
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | CIRO CANNAVACCIUOLO | -- | 2049 | 2023-01-07 15:10:43 | | | |
| 2 | Amina Yu | + 15 word(s) | 2064 | 2023-01-09 03:05:29 | | |
Video Upload Options
The analysis of foods is a comprehensive process of extraction, identification, and quantification of several classes of compounds from natural matrices. The detection and quantification of primary metabolites (sugars, amino acids, vitamins, and lipids), contaminants (toxins, heavy metals, and allergens), and secondary metabolites (polyphenolics, flavonoids, terpenes, and alkaloids) is a crucial practice for ensuring the safety and quality of foods and related functional products. Due to the variable structure of food analytes, a gap in a universal method suitable for the extraction and analysis of all compounds is lacking. Moreover, conventional extractants are usually made of organic solvents and common extraction techniques usually require a long extraction time to exhaust the matrix. The actual discussions about climatic changes provide a growing awareness of the scientific and industrial community to reduce the environmental impact by using sustainable processes. In general, the main principles of “green chemistry” are based on the design of processes aimed to reduce energy consumption and the use of eco-friendly solvents with less toxicity to the environment and human health.
1. Physicochemical Properties of Natural Deep Eutectic Solvents (NADESs) for the Extraction Process
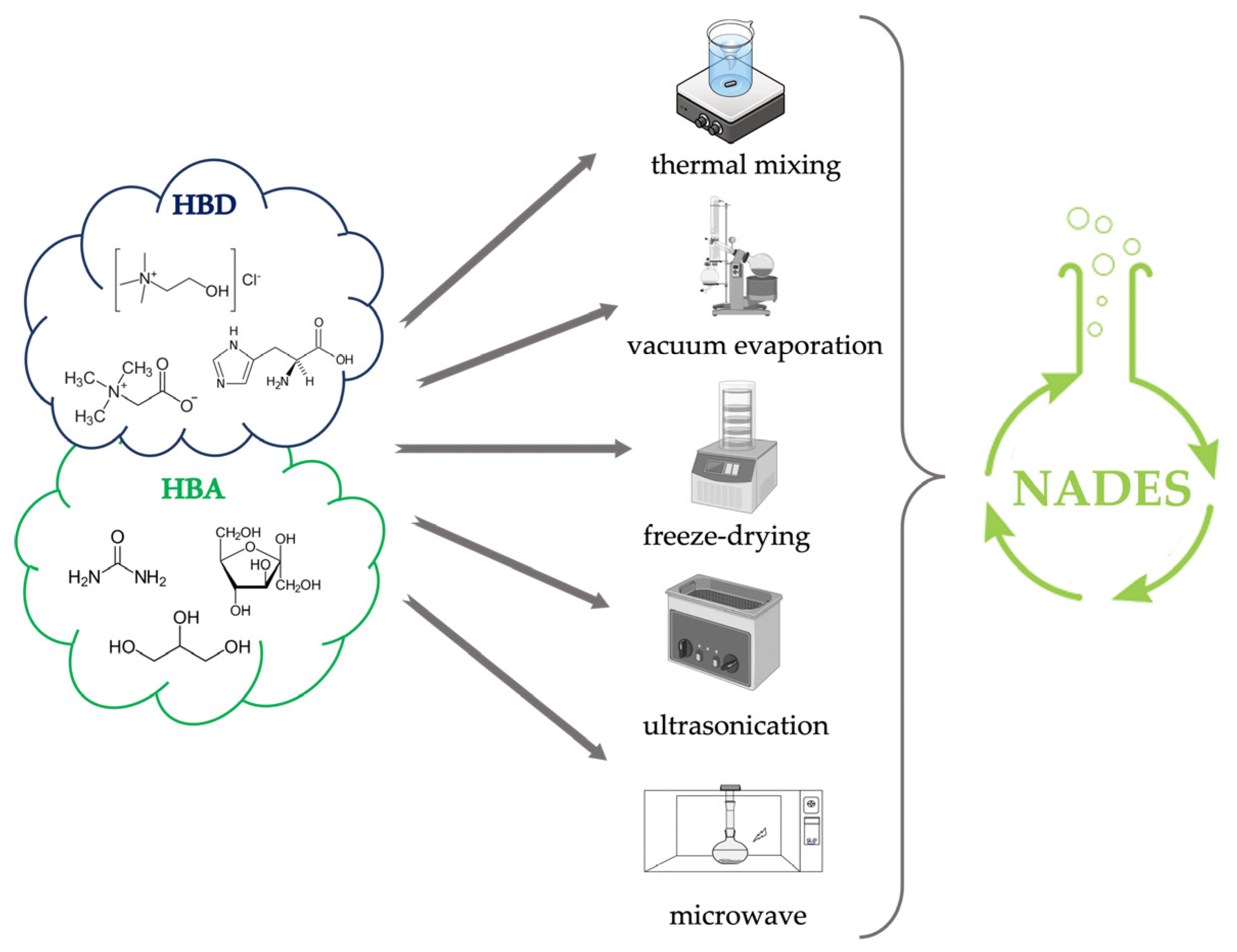
2. Preparation of NADES
-
Microwave-assisted synthesis exploits the production of microwaves that upon interaction with precursors generate collisions between molecules and between the hydrogen bond donor and hydrogen bond acceptor components due to dipole rotation resulting in dielectric heating that speeds up the synthesis time [23][24]. According to Popovic et al., 2022, microwaves could be one of the fastest methods for the preparation of some NADES, taking even less than a minute [20]. However, because of the possible overheating caused by the technique, it is advisable to divide the process into several cycles of a few seconds interspersed with cooling pauses [25]. The entire preparation is carried out in closed systems with controlled pressure and temperature.
