
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandro Percario | -- | 4328 | 2023-01-08 18:49:41 | | | |
| 2 | Peter Tang | Meta information modification | 4328 | 2023-01-09 02:20:07 | | |
Video Upload Options
Malaria is a disease that affects thousands of people around the world every year. Its pathogenesis is associated with the production of reactive oxygen and nitrogen species (RONS) and lower levels of micronutrients and antioxidants. Patients under drug treatment have high levels of oxidative stress biomarkers in the body tissues, which limits the use of these drugs. Therefore, several studies have suggested that RONS inhibition may represent an adjuvant therapeutic strategy in the treatment of these patients by increasing the antioxidant capacity of the host. In this sense, supplementation with antioxidant compounds such as zinc, selenium, and vitamins A, C, and E has been suggested as part of the treatment. Among dietary antioxidants, lycopene is the most powerful antioxidant among the main carotenoids.
1. Introduction
2. Oxidative Stress
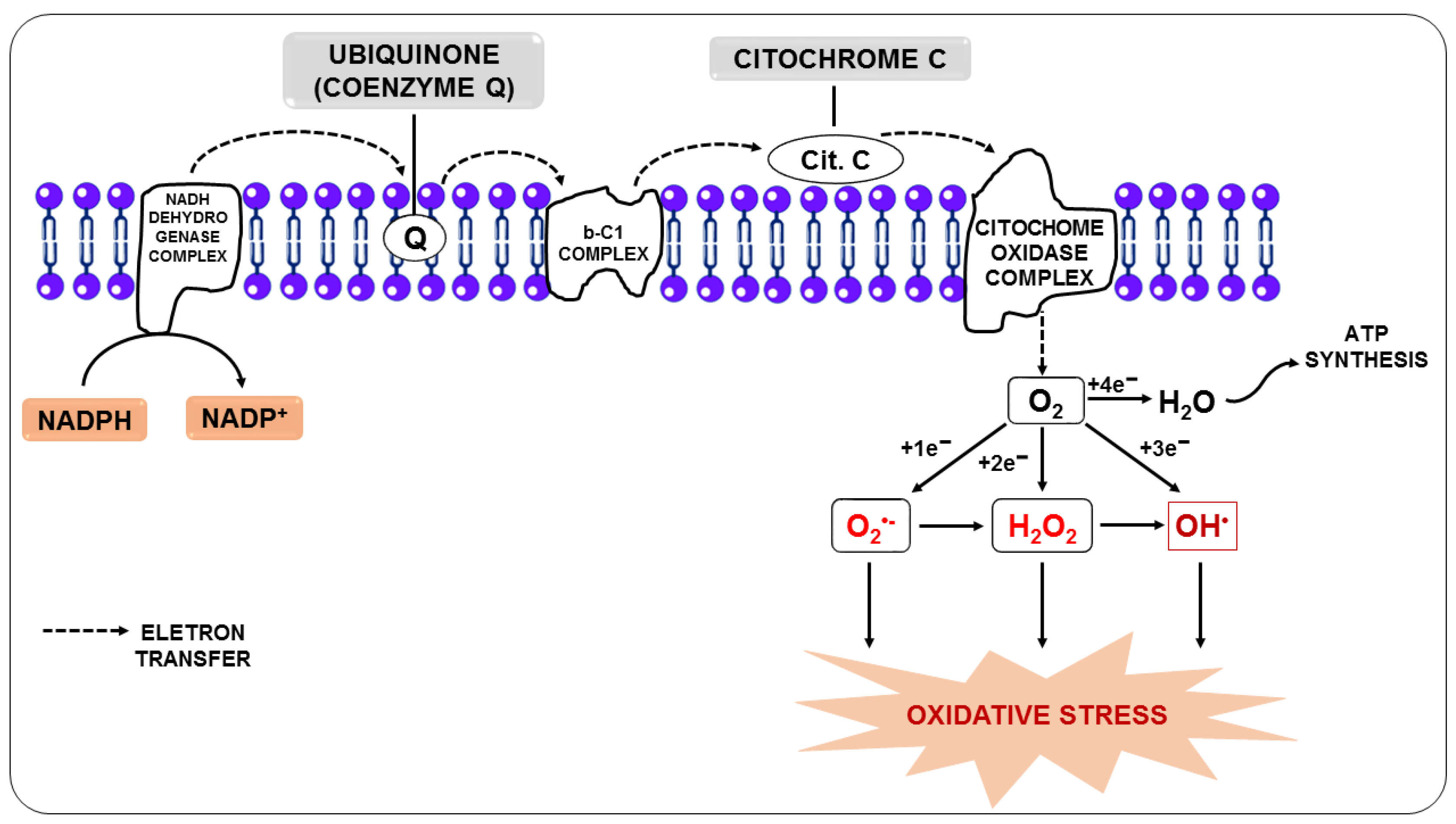
3. Oxidative Stress in Malaria
3.1. Oxidative Stress as a Host Defense Mechanism
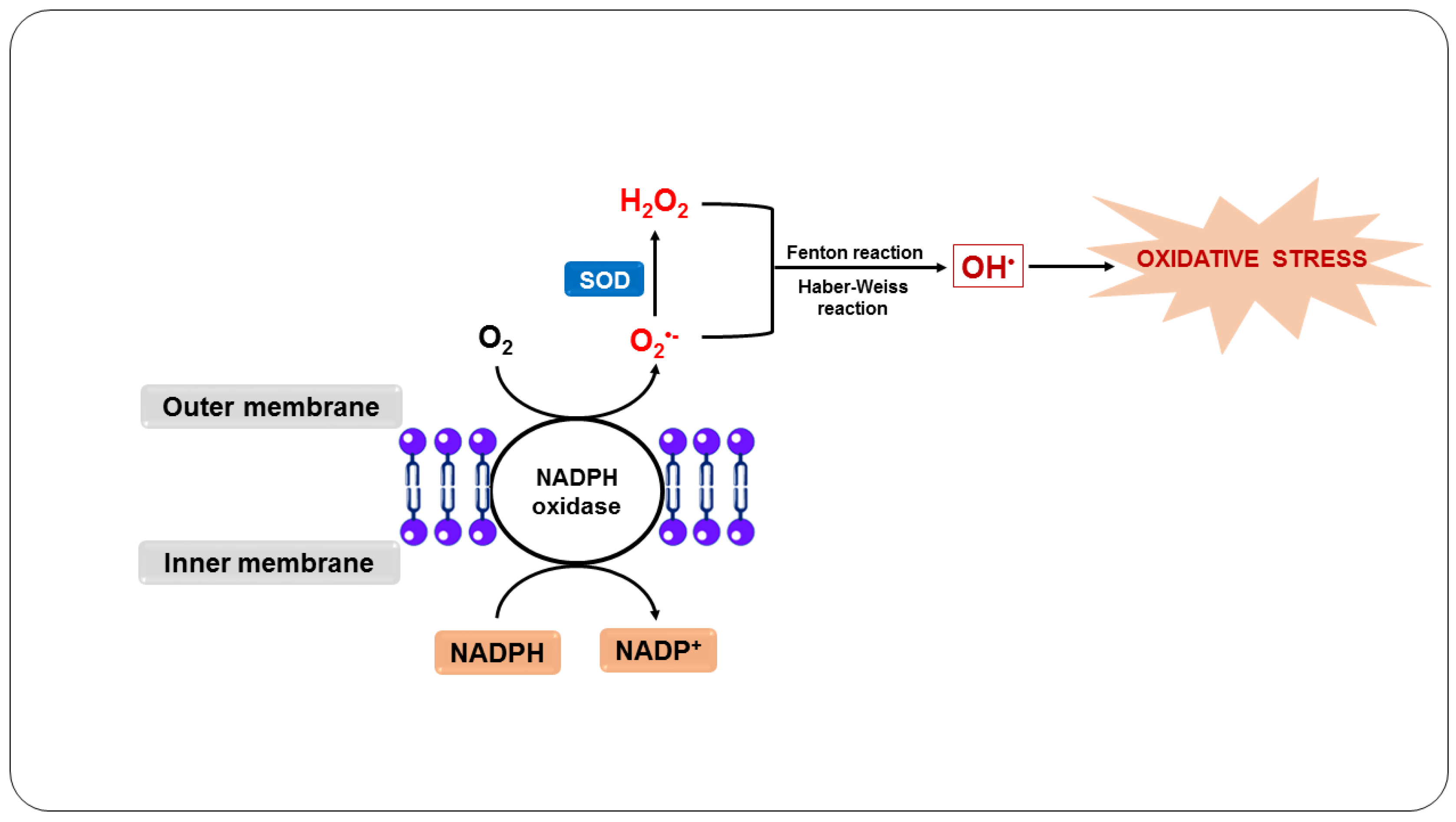
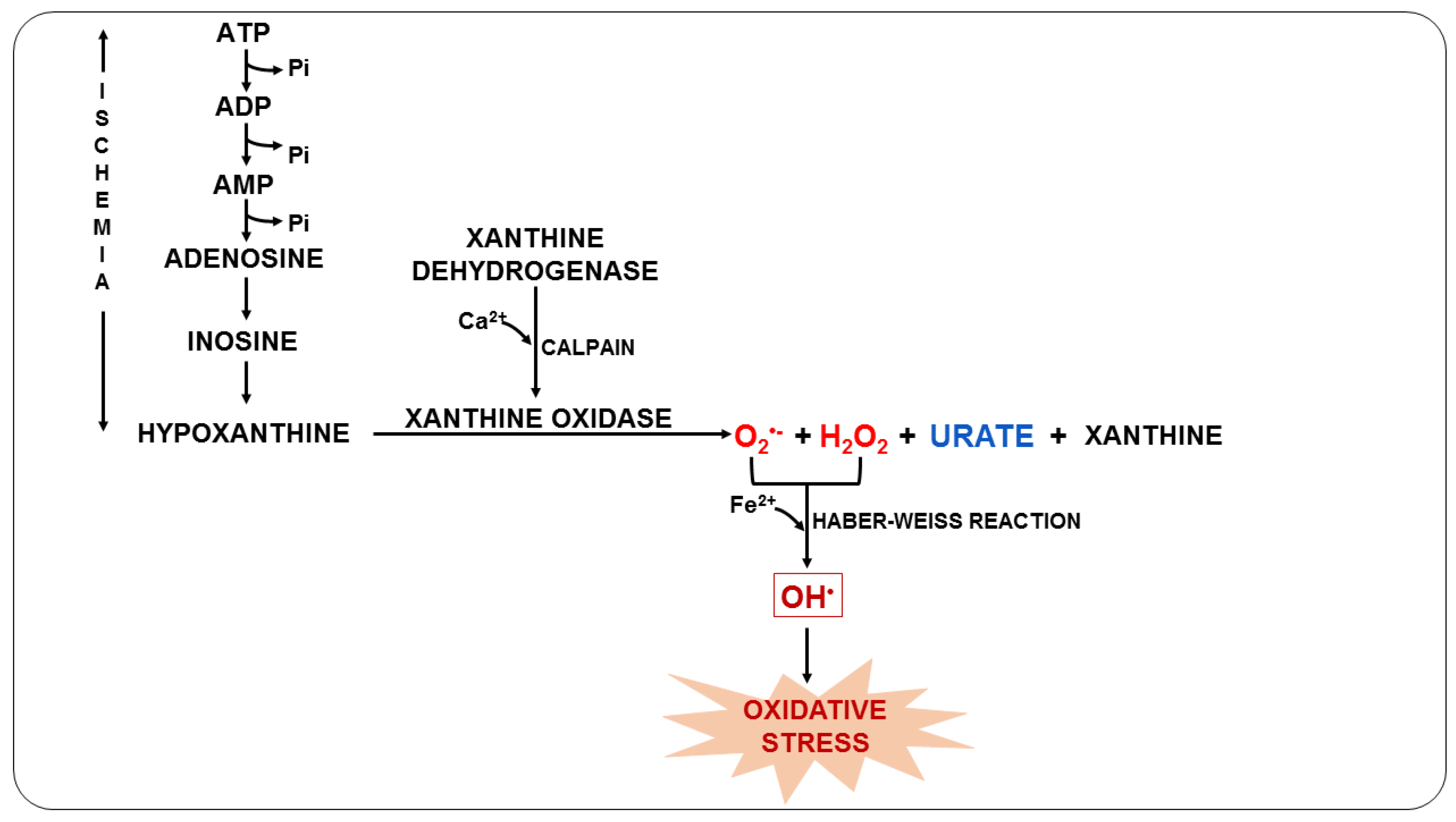
3.2. Oxidative Stress Due to Ischemia-Reperfusion Syndrome
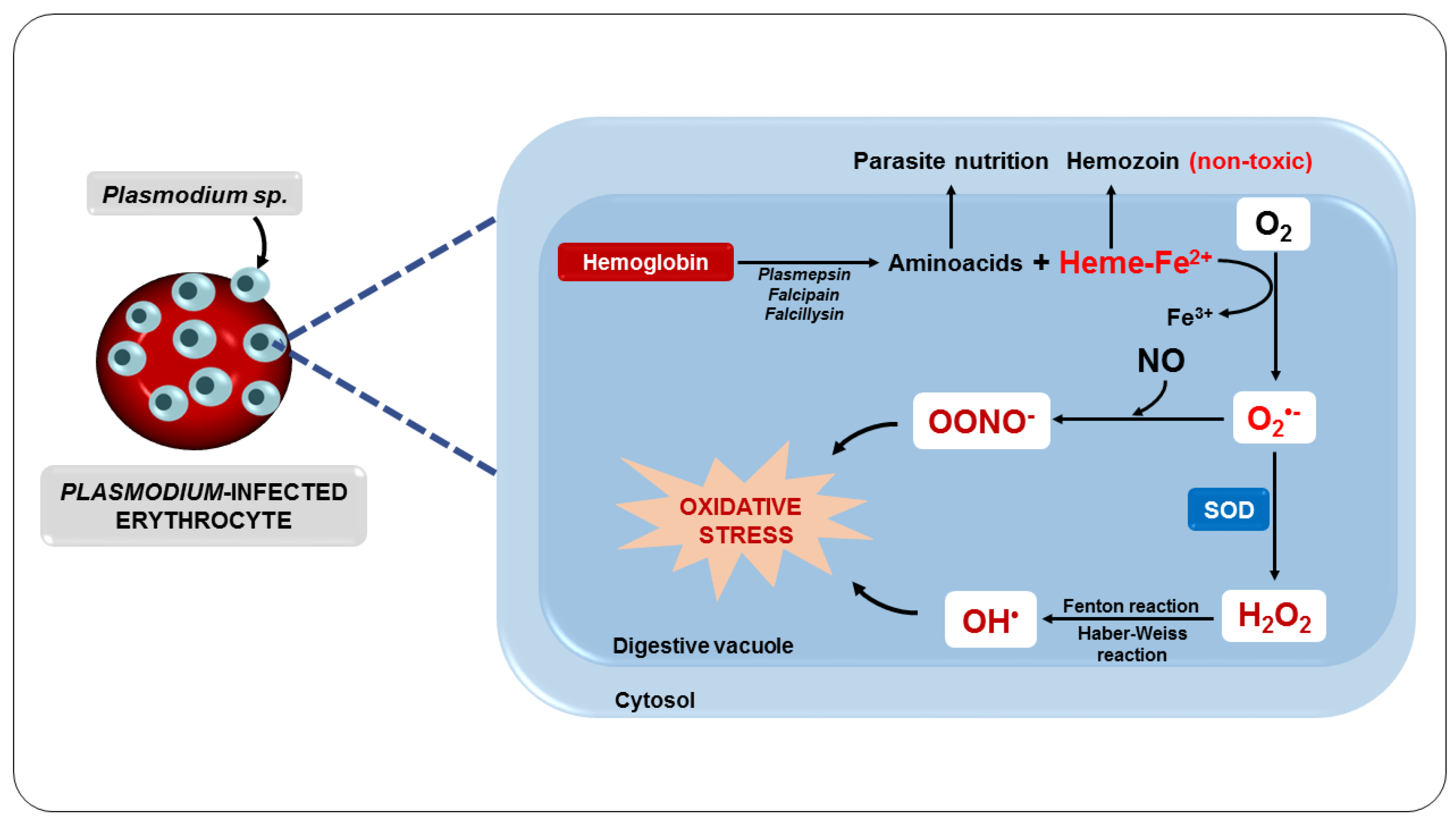
