
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lixin Tang | -- | 1322 | 2023-01-06 12:27:35 | | | |
| 2 | Lixin Tang | + 1721 word(s) | 3043 | 2023-01-06 12:41:05 | | | | |
| 3 | Jessie Wu | -790 word(s) | 2253 | 2023-01-09 05:19:02 | | | | |
| 4 | Lixin Tang | Meta information modification | 2253 | 2023-01-09 06:46:43 | | | | |
| 5 | Jessie Wu | Meta information modification | 2253 | 2023-01-09 09:38:15 | | |
Video Upload Options
Velvet deer are not only a representative special economic animal but also an important part of livestock. With the increasing awareness of international competition for germplasm resources in China, more and more attention has been paid to the protection and utilization of germplasm resources.
1. Introduction
2. Present Situation of Velvet Deer Germplasm Resources

2.1. Present Situation of Wild Sika Deer Germplasm Resources
| Subspecies of Sika Deer | Distribution | Number |
|---|---|---|
| Cervus nippon hortulorum | Heilongjiang, Jilin province | --- |
| Cervus nippon kopshi | Qingliangfeng National Nature Reserve, Taohongling National Nature Reserve and south of Anhui | More than 874 |
| Cervus nippon sinchuanicus | Sichuan Tiebu Sike Deer Nature Reserve | About 800 |
| Cervus nippon taiouanus | Kenting National Park, Taiwan | About 1000 |
2.2. Present Situation of Wild Red Deer Germplasm Resources
| Subspecies of Red Deer | Distribution | Number |
|---|---|---|
| Cervus elaphus wallichi [11] | Sangri County, Tibet | About 300 |
| Cervus elaphus macneilli [1] | Aba and Ganzi, Sichuan Province | --- |
| Cervus elaphus kansuensis [12] | Qilian Mountain, Gansu, Qinhai, Ningxia, Southwest Sichuan and East Tibet | --- |
| Cervus elaphus yarkandensis [13] | Northern Tarim Basin and desert of the southern plain, Xinjiang | About 450 |
| Cervus elaphus songaricus [14] | Urumqi Nanshan, Hami Mountain and northern Tianshan Mountains, Xinjiang | Fewer than 10,000 |
| Cervus elaphus alashanicus [15] | Helan Mountain, Ningxia | --- |
| Cervus elaphus sibiricus [16] | Western and northern Xinjiang, Tianshan Mountain and forest and grassland in Altay | About 30,000 |
| Cervus elaphus xanthopygus [17][18] | Saihanwula Nature Reserve, Inner Mongolia and Muling forest area, Heilongjiang | --- |
2.3. Current Situation of Domestic Sika Deer Germplasm Resources
2.4. Current Situation of Domestic Red Deer Germplasm Resources
2.5. Current Situation of Other Kinds of Velvet Deer Germplasm Resources
3. Utilization of Velvet Deer Germplasm Resources
Germplasm resources are the material basis of modern breeding. Different types of germplasm resources have different genetic characteristics. An in-depth study of velvet deer germplasm resources will not only help to clarify the origin, evolution, classification and other issues, but it will also lay a great foundation for breeding innovation.
3.1. Application of Velvet Deer Germplasm Resources in Traditional Breeding
3.1.1. Pure Breeding
As a basic breeding method, pure breeding plays a role in maintaining and improving the good quality of varieties. It has been widely used in the breeding of pigs [30], sheep [31], cattle [32] and other domestic animals. Shuangyang sika deer are the first velvet-used variety in China. They have been selected and bred through large-scale pure breeding for 23 years and have been introduced around China [33]. In the 1960s, the average velvet antler yield of Shuangyang sika deer exceeded 1 kg, ranking first in antler production in China [34].
3.1.2. Cross-Breeding
Nowadays, antler velvet is a major source of income for the Chinese velvet deer industry. Therefore, velvet antler production performance has become the main goal of velvet deer breeding. As an important breeding method, hybridization is widely used in the modern livestock breeding industry and aims to breed new varieties with high production performance and improve the production efficiency of livestock, thereby achieving improved industrial gains. In order to improve the velvet antler-producing performance of velvet deer, China has carried out more than 70 years of cross-breeding with nearly 30 kinds of cross combinations, including hybridizations between red deer subspecies, sika deer varieties and hybridizations of sika deer and red deer [35].