-
Ultrasound-assisted is a little-explored but effective way of preparing NADESs. The cavitation process promotes, through the release of heat and pressure exerted because of bubbles implosion, the interaction between the hydrogen bond acceptor (HBA) and the hydrogen bond donor (HBD) [21]. According to when described by Santana et al., 2019, the preparation of NADES by ultrasound can also be performed by heating the mixture around 50 °C [21]. This approach requires several minutes with intermediate times between microwaves and the remaining techniques described above.
3. Use of NADES as Green Solvent in the Extraction Techniques
4. Toxicity and Sustainability
References
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134.
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced Extraction of Bioactive Natural Products Using Tailor-Made Deep Eutectic Solvents: Application to Flavonoid Extraction from Flos Sophorae. Green Chem. 2015, 17, 1718–1727.
- Rente, D.; Paiva, A.; Duarte, A.R. The Role of Hydrogen Bond Donor on the Extraction of Phenolic Compounds from Natural Matrices Using Deep Eutectic Systems. Molecules 2021, 26, 2336.
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of Type III Deep Eutectic Solvents and Effect of Water on Their Intermolecular Interactions. Fluid Phase Equilib. 2017, 441, 43–48.
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and Thermal Behavior of Natural Deep Eutectic Solvents. J. Mol. Liq. 2016, 215, 534–540.
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68.
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and Efficient Extraction of Rutin from Tartary Buckwheat Hull by Using Natural Deep Eutectic Solvents. Food Chem. 2017, 221, 1400–1405.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Tuntarawongsa, S.; Phaechamud, T. Menthol, Borneol, Camphor and WS-3 Eutectic Mixture. Adv. Mater. Res. 2012, 506, 355–358.
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van Den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17, 4518–4521.
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942.
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of Green Betaine-Based Deep Eutectic Solvent Aqueous Two-Phase System for the Extraction of Protein. Talanta 2016, 152, 23–32.
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A Green Deep Eutectic Solvent-Based Aqueous Two-Phase System for Protein Extracting. Anal. Chim. Acta 2015, 864, 9–20.
- Pang, J.; Sha, X.; Chao, Y.; Chen, G.; Han, C.; Zhu, W.; Li, H.; Zhang, Q. Green Aqueous Biphasic Systems Containing Deep Eutectic Solvents and Sodium Salts for the Extraction of Protein. RSC Adv. 2017, 7, 49361–49367.
- Deng, W.W.; Zong, Y.; Xiao, Y.X. Hexafluoroisopropanol-Based Deep Eutectic Solvent/Salt Aqueous Two-Phase Systems for Extraction of Anthraquinones from Rhei Radix et Rhizoma Samples. ACS Sustain. Chem. Eng. 2017, 5, 4267–4275.
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Two-Phase Systems Developed with Hydrophilic and Hydrophobic Deep Eutectic Solvents for Simultaneously Extracting Various Bioactive Compounds with Different Polarities. Green Chem. 2018, 20, 1879–1886.
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. Adv. Biochem. Eng. Biotechnol. 2019, 168, 31–59.
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents—Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562.
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable Synthesis of Natural Deep Eutectic Solvents (NADES) by Different Methods. J. Mol. Liq. 2019, 293, 111452.
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; Monte, F. Del Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir 2009, 25, 5509–5515.
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A Greener Approach to Prepare Natural Deep Eutectic Solvents. ChemistrySelect 2018, 3, 6122–6125.
- Zhu, X.H.; Hang, Q.M. Microscopical and Physical Characterization of Microwave and Microwave-Hydrothermal Synthesis Products. Micron 2013, 44, 21–44.
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344.
- Bajkacz, S.; Adamek, J. Evaluation of New Natural Deep Eutectic Solvents for the Extraction of Isoflavones from Soy Products. Talanta 2017, 168, 329–335.
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2023, 398, 133871.
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers Based on Natural Deep Eutectic Mixtures to Enhance Anthocyanins Isolation from Grape Pomace by Pressurized Hot Water Extraction. LWT 2021, 149, 111889.
- Fan, Y.; Li, Q. An Efficient Extraction Method for Essential Oil from Angelica Sinensis Radix by Natural Deep Eutectic Solvents-Assisted Microwave Hydrodistillation. Sustain. Chem. Pharm. 2022, 29, 100792.
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of Thermal Behavior of Deep Eutectic Solvents and Their Potential as Drug Solubilization Vehicles. Int. J. Pharm. 2009, 378, 136–139.
- De Morais, P.; Gonçalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of Cholinium-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404.
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT 2016, 73, 45–51.
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are Deep Eutectic Solvents Benign or Toxic? Chemosphere 2013, 90, 2193–2195.
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Redovniković, R.I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53.




