.; Slimani, N.; Clavel-Chapelon, F.; et al. Is Concordance with World Cancer Research Fund/American Institute for Cancer Research Guidelines for Cancer Prevention Related to Subsequent Risk of Cancer? Results from the EPIC Study. Am. J. Clin. Nutr. 2012, 96, 150–163.
- Norat, T.; Aune, D.; Chan, D.; Romaguera, D. Fruits and Vegetables: Updating the Epidemiologic Evidence for the WCRF/AICR Lifestyle Recommendations for Cancer Prevention. In Advances in Nutrition and Cancer; Springer: Berlin/Heidelberg, Germany, 2014; pp. 35–50.
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Coronary Heart Disease: Meta-Analysis of Cohort Studies. J. Hum. Hypertens. 2007, 21, 717–728.
- Bazzano, L.A.; Serdula, M.K.; Liu, S. Dietary Intake of Fruits and Vegetables and Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2003, 5, 492–499.
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and Vegetable Consumption and Stroke: Meta-Analysis of Cohort Studies. Lancet 2006, 367, 320–326.
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruit and Vegetable Consumption and Risk of Stroke: A Meta-Analysis of Cohort Studies. Neurology 2005, 65, 1193–1197.
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and Vegetable Intake and Incidence of Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. BMJ 2010, 341, 543.
- Cooper, A.J.; Forouhi, N.G.; Ye, Z.; Buijsse, B.; Arriola, L.; Balkau, B.; Barricarte, A.; Beulens, J.W.J.; Boeing, H.; Büchner, F.L.; et al. Fruit and Vegetable Intake and Type 2 Diabetes: EPIC-InterAct Prospective Study and Meta-Analysis. Eur. J. Clin. Nutr. 2012, 66, 1082–1092.
- Hamer, M.; Chida, Y. Intake of Fruit, Vegetables, and Antioxidants and Risk of Type 2 Diabetes: Systematic Review and Meta-Analysis. J. Hypertens. 2007, 25, 2361–2369.
- Liu, S.; Serdula, M.; Janket, S.-J.; Cook, N.R.; Sesso, H.D.; Willett, W.C.; Manson, J.E.; Buring, J.E. A Prospective Study of Fruit and Vegetable Intake and the Risk of Type 2 Diabetes in Women. Diabetes Care 2004, 27, 2993–2996.
- Seyedrezazadeh, E.; Pour Moghaddam, M.; Ansarin, K.; Reza Vafa, M.; Sharma, S.; Kolahdooz, F. Fruit and Vegetable Intake and Risk of Wheezing and Asthma: A Systematic Review and Meta-Analysis. Nutr. Rev. 2014, 72, 411–428.
- O’Neill, L.; Lynch, P.; McNamara, M.; Byrne, H.J. Structure Property Relationships in Conjugated Organic Systems. Synth. Met. 2005, 153, 289–292.
- Lu, L.; Shi, L.; Secor, J.; Alfano, R. Resonance Raman Scattering of β-Carotene Solution Excited by Visible Laser Beams into Second Singlet State. J. Photochem. Photobiol. B 2018, 179, 18–22.
- Kiokias, S.; Proestos, C.; Varzakas, T. A Review of the Structure, Biosynthesis, Absorption of Carotenoids-Analysis and Properties of Their Common Natural Extracts. Curr. Res. Nutr. Food Sci. J. 2016, 4, 25–37.
- Amengual Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388.
- Widomska, J.; Gruszecki, W.I.; Subczynski, W.K. Factors Differentiating the Antioxidant Activity of Macular Xanthophylls in the Human Eye Retina. Antioxidants 2021, 10, 601.
- Lu, L.; Ni, X.; Luo, X. Influence of Phenylalanine on Carotenoid Aggregation. J. Appl. Spectrosc. 2015, 81, 1068–1072.
- Lu, L.; Liu, G.; Ni, X.; Luo, X.; Lu, L.; Liu, G.; Ni, X.; Luo, X. Spectral Analysis of Interaction between Carotenoid and Tyrosine in Ethanol-Water Solution. RJPCA 2015, 89, 417–422.