3.3. Oxidative Stress Due to the Metabolism of the Parasite
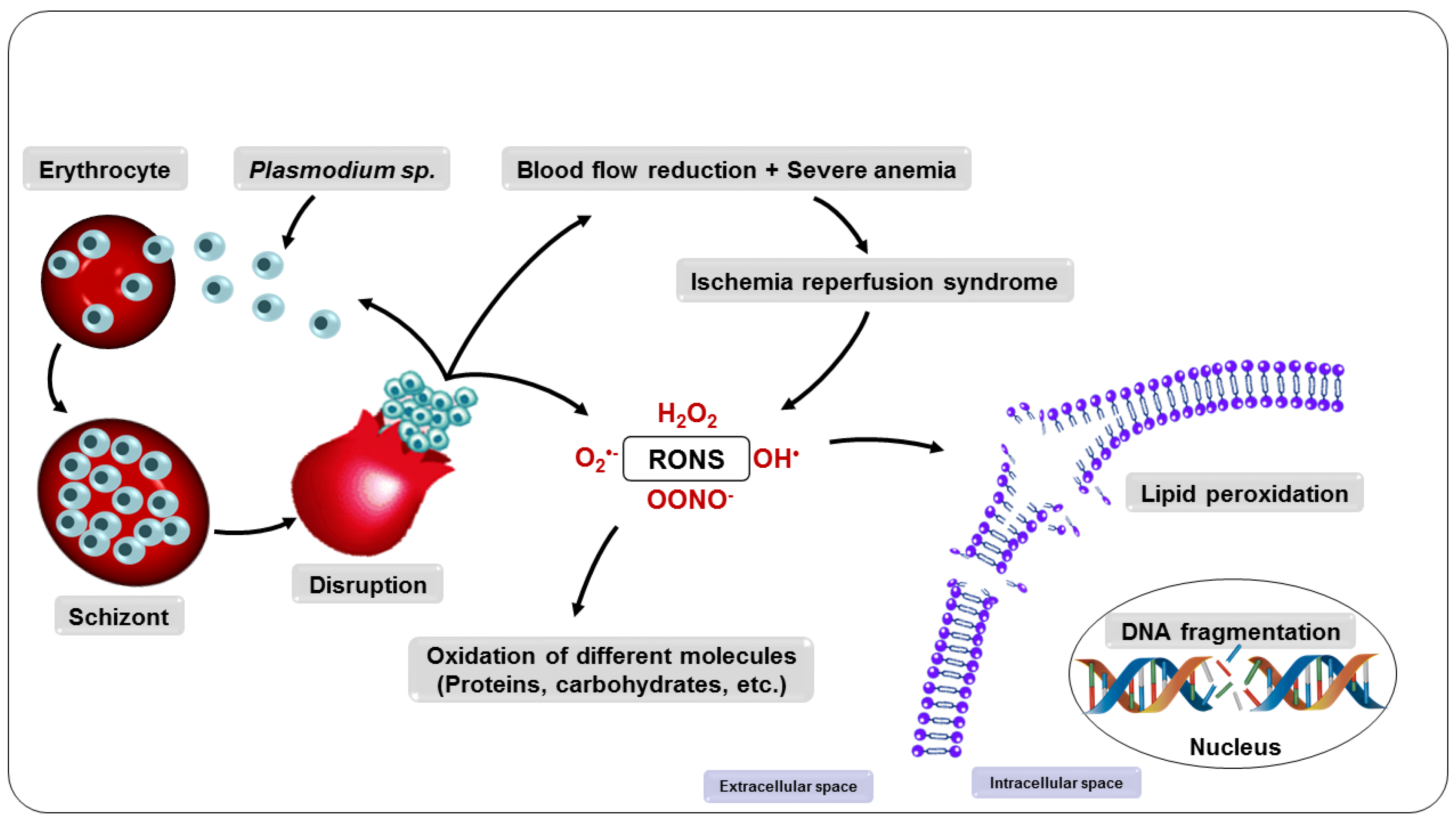
3.4. Oxidative Stress as a Consequence of the Metabolization of Antimalarial Drugs
3.5. Nitric Oxide in Malaria
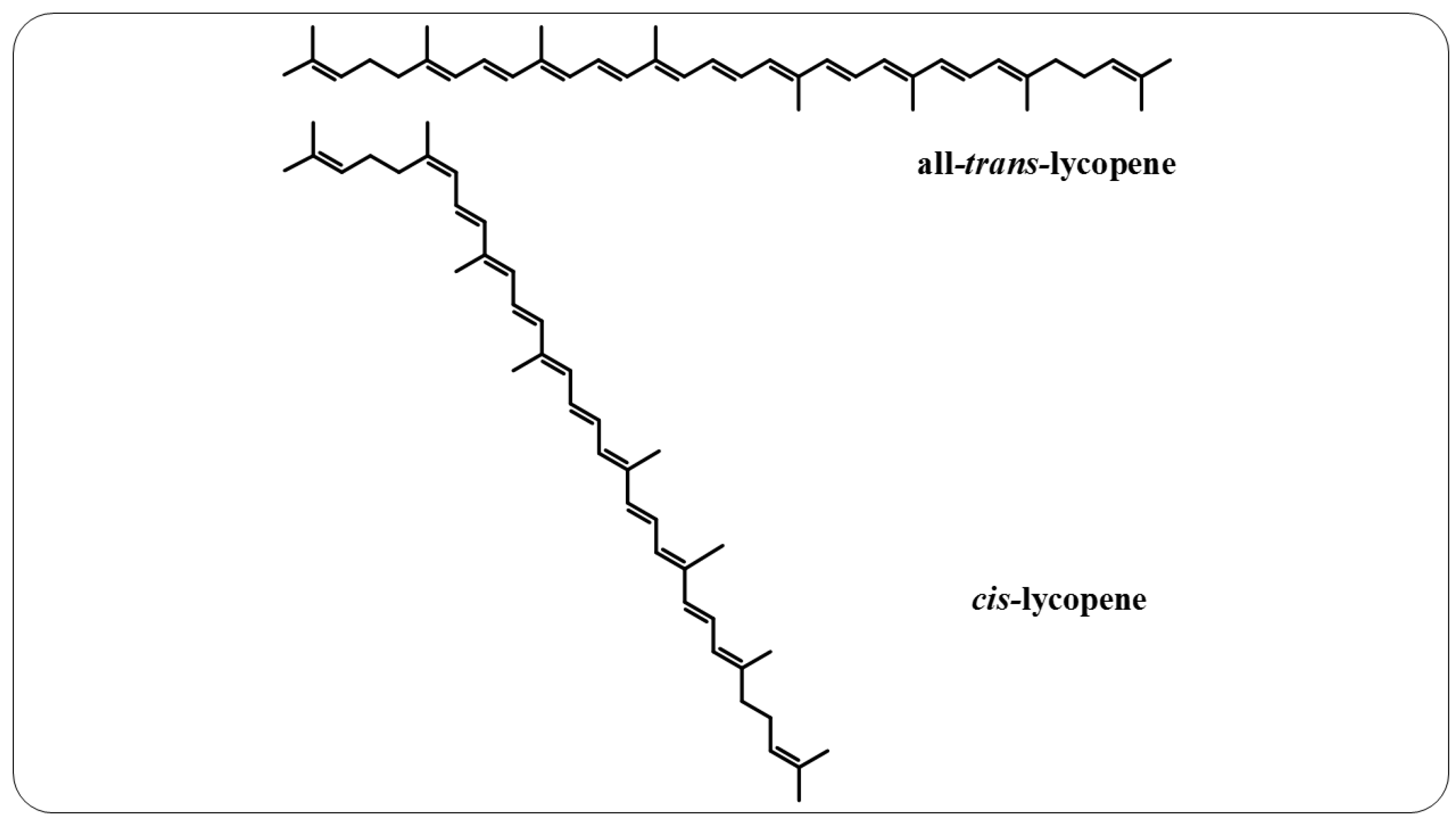
4. Lycopene
5. Antioxidant Effects of Lycopene
6. Effects of Lycopene on Malaria
References
- WHO. Word Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Griffiths, M.J.; Shafi, M.J.; Popper, S.J.; Hemingway, C.A.; Kortok, M.M.; Wathen, A.; Rockett, K.A.; Mott, R.; Levin, M.; Newton, C.R.; et al. Genomewide Analysis of the Host Response to Malaria in Kenyan Children. J. Infect. Dis. 2005, 191, 1599–1611.
- van Wolfswinkel, M.E.; Langenberg, M.C.C.; Wammes, L.J.; Sauerwein, R.W.; Koelewijn, R.; Hermsen, C.C.; van Hellemond, J.J.; van Genderen, P.J. Changes in Total and Differential Leukocyte Counts during the Clinically Silent Liver Phase in a Controlled Human Malaria Infection in Malaria-Naïve Dutch Volunteers. Malar. J. 2017, 16, 457.
- Aitken, E.H.; Alemu, A.; Rogerson, S.J. Neutrophils and Malaria. Front. Immunol. 2018, 9, 3005.
- Gonçalves, R.M.; Scopel, K.K.G.; Bastos, M.S.; Ferreira, M.U. Cytokine Balance in Human Malaria: Does Plasmodium Vivax Elicit More Inflammatory Responses than Plasmodium falciparum? PLoS ONE 2012, 7, e44394.