Because of reproductive isolation, the offspring of most interspecific hybrids are not as adaptable as their parents. They are sterile or even lethal [36]. However, there is barely any hybridization incompatibility among velvet deer interspecies, which provides a feasible basis for variety improvement and breeding innovation of velvet deer. The characteristics of sika deer are relatively stable genetic performance, and the quality of their velvet antlers is better than that of red deer [37]. Sika deer are small in size and low in pilose antler yield, while red deer are large in size and high in pilose antler yield. Therefore, hybridization between sika deer and red deer has always been the main breeding method to improve production performance and breed new varieties. The physical appearance of F1s (Figure 2C,D) lies between the sika deer (Figure 2B) and the red deer (Figure 2A). F1s have normal reproductive capacity, the fertilityand the performance of antler-producing reflect the great economic heterosis [38]. In addition, the growth performance of F1s, such as dressing percentage, carcass weight and net meat weight [39], show extremely significant heterosis [40]. The cytogenetic basis for the fertility of interspecific hybridization in velvet deer is the formation of a cis-trivalent structure of the telocentric/centromere chromosome during the meiosis of F1, which may be related to the balanced distribution of gametes [41]. The testis sections of F1s also show normal morphology and viable sperm; the testes are fully developed [42]. The fertility of the interspecific hybridization greatly improves the breeding innovation efficiency of the velvet deer.
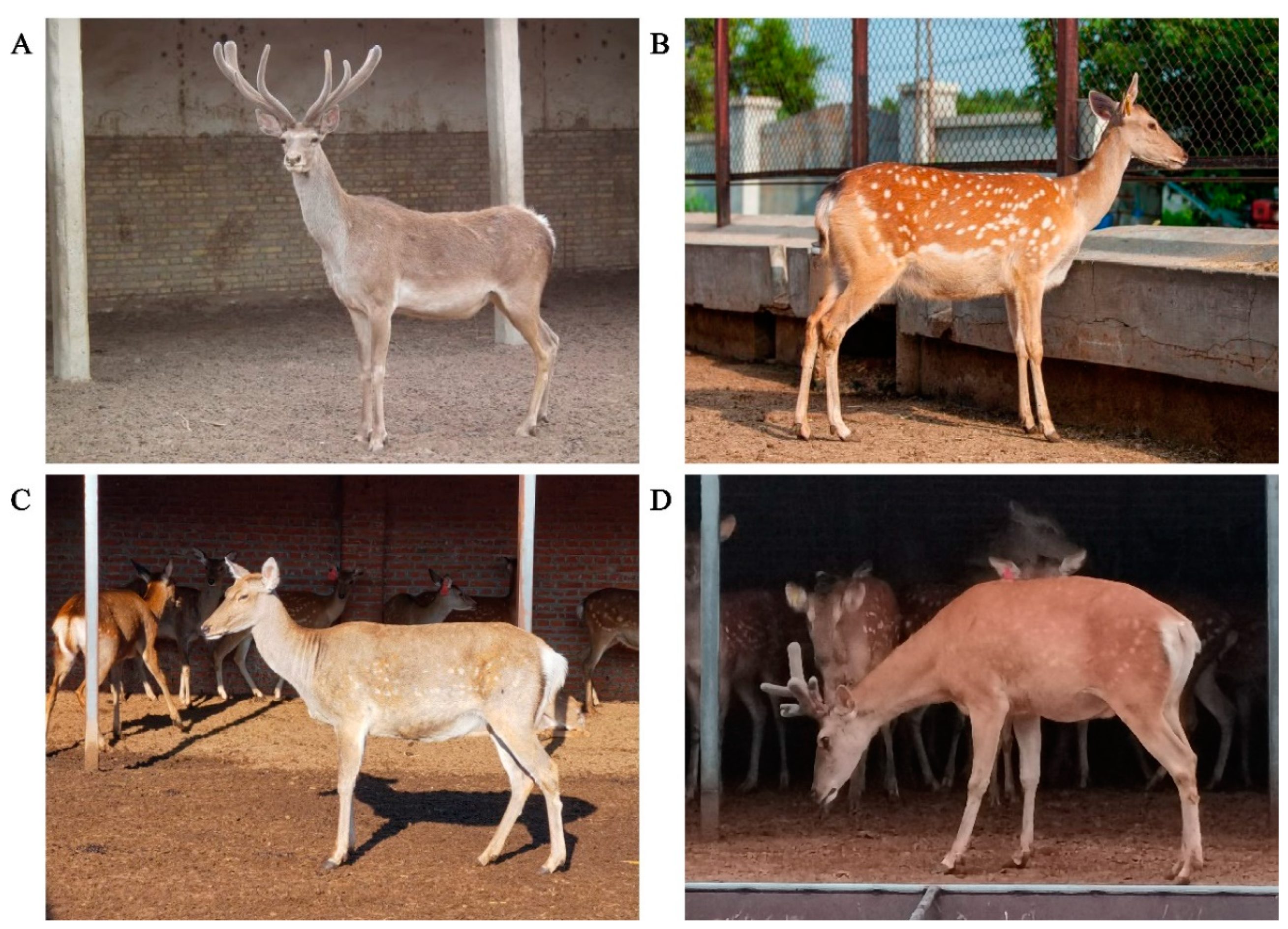
Figure 2. Appearance of F1s and their parents: (A) male red deer and (B) female sika deer; (C,D) F1 offspring.