- Qu, F.; Fu, H.; Li, Y.; Sun, C.; Li, Z.; Gong, N.; Men, Z. Temperature Effect on Electronic and Vibrational Properties of β-Carotene Aggregates in Aqueous Ethanol Solution. Dye. Pigments 2019, 166, 323–329.
- Meinhardt-Wollweber, M.; Suhr, C.; Kniggendorf, A.K.; Roth, B. Absorption and Resonance Raman Characteristics of β-Carotene in Water-Ethanol Mixtures, Emulsion and Hydrogel. AIP Adv. 2018, 8, 055320.
- Clevidence, B.A.; Bieri, J.G. Association of Carotenoids with Human Plasma Lipoproteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1993; pp. 33–46.
- Parker, R.S. Absorption, Metabolism, and Transport of Carotenoids. FASEB J. 1996, 10, 542–551.
- Mendes-Pinto, M.M.; Sansiaume, E.; Hashimoto, H.; Pascal, A.A.; Gall, A.; Robert, B. Electronic Absorption and Ground State Structure of Carotenoid Molecules. J. Phys. Chem. B 2013, 117, 11015–11021.
- Shreve, A.P.; Trautman, J.K.; Owens, T.G.; Albrecht, A.C. Determination of the S2 Lifetime of β-Carotene. Chem. Phys. Lett. 1991, 178, 89–96.
- Arteni, A.-A.; Fradot, M.; Galzerano, D.; Mendes-Pinto, M.M.; Sahel, J.-A.; Picaud, S.; Robert, B.; Pascal, A.A. Structure and Conformation of the Carotenoids in Human Retinal Macular Pigment. PLoS ONE 2015, 10, e0135779.
- Hempel, J.; Schädle, C.N.; Leptihn, S.; Carle, R.; Schweiggert, R.M. Structure Related Aggregation Behavior of Carotenoids and Carotenoid Esters. J. Photochem. Photobiol. A Chem. 2016, 317, 161–174.
- Billsten, H.H.; Sundström, V.; Polívka, T. Self-Assembled Aggregates of the Carotenoid Zeaxanthin: Time-Resolved Study of Excited States. J. Phys. Chem. A 2005, 109, 1521–1529.
- Aspinall-O’Dea, M.; Wentworth, M.; Pascal, A.; Robert, B.; Ruban, A.; Horton, P. In Vitro Reconstitution of the Activated Zeaxanthin State Associated with Energy Dissipation in Plants. Proc. Natl. Acad. Sci. USA 2002, 99, 16331–16335.
- Moschetti, A.; Fox, C.A.; McGowen, S.; Ryan, R.O. Lutein Nanodisks Protect Human Retinal Pigment Epithelial Cells from UV Light-Induced Damage. Front. Nanotechnol. 2022, 4, 955022.
- dos Santos, R.C.; Ombredane, A.S.; Souza, M.T.; Vasconcelos, A.G.; Plácido, A.; Das, A.; Amorim, G.N.; Alves Barbosa, E.; Lima, F.C.D.A.; Ropke, C.D.; et al. Lycopene-Rich Extract from Red Guava (Psidium guajava L.) Displays Cytotoxic Effect against Human Breast Adenocarcinoma Cell Line MCF-7 via an Apoptotic-like Pathway. Food Res. Int. 2018, 105, 184–196.
- Udensi, J.; Loskutova, E.; Loughman, J.; Byrne, H.J. Quantitative Raman Analysis of Carotenoid Protein Complexes in Aqueous Solution. Molecules 2022, 27, 4724.
- Avendaño, C.; Menéndez, J.C. Cancer Chemoprevention. In Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2008; pp. 417–429.
- Beta-Carotene|C40H56-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280489 (accessed on 8 December 2022).
- Lycopene|C40H56-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/446925 (accessed on 8 December 2022).
- Widomska, J.; Sangiovanni, J.P.; Subczynski, W.K. Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients 2020, 12, 1333.