- Oyegue-Liabagui, S.L.; Bouopda-Tuedom, A.G.; Kouna, L.C.; Maghendji-Nzondo, S.; Nzoughe, H.; Tchitoula-Makaya, N.; Pegha-Moukandja, I.; Lekana-Douki, J.-B. Pro- and Anti-Inflammatory Cytokines in Children with Malaria in Franceville, Gabon. Am. J. Clin. Exp. Immunol. 2017, 6, 9–20.
- Nahrendorf, W.; Ivens, A.; Spence, P.J. Inducible Mechanisms of Disease Tolerance Provide an Alternative Strategy of Acquired Immunity to Malaria. eLife 2021, 10, e63838.
- Torre, D.; Speranza, F.; Giola, M.; Matteelli, A.; Tambini, R.; Biondi, G. Role of Th1 and Th2 Cytokines in Immune Response to Uncomplicated Plasmodium falciparum Malaria. Clin. Diagn. Lab. Immunol. 2002, 9, 348–351.
- John, C.C.; Park, G.S.; Sam-Agudu, N.; Opoka, R.O.; Boivin, M.J. Elevated Serum Levels of IL-1ra in Children with Plasmodium falciparum Malaria Are Associated with Increased Severity of Disease. Cytokine 2008, 41, 204–208.
- Dieye, Y.; Mbengue, B.; Dagamajalu, S.; Fall, M.M.; Loke, M.F.; Nguer, C.M.; Thiam, A.; Vadivelu, J.; Dieye, A. Cytokine Response during Non-Cerebral and Cerebral Malaria: Evidence of a Failure to Control Inflammation as a Cause of Death in African Adults. PeerJ 2016, 2016, e1965.
- Mandala, W.L.; Msefula, C.L.; Gondwe, E.N.; Drayson, M.T.; Molyneux, M.E.; MacLennan, C.A. Cytokine Profiles in Malawian Children Presenting with Uncomplicated Malaria, Severe Malarial Anemia, and Cerebral Malaria. Clin. Vaccine Immunol. 2017, 24, e00533-16.