3.2. Application of Velvet Deer Germplasm Resources in Molecular Breeding
3.2.1. Molecular Assisted Breeding
3.2.2. Genomic Selection
Genomic selection (GS) is a molecular breeding technology that uses the whole genome’s genetic markers of reference groups with genotypes and phenotypes to build models and predict the phenotypic value of genotype-only individuals [52]. It has become an important method for improving the economic traits of livestock and poultry due to its high accuracy, good character-selection effect and reductions in the economic and time costs of breeding. The application of genomic selection cannot be separated from the popularization of whole-genome-sequencing technology. Obtaining a high-quality whole genome of a species is the prerequisite for genomic selection. The high-quality chromosome-level genome of sika deer has been published [53] and used as a reference genome for a genome-wide association study(GWAS). A total of 94 SNPs significantly related to velvet antler production performance have been obtained through genomic selection; they are located in the exon regions of OAS2 and ALYREF/THOC4 [54], and it is speculated that OAS2 and ALYREF/THOC4 may be related to the weight of velvet antlers [55]. As a member of Cervidae with special evolutionary status, the chromosome-level genome of Tarim red deer has also been published [56]. However, it was mainly used for study on adaptive evolution. There are no reports on genomic selection and molecular breeding of red deer.
References
- Sheng, H.L. The Deer in China, 1st ed.; East China Normal University Press: Shanghai, China, 1992; pp. 20–22.
- Zhao, S.Z. The main species of antler deer raised in China. Spec. Econ. Anim. Plant 2000, 3, 8.
- Guo, Y.S.; Zheng, H.Z. On the Geological distribution, Taxonomic status of species and evolutionary history of sika deer in China. Acta Theriol. Sin. 2000, 20, 168–179.
- Tian, X.M.; Liu, X.X.; Zhou, S.C.; Zhang, M.H.; Wang, X.L. Molecular Identification of the Population Distribution and Genetic Variation of Cervus nippon hortulorum. Chin. J. Zool. 2020, 55, 329–338.
- Zhang, S.Y.; Guo, R.; Liu, W.; Weng, D.M.; Cheng, Z.F. Research Progress and Prospect of Cervus nippon kopschi. J. Zhejiang For. Sci. Technol. 2016, 36, 90–94.
- Yen, S.C.; Chen, K.H.; Wang, Y.; Wang, C.P. Residents’ attitudes toward reintroduced sika deer in Kenting National Park, Taiwan. Wildl. Biol. 2015, 21, 220–226.
- Guo, Y.S. Distribution, Numbers and Habitat of Sichuan Sika deer (Cervus nippon Sichuanicus). Acta Theriol. Sin. 2000, 20, 81–87.
- Announcement of the Ministry of Agriculture and Rural Affairs of the State Forestry and Grassland Administration. Available online: https://www.forestry.gov.cn/main/5461/20210205/122418860831352.html (accessed on 16 November 2022).
- Yang, B.Y.; Guan, J.Y. From 60 to 624. Jiangxi Dly. 2021, 5, 3.
- Ma, Y.Q.; Zhao, Y.F.; Yu, Y.M. Biological characteristics and subspecies classification of Red deer (Cervus elaphus). Territ. Nat. Resour. Study 1998, 4, 63–66.
- Liu, W.L. Critically endangered rare animal species—Tibet red deer. Tibet. Sci. Technol. 2009, 6, 65–68.
- Zhao, S.Z. Kansu Red Deer. Spec. Econ. Anim. Plant 2010, 13, 7.
- Mahmut, H.; Omar, A.; Anwar, T.; Zhu, F.; Noriyuki, O. The Present Living Status of Tarim Red Deer (Cervus elaphus yarkandensis) and Its Protective Countermeasures. Acta Theriol. Sin. 2004, 24, 329–332.
- Cui, J.G.; Zhao, H.Y. Investigation Report on Tianshan Red Deer Variety Resources. Chin. Abstr. Anim. Husb. Vet. Med. 2012, 4, 53.
- Gao, H.; Teng, L.W.; Wang, Y.; Wang, J.F. Habitat assessment of red deer (Cervus alashanicus) in the Helan Mountains, China. Acta Theriol. Sin. 2017, 37, 3926–3931.
- Lei, Q.K.; Shen, W.J. An Introduction to Cervus Canadensis Sibiricus Mescn. J. Shi Hezi Agric. Coll. 1993, 4, 44–47.
- Zhang, Z.; Zhang, R.; Li, X.Y.; Yang, Z.D.; Han, Z.Q. Genetic diversity and sex structure of red deer population in Saihanwula Nature Reserve, Inner Mongolia. Acta Theriol. Sin. 2021, 41, 42–50.