- Ouyang, S.; Sun, C.; Zhou, M.; Li, D.; Wang, W.; Qu, G.; Li, Z.; Gao, S.; Yang, J.; Ouyang, S.; et al. Effect of Solution Concentration on the Structured Order and Optical Properties of Short-Chain Polyene Biomolecules. SCPMA 2010, 53, 1646–1650.
- Gong, N.; Fu, H.; Wang, S.; Cao, X.; Li, Z.; Sun, C.; Men, Z. All-Trans-β-Carotene Absorption Shift and Electron-Phonon Coupling Modulated by Solvent Polarizability. J. Mol. Liq. 2018, 251, 417–422.
- Mendes-Pinto, M.M.; LaFountain, A.M.; Stoddard, M.C.; Prum, R.O.; Frank, H.A.; Robert, B. Variation in Carotenoid–Protein Interaction in Bird Feathers Produces Novel Plumage Coloration. J. R. Soc. Interface 2012, 9, 3338–3350.
- Liu, W.L.; Zheng, Z.R.; Zhu, R.B.; Liu, Z.G.; Xu, D.P.; Yu, H.M.; Wu, W.Z.; Li, A.H.; Yang, Y.Q.; Su, W.H. Effect of Pressure and Solvent on Raman Spectra of All-Trans-Beta-Carotene. J. Phys. Chem. A 2007, 111, 10044–10049.
- Dudek, M.; Zajac, G.; Kaczor, A.; Baranska, M. Resonance Raman Optical Activity of Zeaxanthin Aggregates. J. Raman Spectrosc. 2017, 48, 673–679.
- Wang, C.; Berg, C.J.; Hsu, C.C.; Merrill, B.A.; Tauber, M.J. Characterization of Carotenoid Aggregates by Steady-State Optical Spectroscopy. J. Phys. Chem. B 2012, 116, 10617–10630.
- Zajac, G.; Lasota, J.; Dudek, M.; Kaczor, A.; Baranska, M. Pre-Resonance Enhancement of Exceptional Intensity in Aggregation-Induced Raman Optical Activity (AIROA) Spectra of Lutein Derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 356–360.
- Parachalil, D.R.; Bruno, C.; Bonnier, F.; Blasco, H.; Chourpa, I.; McIntyre, J.; Byrne, H.J. Raman Spectroscopic Screening of High and Low Molecular Weight Fractions of Human Serum. Analyst 2019, 144, 4295–4311.
- Li, X.; Wang, G.; Chen, D.; Lu, Y. β-Carotene and Astaxanthin with Human and Bovine Serum Albumins. Food Chem. 2015, 179, 213–221.
- Lopez, M.F.; Krastins, B.; Sarracino, D.A.; Byram, G.; Vogelsang, M.S.; Prakash, A.; Peterman, S.; Ahmad, S.; Vadali, G.; Deng, W.; et al. Proteomic Signatures of Serum Albumin-Bound Proteins from Stroke Patients with and without Endovascular Closure of PFO Are Significantly Different and Suggest a Novel Mechanism for Cholesterol Efflux. Clin. Proteom. 2015, 12, 2.
- Chang, H.T.; Cheng, H.; Han, R.M.; Zhang, J.P.; Skibsted, L.H. Binding to Bovine Serum Albumin Protects β-Carotene against Oxidative Degradation. J. Agric. Food Chem. 2016, 64, 5951–5957.
- Reszczynska, E.; Welc, R.; Grudzinski, W.; Trebacz, K.; Gruszecki, W.I. Carotenoid Binding to Proteins: Modeling Pigment Transport to Lipid Membranes. Arch. Biochem. Biophys. 2015, 584, 125–133.
- Phuong, P.T.T.; Lee, S.; Lee, C.; Seo, B.; Park, S.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Shin, B.S.; Youn, Y.S. Beta-Carotene-Bound Albumin Nanoparticles Modified with Chlorin E6 for Breast Tumor Ablation Based on Photodynamic Therapy. Colloids Surf. B Biointerfaces 2018, 171, 123–133.
More
Information
Subjects:
Optics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
09 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