- Couper, K.N.; Blount, D.G.; Wilson, M.S.; Hafalla, J.C.; Belkaid, Y.; Kamanaka, M.; Flavell, R.A.; De Souza, J.B.; Riley, E.M. IL-10 from CD4+CD25-Foxp3-CD127—Adaptive Regulatory T Cells Modulates Parasite Clearance and Pathology during Malaria Infection. PLoS Pathog. 2008, 4, e1000004.
- Bakir, H.Y.; Tomiyama, C.; Abo, T. Cytokine Profile of Murine Malaria: Stage-Related Production of Inflammatory and Anti-Inflammatory Cytokines. Biomed. Res. 2011, 32, 203–208.
- Gonçalves, R.M.; Lima, N.F.; Ferreira, M.U. Parasite Virulence, Co-Infections and Cytokine Balance in Malaria. Pathog. Glob. Health 2014, 108, 173–178.
- Perera, M.K.; Herath, N.P.; Pathirana, S.L.; Phone-Kyaw, M.; Alles, H.K.; Mendis, K.N.; Premawansa, S.; Handunnetti, S.M. Association of High Plasma TNF-Alpha Levels and TNF-Alpha/IL-10 Ratios with TNF2 Allele in Severe P. Falciparum Malaria Patients in Sri Lanka. Pathog. Glob. Health 2013, 107, 21–29.
- Herbert, F.; Tchitchek, N.; Bansal, D.; Jacques, J.; Pathak, S.; Bécavin, C.; Fesel, C.; Dalko, E.; Cazenave, P.A.; Preda, C.; et al. Evidence of IL-17, IP-10, and IL-10 Involvement in Multiple-Organ Dysfunction and IL-17 Pathway in Acute Renal Failure Associated to Plasmodium falciparum Malaria. J. Transl. Med. 2015, 13, 369.
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 2014, 395, 203–230.
- Ashok, G.R.; Samruddhi, M.; Shreewardhan, R.; Mira, R.; Abhay, C.; Ranjana, D. Influence of MDA and Pro-Inflammatory Cytokine Levels in the Pathogenesis of Severe Malaria in Experimental Murine Model. Scholars Acad. J. Biosc. 2016, 4, 617–626.
- Ty, M.C.; Zuniga, M.; Götz, A.; Kayal, S.; Sahu, P.K.; Mohanty, A.; Mohanty, S.; Wassmer, S.C.; Rodriguez, A. Malaria Inflammation by Xanthine Oxidase-produced Reactive Oxygen Species. EMBO Mol. Med. 2019, 11, e9903.
- Haldar, K.; Murphy, S.C.; Milner, D.A.; Taylor, T.E. Malaria: Mechanisms of Erythrocytic Infection and Pathological Correlates of Severe Disease. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 217–249.
- Narsaria, N.; Mohanty, C.; Das, B.K.; Mishra, S.P.; Prasad, R. Oxidative Stress in Children with Severe Malaria. J. Trop. Pediatr. 2012, 58, 147–150.
- Srivastava, A.; Creek, D.J.; Evans, K.J.; De Souza, D.; Schofield, L.; Müller, S.; Barrett, M.P.; McConville, M.J.; Waters, A.P. Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites. PLoS Pathog. 2015, 11, e1004882.
- Clark, I.A.; Budd, A.C.; Alleva, L.M.; Cowden, W.B. Human Malarial Disease: A Consequence of Inflammatory Cytokine Release. Malar. J. 2006, 5, 85.
- Jain, V.; Singh, P.P.; Silawat, N.; Patel, R.; Saxena, A.; Bharti, P.K.; Shukla, M.; Biswas, S.; Singh, N. A Preliminary Study on Pro- and Anti-Inflammatory Cytokine Profiles in Plasmodium Vivax Malaria Patients from Central Zone of India. Acta Trop. 2010, 113, 263–268.
- Portugal, S.; Moebius, J.; Skinner, J.; Doumbo, S.; Doumtabe, D.; Kone, Y.; Dia, S.; Kanakabandi, K.; Sturdevant, D.E.; Virtaneva, K.; et al. Exposure-Dependent Control of Malaria-Induced Inflammation in Children. PLoS Pathog. 2014, 10, e1004079.
- Tekwani, B.L.; Walker, L.A. Targeting the Hemozoin Synthesis Pathway for New Antimalarial Drug Discovery: Technologies for in Vitro β-Hematin Formation Assay. Comb. Chem. High Throughput Screen. 2005, 8, 63–79.
- Gomes, A.R.Q.; Cunha, N.; Varela, E.L.P.; Brígido, H.P.C.; Vale, V.V.; Dolabela, M.F.; De Carvalho, E.P.; Percário, S. Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int. J. Mol. Sci. 2022, 23, 5949.
- Nussenblatt, V.; Mukasa, G.; Metzger, A.; Ndeezi, G.; Eisinger, W.; Semba, R.D. Relationship between Carotenoids and Anaemia during Acute Uncomplicated Plasmodium falciparum Malaria in Children. J. Health Popul. Nutr. 2002, 20, 205–214.
- Gies, S.; Diallo, S.; Roberts, S.A.; Kazienga, A.; Powney, M.; Brabin, L.; Ouedraogo, S.; Swinkels, D.W.; Geurts-Moespot, A.J.; Claeys, Y.; et al. Effects of Weekly Iron and Folic Acid Supplements on Malaria Risk in Nulliparous Women in Burkina Faso: A Periconceptional, Double-Blind, Randomized Controlled Noninferiority Trial. J. Infect. Dis. 2018, 218, 1099–1109.
- Iribhogbe, O.I.; Agbaje, E.O.; Oreagba, I.A.; Aina, O.O.; Ota, A.D. Oxidative Stress and Micronutrient Therapy in Malaria: An In Vivo Study in Plasmodium Berghei Infected Mice. Pak. J. Biol. Sci. 2013, 16, 160–167.
- Shankar, A.H.; Genton, B.; Baisor, M.; Jaino, P.; Tamja, S.; Adiguma, T.; Wu, L.; Rare, L.; Bannon, D.; Tielsch, J.M.; et al. The Influence of Zinc Supplementation on Morbidity Due to Plasmodium falciparum: A Randomized Trial in Preschool Children in Papua New Guinea. Am. J. Trop. Med. Hyg. 2000, 62, 663–669.
- Sondo, P.; Tahita, M.C.; Rouamba, T.; Derra, K.; Kaboré, B.; Compaoré, C.S.; Ouédraogo, F.; Rouamba, E.; Ilboudo, H.; Bambara, E.A.; et al. Assessment of a Combined Strategy of Seasonal Malaria Chemoprevention and Supplementation with Vitamin A, Zinc and Plumpy’DozTM to Prevent Malaria and Malnutrition in Children under 5 Years Old in Burkina Faso: A Randomized Open-Label Trial (SMC-NUT). Trials 2021, 22, 360.
- Das, B.S.; Thurnham, D.I.; Das, D.B. Plasma α-Tocopherol, Retinol, and Carotenoids in Children with Falciparum Malaria. Am. J. Clin. Nutr. 1996, 64, 94–100.
- Metzger, A.; Mukasa, G.; Shankar, A.H.; Ndeezi, G.; Melikian, G.; Semba, R.D. Antioxidant Status and Acute Malaria in Children in Kampala, Uganda. Am. J. Trop. Med. Hyg. 2001, 65, 115–119.
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35, 1147–1150.
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748.
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free Radic. Biol. Med. 2009, 47, 333–343.
- Scherz-Shouval, R.; Elazar, Z. Regulation of Autophagy by ROS: Physiology and Pathology. Trends Biochem. Sci. 2011, 36, 30–38.
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276.
- Nathan, C.; Ding, A. SnapShot: Reactive Oxygen Intermediates (ROI). Cell 2010, 140, 952.e2.
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alexandria J. Med. 2018, 54, 287–293.
- York-Duran, M.J.; Godoy-Gallardo, M.; Jansman, M.M.T.; Hosta-Rigau, L. A Dual-Component Carrier with Both Non-Enzymatic and Enzymatic Antioxidant Activity towards ROS Depletion. Biomater. Sci. 2019, 7, 4813–4826.
- Rizvi, S.I.; Maurya, P.K. Alterations in Antioxidant Enzymes During Aging in Humans. Mol. Biotechnol. 2007, 37, 58–61.
- Gęgotek, A.; Nikliński, J.; Žarković, N.; Žarković, K.; Waeg, G.; Łuczaj, W.; Charkiewicz, R.; Skrzydlewska, E. Lipid Mediators Involved in the Oxidative Stress and Antioxidant Defence of Human Lung Cancer Cells. Redox Biol. 2016, 9, 210–219.
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71.
- Aninagyei, E.; Tettey, C.O.; Kwansa-Bentum, H.; Boakye, A.A.; Ghartey-Kwansah, G.; Boye, A.; Acheampong, D.O. Oxidative Stress and Associated Clinical Manifestations in Malaria and Sickle Cell (HbSS) Comorbidity. PLoS ONE 2022, 17, e0269720.
- Kasperczyk, S.; Dobrakowski, M.; Kasperczyk, A.; Zalejska-Fiolka, J.; Pawlas, N.; Kapka-Skrzypczak, L.; Birkner, E. Effect of Treatment with N-Acetylcysteine on Non-Enzymatic Antioxidant Reserves and Lipid Peroxidation in Workers Exposed to Lead. Ann. Agric. Environ. Med. 2014, 21, 272–277.
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78.
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006.
- Csányi, G.; Miller, F.J., Jr. Oxidative Stress in Cardiovascular Disease. Int. J. Mol. Sci. 2014, 15, 6002–6008.
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate Extract Alleviates Disease Activity and Some Blood Biomarkers of Inflammation and Oxidative Stress in Rheumatoid Arthritis Patients. Eur. J. Clin. Nutr. 2017, 71, 92–96.
- Kremsner, P.G.; Greve, B.; Lell, B.; Luckner, D.; Schmid, D. Malarial Anaemia in African Children Associated with High Oxygen-Radical Production. Lancet 2000, 355, 40–41.
- Guha, M.; Kumar, S.; Choubey, V.; Maity, P.; Bandyopadhyay, U.; Guha, M.; Kumar, S.; Choubey, V.; Maity, P.; Bandyopadhyay, U. Apoptosis in Liver during Malaria: Role of Oxidative Stress and Implication of Mitochondrial Pathway. FASEB J. 2006, 20, 1224–1226.
- Percário, S.; Moreira, D.; Gomes, B.; Ferreira, M.; Gonçalves, A.; Laurindo, P.; Vilhena, T.; Dolabela, M.; Green, M. Oxidative Stress in Malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372.
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive Oxygen Species in the Immune System. Int. Rev. Immunol. 2013, 32, 249–270.
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828.
- Leoratti, F.M.D.S.; Trevelin, S.C.; Cunha, F.Q.; Rocha, B.C.; Costa, P.A.C.; Gravina, H.D.; Tada, M.S.; Pereira, D.B.; Golenbock, D.T.; do Valle Antonelli, L.R.; et al. Neutrophil Paralysis in Plasmodium Vivax Malaria. PLoS Negl. Trop. Dis. 2012, 6, e1710.
- Chua, C.L.L.; Ng, I.M.J.; Yap, B.J.M.; Teo, A. Factors Influencing Phagocytosis of Malaria Parasites: The Story so Far. Malar. J. 2021, 20, 319.
- Zelter, T.; Strahilevitz, J.; Simantov, K.; Yajuk, O.; Adams, Y.; Ramstedt Jensen, A.; Dzikowski, R.; Granot, Z. Neutrophils Impose Strong Immune Pressure against PfEMP1 Variants Implicated in Cerebral Malaria. EMBO Rep. 2022, 23, e53641.
- Joos, C.; Marrama, L.; Polson, H.E.J.; Corre, S.; Diatta, A.M.; Diouf, B.; Trape, J.F.; Tall, A.; Longacre, S.; Perraut, R. Clinical Protection from Falciparum Malaria Correlates with Neutrophil Respiratory Bursts Induced by Merozoites Opsonized with Human Serum Antibodies. PLoS ONE 2010, 5, e9871.
- Chen, L.; Zhang, Z.-H.; Sendo, F. Neutrophils Play a Critical Role in the Pathogenesis of Experimental Cerebral Malaria. Clin. Exp. Immunol. 2001, 120, 125–133.
- Su, Z.; Fortin, A.; Gros, P.; Stevenson, M.M. Opsonin-Independent Phagocytosis: An Effector Mechanism against Acute Blood-Stage Plasmodium chabaudi AS Infection. J. Infect. Dis. 2002, 186, 1321–1329.
- Sponaas, A.M.; Do Rosario, A.P.F.; Voisine, C.; Mastelic, B.; Thompson, J.; Koernig, S.; Jarra, W.; Renia, L.; Mauduit, M.; Potocnik, A.J.; et al. Migrating Monocytes Recruited to the Spleen Play an Important Role in Control of Blood Stage Malaria. Blood 2009, 114, 5522–5531.
- Weinberg, J.B.; Volkheimer, A.D.; Rubach, M.P.; Florence, S.M.; Mukemba, J.P.; Kalingonji, A.R.; Langelier, C.; Chen, Y.; Bush, M.; Yeo, T.W.; et al. Monocyte Polarization in Children with Falciparum Malaria: Relationship to Nitric Oxide Insufficiency and Disease Severity. Sci. Rep. 2016, 6, 29151.
- Molina-Cruz, A.; DeJong, R.J.; Charles, B.; Gupta, L.; Kumar, S.; Jaramillo-Gutierrez, G.; Barillas-Mury, C. Reactive Oxygen Species Modulate Anopheles Gambiae Immunity against Bacteria and Plasmodium. J. Biol. Chem. 2008, 283, 3217–3223.
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS Production in Phagocytes: Why, When, and Where? J. Leukoc. Biol. 2013, 94, 657–670.
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative Stress in Malaria Parasite-Infected Erythrocytes: Host–Parasite Interactions. Int. J. Parasitol. 2004, 34, 163–189.
- Ortega-Pajares, A.; Rogerson, S.J. The Rough Guide to Monocytes in Malaria Infection. Front. Immunol. 2018, 9, 2888.
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401.
- Sanni, L.A.; Rae, C.; Maitland, A.; Stocker, R.; Hunt, N.H. Is Ischemia Involved in the Pathogenesis of Murine Cerebral Malaria? Am. J. Pathol. 2001, 159, 1105–1112.
- Carden, D.L.; Granger, D.N. Pathophysiology of Ischaemia-Reperfusion Injury. J. Pathol. 2000, 190, 255–266.
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317.
- Kelley, E.E.; Khoo, N.K.H.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen Peroxide Is the Major Oxidant Product of Xanthine Oxidase. Free Radic. Biol. Med. 2010, 48, 493–498.
- Schmidt, H.M.; Kelley, E.E.; Straub, A.C. The Impact of Xanthine Oxidase (XO) on Hemolytic Diseases. Redox Biol. 2019, 21, 101072.
- Orengo, J.M.; Leliwa-Sytek, A.; Evans, J.E.; Evans, B.; van de Hoef, D.; Nyako, M.; Day, K.; Rodriguez, A. Uric Acid Is a Mediator of the Plasmodium falciparum-Induced Inflammatory Response. PLoS ONE 2009, 4, e5194.
- Hong, Q.; Qi, K.; Feng, Z.; Huang, Z.; Cui, S.; Wang, L.; Fu, B.; Ding, R.; Yang, J.; Chen, X.; et al. Hyperuricemia Induces Endothelial Dysfunction via Mitochondrial Na+/Ca2+ Exchanger-Mediated Mitochondrial Calcium Overload. Cell Calcium 2012, 51, 402–410.
- Ayede, A.; Amoo, B.; Anetor, J.; Adeola, A. Status of Some Basic Antioxidants in Pre- and Postmalaria Treatment in Children. J. Child Sci. 2018, 08, e31–e35.
- Lopera-Mesa, T.M.; Mita-Mendoza, N.K.; van de Hoef, D.L.; Doumbia, S.; Konaté, D.; Doumbouya, M.; Gu, W.; Traoré, K.; Diakité, S.A.S.; Remaley, A.T.; et al. Plasma Uric Acid Levels Correlate with Inflammation and Disease Severity in Malian Children with Plasmodium falciparum Malaria. PLoS ONE 2012, 7, e46424.
- Mita-Mendoza, N.K.; van de Hoef, D.L.; Lopera-Mesa, T.M.; Doumbia, S.; Konate, D.; Doumbouya, M.; Gu, W.; Anderson, J.M.; Santos-Argumedo, L.; Rodriguez, A.; et al. A Potential Role for Plasma Uric Acid in the Endothelial Pathology of Plasmodium falciparum Malaria. PLoS ONE 2013, 8, e54481.
- Meneshian, A.; Bulkley, G.B. The Physiology of Endothelial Xanthine Oxidase: From Urate Catabolism to Reperfusion Injury to Inflammatory Signal Transduction. Microcirculation 2002, 9, 161–175.
- Bakar, N.A.; Klonis, N.; Hanssen, E.; Chan, C.; Tilley, L. Digestive-Vacuole Genesis and Endocytic Processes in the Early Intraerythrocytic Stages of Plasmodium falciparum. J. Cell Sci. 2010, 123, 441–450.
- Jonscher, E.; Flemming, S.; Schmitt, M.; Sabitzki, R.; Reichard, N.; Birnbaum, J.; Bergmann, B.; Höhn, K.; Spielmann, T. PfVPS45 Is Required for Host Cell Cytosol Uptake by Malaria Blood Stage Parasites. Cell Host Microbe 2019, 25, 166–173.e5.
- Kumar, S.; Bandyopadhyay, U. Free Heme Toxicity and Its Detoxification Systems in Human. Toxicol. Lett. 2005, 157, 175–188.
- Kehrer, J.P. The Haber-Weiss Reaction and Mechanisms of Toxicity. Toxicology 2000, 149, 43–50.
- Gupta, M.; Kumar, S.; Kumar, R.; Kumar, A.; Verma, R.; Darokar, M.P.; Rout, P.; Pal, A. Inhibition of Heme Detoxification Pathway in Malaria Parasite by 3-Hydroxy-11-Keto-β-Boswellic Acid Isolated from Boswellia Serrata. Biomed. Pharmacother. 2021, 144, 112302.
- Dondorp, A.M.; Omodeo-Salè, F.; Chotivanich, K.; Taramelli, D.; White, N.J. Oxidative Stress and Rheology in Severe Malaria. Red. Rep. 2003, 8, 292–294.
- Nuchsongsin, F.; Chotivanich, K.; Charunwatthana, P.; Fausta, O.S.; Taramelli, D.; Day, N.P.; White, N.J.; Dondorp, A.M. Effects of Malaria Heme Products on Red Blood Cell Deformability. Am. J. Trop. Med. Hyg. 2007, 77, 617–622.
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Base Damage to Cellular DNA. Free Radic. Biol. Med. 2010, 49, 9–21.
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed Res. Int. 2014, 2014, 761264.
- Dondorp, A.M.; Kager, P.A.; Vreeken, J.; White, N.J. Abnormal Blood Flow and Red Blood Cell Deformability in Severe Malaria. Parasitol. Today 2000, 16, 228–232.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Kavishe, R.A.; Koenderink, J.B.; Alifrangis, M. Oxidative Stress in Malaria and Artemisinin Combination Therapy: Pros and Cons. FEBS J. 2017, 284, 2579–2591.
- Campo, B.; Vandal, O.; Wesche, D.L.; Burrows, J.N. Killing the Hypnozoite—Drug Discovery Approaches to Prevent Relapse in Plasmodium Vivax. Pathog. Glob. Health 2015, 109, 107–122.
- John, C.C. Primaquine plus Artemisinin Combination Therapy for Reduction of Malaria Transmission: Promise and Risk. BMC Med. 2016, 14, 65.
- Sullivan, D.J. Theories on Malarial Pigment Formation and Quinoline Action. Int. J. Parasitol. 2002, 32, 1645–1653.
- Roepe, P.D. Molecular and Physiologic Basis of Quinoline Drug Resistance in Plasmodium falciparum Malaria. Future Microbiol. 2009, 4, 441–455.
- Fitch, C.D. Ferriprotoporphyrin IX, Phospholipids, and the Antimalarial Actions of Quinoline Drugs. Life Sci. 2004, 74, 1957–1972.
- Radfar, A.; Diez, A.; Bautista, J.M. Chloroquine Mediates Specific Proteome Oxidative Damage across the Erythrocytic Cycle of Resistant Plasmodium falciparum. Free Radic. Biol. Med. 2008, 44, 2034–2042.
- Acosta, M.E.; Gotopo, L.; Gamboa, N.; Rodrigues, J.R.; Henriques, G.C.; Cabrera, G.; Romero, A.H. Antimalarial Activity of Highly Coordinative Fused Heterocycles Targeting β–Hematin Crystallization. ACS Omega 2022, 7, 7499–7514.
- Durrand, V.; Berry, A.; Sem, R.; Glaziou, P.; Beaudou, J.; Fandeur, T. Variations in the Sequence and Expression of the Plasmodium falciparum Chloroquine Resistance Transporter (Pfcrt) and Their Relationship to Chloroquine Resistance in Vitro. Mol. Biochem. Parasitol. 2004, 136, 273–285.
- Lakshmanan, V.; Bray, P.G.; Verdier-Pinard, D.; Johnson, D.J.; Horrocks, P.; Muhle, R.A.; Alakpa, G.E.; Hughes, R.H.; Ward, S.A.; Krogstad, D.J.; et al. A Critical Role for PfCRT K76T in Plasmodium falciparum Verapamil-Reversible Chloroquine Resistance. EMBO J. 2005, 24, 2294–2305.
- Callaghan, P.S.; Hassett, M.R.; Roepe, P.D. Functional Comparison of 45 Naturally Occurring Isoforms of the Plasmodium falciparum Chloroquine Resistance Transporter (PfCRT). Biochemistry 2015, 54, 5083–5094.
- Gorka, A.P.; De Dios, A.; Roepe, P.D. Quinoline Drug-Heme Interactions and Implications for Antimalarial Cytostatic versus Cytocidal Activities. J. Med. Chem. 2013, 56, 5231–5246.
- Parhizgar, A.R.; Tahghighi, A. Introducing New Antimalarial Analogues of Chloroquine and Amodiaquine: A Narrative Review. Iran. J. Med. Sci. 2017, 42, 115–128.
- Herraiz, T.; Guillén, H.; González-Peña, D.; Arán, V.J. Antimalarial Quinoline Drugs Inhibit β-Hematin and Increase Free Hemin Catalyzing Peroxidative Reactions and Inhibition of Cysteine Proteases. Sci. Rep. 2019, 9, 15398.
- Falade, C.O.; Ogundele, A.O.; Yusuf, B.O.; Ademowo, O.G.; Ladipo, S.M. High Efficacy of Two Artemisinin-Based Combinations (Artemether- Lumefantrine and Artesunate plus Amodiaquine) for Acute Uncomplicated Malaria in Ibadan, Nigeria. Trop. Med. Int. Health 2008, 13, 635–643.
- Makanga, M.; Krudsood, S. The Clinical Efficacy of Artemether/Lumefantrine (Coartem®). Malar. J. 2009, 8, S5.
- Egunsola, O.; Oshikoya, K.A. Comparative Safety of Artemether-Lumefantrine and Other Artemisinin-Based Combinations in Children: A Systematic Review. Malar. J. 2013, 12, 385.
- Wang, J.; Huang, L.; Li, J.; Fan, Q.; Long, Y.; Li, Y.; Zhou, B. Artemisinin Directly Targets Malarial Mitochondria through Its Specific Mitochondrial Activation. PLoS ONE 2010, 5, e9582.
- Klonis, N.; Crespo-Ortiz, M.P.; Bottova, I.; Abu-Bakar, N.; Kenny, S.; Rosenthal, P.J.; Tilley, L. Artemisinin Activity against Plasmodium falciparum Requires Hemoglobin Uptake and Digestion. Proc. Natl. Acad. Sci. USA 2011, 108, 11405–11410.
- Yang, J.; He, Y.; Li, Y.; Zhang, X.; Wong, Y.-K.; Shen, S.; Zhong, T.; Zhang, J.; Liu, Q.; Wang, J. Advances in the Research on the Targets of Anti-Malaria Actions of Artemisinin. Pharmacol. Ther. 2020, 216, 107697.
- Pukrittayakamee, S.; Chotivanich, K.; Chantra, A.; Clemens, R.; Looareesuwan, S.; White, N.J. Activities of Artesunate and Primaquine against Asexual- and Sexual-Stage Parasites in Falciparum Malaria. Antimicrob. Agents Chemother. 2004, 48, 1329–1334.
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.A.; Sinden, R.E.; Leroy, D. The Activities of Current Antimalarial Drugs on the Life Cycle Stages of Plasmodium: A Comparative Study with Human and Rodent Parasites. PLoS Med. 2012, 9, e1001169.
- Okell, L.C.; Drakeley, C.J.; Bousema, T.; Whitty, C.J.M.; Ghani, A.C. Modelling the Impact of Artemisinin Combination Therapy and Long-Acting Treatments on Malaria Transmission Intensity. PLoS Med. 2008, 5, 1617–1628.
- Dodoo, A.N.O.; Fogg, C.; Asiimwe, A.; Nartey, E.T.; Kodua, A.; Tenkorang, O.; Ofori-Adjei, D. Pattern of Drug Utilization for Treatment of Uncomplicated Malaria in Urban Ghana Following National Treatment Policy Change to Artemisinin-Combination Therapy. Malar. J. 2009, 8, 2.
- Maude, R.J.; Socheat, D.; Nguon, C.; Saroth, P.; Dara, P.; Li, G.; Song, J.; Yeung, S.; Dondorp, A.M.; Day, N.P.; et al. Optimising Strategies for Plasmodium falciparum Malaria Elimination in Cambodia: Primaquine, Mass Drug Administration and Artemisinin Resistance. PLoS ONE 2012, 7, e37166.
- Lödige, M.; Lewis, M.D.; Paulsen, E.S.; Esch, H.L.; Pradel, G.; Lehmann, L.; Brun, R.; Bringmann, G.; Mueller, A.K. A Primaquine-Chloroquine Hybrid with Dual Activity against Plasmodium Liver and Blood Stages. Int. J. Med. Microbiol. 2013, 303, 539–547.
- Milner, E.E.; Berman, J.; Caridha, D.; Dickson, S.P.; Hickman, M.; Lee, P.J.; Marcsisin, S.R.; Read, L.T.; Roncal, N.; Vesely, B.A.; et al. Cytochrome P450 2D-Mediated Metabolism Is Not Necessary for Tafenoquine and Primaquine to Eradicate the Erythrocytic Stages of Plasmodium berghei. Malar. J. 2016, 15, 588.
- Shekalaghe, S.A.; Braak, R.T.; Daou, M.; Kavishe, R.; Van Bijllaardt, W.D.; Van Bosch, S.D.; Koenderink, J.B.; Luty, A.J.F.; Whitty, C.J.M.; Drakeley, C.; et al. In Tanzania, Hemolysis after a Single Dose of Primaquine Coadministered with an Artemisinin Is Not Restricted to Glucose-6-Phosphate Dehydrogenase-Deficient (G6PD A-) Individuals. Antimicrob. Agents Chemother. 2010, 54, 1762–1768.
- Brito-Sousa, J.D.; Santos, T.C.; Avalos, S.; Fontecha, G.; Melo, G.C.; Val, F.; Siqueira, A.M.; Alecrim, G.C.; Bassat, Q.; Lacerda, M.V.G.; et al. Clinical Spectrum of Primaquine-Induced Hemolysis in Glucose-6-Phosphate Dehydrogenase Deficiency: A 9-Year Hospitalization-Based Study from the Brazilian Amazon. Clin. Infec. Dis. 2019, 69, 1440–1442.
- Farombi, E.O.; Shyntum, Y.Y.; Emerole, G.O. Influence of Chloroquine Treatment and Plasmodium falciparum Malaria Infection on Some Enzymatic and Non-Enzymatic Antioxidant Defense Indices in Humans. Drug Chem. Toxicol. 2003, 26, 59–71.
- Zanini, G.M.; Cabrales, P.; Barkho, W.; Frangos, J.A.; Carvalho, L.J.M. Exogenous Nitric Oxide Decreases Brain Vascular Inflammation, Leakage and Venular Resistance during Plasmodium Berghei ANKA Infection in Mice. J. Neuroinflamm. 2011, 8, 66.
- Moreira, D.R.; Uberti, A.C.M.G.; Gomes, A.R.Q.; Ferreira, M.E.S.; da Silva Barbosa, A.; Varela, E.L.P.; Dolabela, M.F.; Percário, S. Dexamethasone Increased the Survival Rate in Plasmodium berghei-Infected Mice. Sci. Rep. 2021, 11, 2623.
- Barbosa, A.D.S.; Temple, M.C.R.; Varela, E.L.P.; Gomes, A.R.Q.; Silveira, E.L.; de Carvalho, E.P.; Dolabela, M.F.; Percario, S. Inhibition of Nitric Oxide Synthesis Promotes Increased Mortality despite the Reduction of Parasitemia in Plasmodium berghei-Infected Mice. Res. Soc. Dev. 2021, 10, e27810111805.
- Fritsche, G.; Larcher, C.; Schennach, H.; Weiss, G. Regulatory Interactions between Iron and Nitric Oxide Metabolism for Immune Defense against Plasmodium falciparum Infection. J. Infect. Dis. 2001, 183, 1388–1394.
- Cabrales, P.; Zanini, G.M.; Meays, D.; Frangos, J.A.; Carvalho, L.J.M. Nitric Oxide Protection Against Murine Cerebral Malaria Is Associated with Improved Cerebral Microcirculatory Physiology. J. Infect. Dis. 2011, 203, 1454–1463.
- Coleman, J.W. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406.
- Bian, K.; Doursout, M.-F.; Murad, F. Vascular System: Role of Nitric Oxide in Cardiovascular Diseases. J. Clin. Hypertens. 2008, 10, 304–310.
- Ahlawat, A.; Rana, A.; Goyal, N.; Sharma, S. Potential Role of Nitric Oxide Synthase Isoforms in Pathophysiology of Neuropathic Pain. Inflammopharmacology 2014, 22, 269–278.
- Bogdan, C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001, 2, 907–916.
- Vallance, P.; Leiper, J. Blocking NO Synthesis: How, Where and Why? Nat. Rev. Drug Discov. 2002, 1, 939–950.
- Forstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837.
- Wong, V.C.; Lerner, E. Nitric Oxide Inhibition Strategies. Future Sci. OA 2015, 1, FSO35.
- Nahrevanian, H.; Dascombe, M.J. Nitric Oxide and Reactive Nitrogen Intermediates during Lethal and Nonlethal Strains of Murine Malaria. Parasite Immunol. 2001, 23, 491–501.
- Luckhart, S.; Crampton, A.L.; Zamora, R.; Lieber, M.J.; Dos Santos, P.C.; Peterson, T.M.L.; Emmith, N.; Lim, J.; Wink, D.A.; Vodovotz, Y. Mammalian Transforming Growth Factor Β1 Activated after Ingestion by Anopheles stephensi Modulates Mosquito Immunity. Infect. Immun. 2003, 71, 3000–3009.
- Kun, J.F.; Mordmüller, B.; Perkins, D.J.; May, J.; Mercereau-Puijalon, O.; Alpers, M.; Weinberg, J.B.; Kremsner, P.G. Nitric Oxide Synthase 2Lambaréné (G-954C), Increased Nitric Oxide Production, and Protection against Malaria. J. Infect. Dis. 2001, 184, 330–336.
- Gramaglia, I.; Sobolewski, P.; Meays, D.; Contreras, R.; Nolan, J.P.; Frangos, J.A.; Intaglietta, M.; Van Der Heyde, H.C. Low Nitric Oxide Bioavailability Contributes to the Genesis of Experimental Cerebral Malaria. Nat. Med. 2006, 12, 1417–1422.
- Peterson, T.M.L.; Gow, A.J.; Luckhart, S. Nitric Oxide Metabolites Induced in Anopheles Stephensi Control Malaria Parasite Infection. Free Radic. Biol. Med. 2007, 42, 132–142.
- Maurizio, P.L.; Fuseini, H.; Tegha, G.; Hosseinipour, M.; De Paris, K. Signatures of Divergent Anti-Malarial Treatment Responses in Peripheral Blood from Adults and Young Children in Malawi. Malar. J. 2019, 18, 205.
- Dzodzomenyo, M.; Ghansah, A.; Ensaw, N.; Dovie, B.; Bimi, L.; Quansah, R.; Gyan, B.A.; Gyakobo, M.; Amoani, B. Inducible Nitric Oxide Synthase 2 Promoter Polymorphism and Malaria Disease Severity in Children in Southern Ghana. PLoS ONE 2018, 13, e0202218.
- Hobbs, M.R.; Udhayakumar, V.; Levesque, M.C.; Booth, J.; Roberts, J.M.; Tkachuk, A.N.; Pole, A.; Coon, H.; Kariuki, S.; Nahlen, B.L.; et al. A New NOS2 Promoter Polymorphism Associated with Increased Nitric Oxide Production and Protection from Severe Malaria in Tanzanian and Kenyan Children. Lancet 2002, 360, 1468–1475.
- Clark, I.A.; Rockett, K.A.; Burgner, D. Genes, Nitric Oxide and Malaria in African Children. Trends Parasitol. 2003, 19, 335–337.
- Serghides, L.; Kim, H.; Lu, Z.; Kain, D.C.; Miller, C.; Francis, R.C.; Liles, W.C.; Zapol, W.M.; Kain, K.C. Inhaled Nitric Oxide Reduces Endothelial Activation and Parasite Accumulation in the Brain, and Enhances Survival in Experimental Cerebral Malaria. PLoS ONE 2011, 6, e27714.
- Ong, P.K.; Melchior, B.; Martins, Y.C.; Hofer, A.; Orjuela-Sánchez, P.; Cabrales, P.; Zanini, G.M.; Frangos, J.A.; Carvalho, L.J.M. Nitric Oxide Synthase Dysfunction Contributes to Impaired Cerebroarteriolar Reactivity in Experimental Cerebral Malaria. PLoS Pathog. 2013, 9, e1003444.
- Cui, L.; Miao, J.; Cui, L. Cytotoxic Effect of Curcumin on Malaria Parasite Plasmodium falciparum: Inhibition of Histone Acetylation and Generation of Reactive Oxygen Species. Antimicrob. Agents Chemother. 2007, 51, 488–494.
- Iribhogbe, O.L.; Agbaje, E.O.; Oreagba, I.A.; Aina, O.O.; Ota, A.D. Oxidant versus Antioxidant Activity in Malaria: Role of Nutritional Therapy. J. Med. Sci. 2012, 12, 229–233.
- Agarwal, S.; Sharma, V.; Kaul, T.; Abdin, M.Z.; Singh, S. Cytotoxic Effect of Carotenoid Phytonutrient Lycopene on P. falciparum Infected Erythrocytes. Mol. Biochem. Parasitol. 2014, 197, 15–20.
- Quadros Gomes, B.A.; Da Silva, L.F.D.; Quadros Gomes, A.R.; Moreira, D.R.; Dolabela, M.F.; Santos, R.S.; Green, M.D.; Carvalho, E.P.; Percário, S. N-Acetyl Cysteine and Mushroom Agaricus sylvaticus Supplementation Decreased Parasitaemia and Pulmonary Oxidative Stress in a Mice Model of Malaria. Malar. J. 2015, 14, 202.
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Aspects Med. 2005, 26, 459–516.
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179.
- Ferreira-Santos, P.; Aparicio, R.; Carrón, R.; Sevilla, M.Á.; Monroy-Ruiz, J.; Montero, M.J. Lycopene-Supplemented Diet Ameliorates Cardiovascular Remodeling and Oxidative Stress in Rats with Hypertension Induced by Angiotensin II. J. Funct. Foods 2018, 47, 279–287.
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Nagata, N.; Xu, L.; Yamamoto, S.; Fuke, N.; Ushida, Y.; Suganuma, H.; Kaneko, S.; et al. Lycopene Prevents the Progression of Lipotoxicity-Induced Nonalcoholic Steatohepatitis by Decreasing Oxidative Stress in Mice. Free Radic. Biol. Med. 2020, 152, 571–582.
- Chen, J.; Pu, Z.; Xiao, Y.; Li, C.; Du, X.; Su, C.; Zhang, X. Lycopene Synthesis via Tri-Cistronic Expression of LeGGPS2, LePSY1 and CrtI in Escherichia coli. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2012, 28, 823–833.
- Hadley, C.W.; Clinton, S.K.; Schwartz, S.J. The Consumption of Processed Tomato Products Enhances Plasma Lycopene Concentrations in Association with a Reduced Lipoprotein Sensitivity to Oxidative Damage. J. Nutr. 2003, 133, 727–732.
- Ganji, V.; Kafai, M.R. Population Determinants of Serum Lycopene Concentrations in the United States: Data from the Third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2005, 135, 567–572.
- Kong, Q.; Yuan, J.; Gao, L.; Liu, P.; Cao, L.; Huang, Y.; Zhao, L.; Lv, H.; Bie, Z. Transcriptional Regulation of Lycopene Metabolism Mediated by Rootstock during the Ripening of Grafted Watermelons. Food Chem. 2017, 214, 406–411.
- Hussain, A.; Pu, H.; Sun, D.-W. Measurements of Lycopene Contents in Fruit: A Review of Recent Developments in Conventional and Novel Techniques. Crit. Rev. Food Sci. Nutr. 2019, 59, 758–769.
- Mayeaux, M.; Xu, Z.; King, J.M.; Prinyawiwatkul, W. Effects of Cooking Conditions on the Lycopene Content in Tomatoes. J. Food Sci. 2006, 71, C461–C464.
- Rodriguez, E.B.; Rodriguez-Amaya, D.B. Lycopene Epoxides and Apo-Lycopenals Formed by Chemical Reactions and Autoxidation in Model Systems and Processed Foods. J. Food Sci. 2009, 74, C674–C682.
- Colle, I.; Lemmens, L.; Van Buggenhout, S.; Van Loey, A.; Hendrickx, M. Effect of Thermal Processing on the Degradation, Isomerization, and Bioaccessibility of Lycopene in Tomato Pulp. J. Food Sci. 2010, 75, C753–C759.
- Chen, H.; Zhong, Q. Thermal and UV Stability of β-Carotene Dissolved in Peppermint Oil Microemulsified by Sunflower Lecithin and Tween 20 Blend. Food Chem. 2015, 174, 630–636.
- Ge, W.; Li, D.; Chen, M.; Wang, X.; Liu, S.; Sun, R. Characterization and Antioxidant Activity of β-Carotene Loaded Chitosan-Graft-Poly(Lactide) Nanomicelles. Carbohydr. Polym. 2015, 117, 169–176.
- Honda, M.; Watanabe, Y.; Murakami, K.; Takemura, R.; Fukaya, T.; Wahyudiono; Kanda, H.; Goto, M. Thermal Isomerization Pre-Treatment to Improve Lycopene Extraction from Tomato Pulp. LWT 2017, 86, 69–75.
- Hernández-Almanza, A.; Montañez, J.; Martínez, G.; Aguilar-Jiménez, A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Lycopene: Progress in Microbial Production. Trends Food Sci. Technol. 2016, 56, 142–148.
- Antonuccio, P.; Micali, A.; Puzzolo, D.; Romeo, C.; Vermiglio, G.; Squadrito, V.; Freni, J.; Pallio, G.; Trichilo, V.; Righi, M.; et al. Nutraceutical Effects of Lycopene in Experimental Varicocele: An “In Vivo” Model to Study Male Infertility. Nutrients 2020, 12, 1536.
- Rao, A.V.; Ray, M.R.; Rao, L.G. Lycopene. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2006; Volume 51, pp. 99–164. ISBN 0120164515.
- Re, R.; Fraser, P.D.; Long, M.; Bramley, P.M.; Rice-Evans, C. Isomerization of Lycopene in the Gastric Milieu. Biochem. Biophys. Res. Commun. 2001, 281, 576–581.
- Honest, K.N.; Zhang, H.W.; Zhang, L. Lycopene: Isomerization Effects on Bioavailability and Bioactivity Properties. Food Rev. Int. 2011, 27, 248–258.
- Anguelova, T.; Warthesen, J. Degradation of Lycopene, α-Carotene, and β-Carotene During Lipid Peroxidation. J. Food Sci. 2000, 65, 71–75.
- Liu, D.; Shi, J.; Colina Ibarra, A.; Kakuda, Y.; Jun Xue, S. The Scavenging Capacity and Synergistic Effects of Lycopene, Vitamin E, Vitamin C, and β-Carotene Mixtures on the DPPH Free Radical. LWT Food Sci. Technol. 2008, 41, 1344–1349.
- Erdman, J.W.; Ford, N.A.; Lindshield, B.L. Are the Health Attributes of Lycopene Related to Its Antioxidant Function? Arch. Biochem. Biophys. 2009, 483, 229–235.
- Yonar, M.E.; Sakin, F. Ameliorative Effect of Lycopene on Antioxidant Status in Cyprinus carpio during Pyrethroid Deltamethrin Exposure. Pestic. Biochem. Physiol. 2011, 99, 226–231.
- Takehara, M.; Nishimura, M.; Kuwa, T.; Inoue, Y.; Kitamura, C.; Kumagai, T.; Honda, M. Characterization and Thermal Isomerization of (All-E)-Lycopene. J. Agric. Food Chem. 2014, 62, 264–269.
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.R.; Menon, V.P. Lycopene as a Natural Protector against γ-Radiation Induced DNA Damage, Lipid Peroxidation and Antioxidant Status in Primary Culture of Isolated Rat Hepatocytes in Vitro. Biochim. Biophys. Acta 2007, 1770, 659–665.
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.F.; Veyrat, C.C.; Borel, P. Effects of Physicochemical Properties of Carotenoids on Their Bioaccessibility, Intestinal Cell Uptake, and Blood and Tissue Concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397.
- Suwannalert, P.; Boonsiri, P.; Khampitak, T.; Khampitak, K.; Sriboonlue, P.; Yongvanit, P. The Levels of Lycopene, Alpha-Tocopherol and a Marker of Oxidative Stress in Healthy Northeast Thai Elderly. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 27–30.
- Kujawska, M.; Ewertowska, M.; Adamska, T.; Sadowski, C.; Ignatowicz, E.; Jodynis-Liebert, J. Antioxidant Effect of Lycopene-Enriched Tomato Paste on N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Physiol. Biochem. 2014, 70, 981–990.
- Aqeel, S.; Naheda, A.; Raza, A.; Khan, K.; Khan, W. Differential Status and Significance of Non-Enzymatic Antioxidants (Reactive Oxygen Species Scavengers) in Malaria and Dengue Patients. Acta Trop. 2019, 195, 127–134.
- Sánchez-Villamil, J.P.; Bautista-Niño, P.K.; Serrano, N.C.; Rincon, M.Y.; Garg, N.J. Potential Role of Antioxidants as Adjunctive Therapy in Chagas Disease. Oxid. Med. Cell. Longev. 2020, 2020, 9081813.
- Ngouela, S.; Lenta, B.N.; Noungoue, D.T.; Ngoupayo, J.; Boyom, F.F.; Tsamo, E.; Gut, J.; Rosenthal, P.J.; Connolly, J.D. Anti-Plasmodial and Antioxidant Activities of Constituents of the Seed Shells of Symphonia Globulifera Linn F. Phytochemistry 2006, 67, 302–306.
- Batista, R.; De Jesus Silva Júnior, A.; De Oliveira, A. Plant-Derived Antimalarial Agents: New Leads and Efficient Phytomedicines. Part II. Non-Alkaloidal Natural Products. Molecules 2009, 14, 3037–3072.
- Soh, P.N.; Witkowski, B.; Olagnier, D.; Nicolau, M.L.; Garcia-Alvarez, M.C.; Berry, A.; Benoit-Vical, F. In Vitro and in Vivo Properties of Ellagic Acid in Malaria Treatment. Antimicrob. Agents Chemother. 2009, 53, 1100–1106.
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170.
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-Level Semi-Synthetic Production of the Potent Antimalarial Artemisinin. Nature 2013, 496, 528–532.
- Richard, S.A.; Black, R.E.; Caulfield, L.E. Undernutrition as An Underlying Cause of Malaria Morbidity and Mortality in Children Less Than Five Years Old. Am. J. Trop. Med. Hyg. 2004, 71, 55–63.
- Akpotuzor, J.O.; Udoh, A.E.; Etukudo, M.H. Total Antioxidant Status, Vitamins A, C and β-Carotene Levels of Children with P. falciparum Infection in University of Calabar Teaching Hospital (UCTH), Calabar. Pakistan J. Nutr. 2007, 6, 485–489.
- Varela, E.L.P.; Gomes, A.R.Q.; Santos, A.S.B.; Cruz, J.N.; Carvalho, E.P.; Prazeres, B.A.P.; Dolabela, M.F.; Percário, S. Antiparasitic Effect of Lycopene in Experimental Malaria. AABC, 2022; submitted.
- Zeba, A.N.; Sorgho, H.; Rouamba, N.; Zongo, I.; Rouamba, J.; Guiguemdé, R.T.; Hamer, D.H.; Mokhtar, N.; Ouedraogo, J.B. Major Reduction of Malaria Morbidity with Combined Vitamin A and Zinc Supplementation in Young Children in Burkina Faso: A Randomized Double Blind Trial. Nutr. J. 2008, 7, 1–7.