- Zhang, W.Q. Winter Nutritional Strategy of the Red Deer (Cervus Elaphus Xanthopygus) in the Muling Forest Region. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2020.
- Meng, M.; Yin, F. Survey of Breeding Status of Sika Deer (Cervus nippon) in Deer Farms in China. Chin. J. Wildl. 2008, 29, 47–49.
- Yu, B.; Wang, B. The Current Situation and Future Prospect of Sika Deer Breeding Industry in Shaanxi Province. J. Xi’an Univ. Nat. Sci. Ed. 2018, 21, 68–70.
- Zhang, R.R.; Sun, Y.S.; Wang, G.W.; Kong, F.T.; Xing, X.M. Thoughts on Accelerating the Development of Sika Deer Industry in Jilin Province. Spec. Wild Econ. Anim. Plant Res. 2021, 44, 151–154.
- Zeng, D.F.; Yang, F.F.; Liang, S.; Xiong, J.J. Development status, problems and countermeasures of Sika deer breeding industry in Hubei Province. Heilongjiang Anim. Sci. Vet. Med. 2017, 24, 189–192.
- Ye, W.B.; Chen, Y.N.; Hu, W.B.; Yang, W.; Zhang, L. Present Situation and Prospect of Cervus nippon Industry in Tianshui and Longnan of Gansu Province. Spec. Wild Econ. Anim. Plant Res. 2021, 43, 105–110.
- Hu, X.L.; Chen, H.J.; Liu, L.; Xu, Y.T.; Zheng, Y.L. Investigation on the Resources and Breeding Industry of Sika Deer in Jiangxi Province. Spec. Wild Econ. Anim. Plant Res. 2021, 43, 119–121.
- Jin, L.; Li, X.; Yang, D. Present Situation Investigation and Development Proposals of the Cervus nippon Industry in Yunnan Province. Guizhou Agric. Sci. 2017, 45, 74–79.
- Hua, X.G. Current Situation and Countermeasures of Red Deer Breeding in Xinjiang. Spec. Econ. Anim. Plant 2007, 10, 8.
- Perhat, S. Investigation of genetic resources of Tarim red deer. Mod. Anim. Husb. 2015, 6, 37–38.
- Zheng, X.T.; Ma, Y.L.; Zhao, M. Natural Character of Artifical Strain Selection for Red Deer. J. Econ. Anim. 2000, 1, 20–22.
- Xue, G.Y.; Xue, S. Present Situation and Countermeasures of Red Deer Breeding Industry in Qingyuan, Fushun. Liaoning For. Sci. Technol. 2011, 4, 50–51.
- Ma, B.E.; Wang, T.B.; Zheng, Y.C. Techniques of purebred breeding, breeding and selection of pigs. Chin. J. Anim. Husb. Vet. Med. 2018, 2, 96–97.
- Liu, D.S.; Zhang, Y.H. Study on Pure Breeding and Hybrid Utilization of Suffolk Sheep. Xinjiang Xumuye 2018, 33, 21–23.
- Li, C.M. Discussion on Main Breeding Methods of Beef Cattle in China. Chin. Livest. Poult. Breed. 2015, 2, 55–56.
- Wang, L.C.; Song, X.Z. Ecological status of sika deer in China. Spec. Econ. Anim. Plant 2014, 3, 12–14.
- Zhao, S.Z.; Han, K.; He, Z.S. Shuangyang Sika Deer. Anim. Husb. Vet. Med. 1985, 2, 64–67.
- Mi, D.D.; Liu, S.; Li, H.P. Main Hybrid Types in Deer Breeding in China. Spec. Econ. Anim. Plant 2014, 17, 7–10.
- Orr, H.A. Dobzhansky, Bateson, and the genetics of speciation. Genetics 1996, 144, 1331–1335.
- Liu, X.Y.; Liu, Y.F.; Chen, H.Y.; Zhao, Y. Determination of Five Nucleotides Contents in Pilos Antler from Cervus Nippon and Cervus Elaphus. Chin. J. Mod. Appl. Pharm. 2018, 35, 1675–1679.
- Bing, G.L.; Zheng, X.T.; Yu, X.Z.; Song, C.L. Characters of Hybrid F1 between Sika Deer and Wapiti. Chin. J. Anim. Vet. Sci. 1988, 4, 244–250.
- Shen, Y.; Jiang, Y.; Zhang, S.; Zou, J.; Gao, X.; Song, Y.; Zhang, Y.; Hu, Y.; Huang, Y.; Jiang, Q. The Effect of Dietary Supplementation with Resveratrol on Growth Performance, Carcass and Meat Quality, Blood Lipid Levels and Ruminal Microbiota in Fattening Goats. Foods 2022, 11, 598.
- Zheng, X.T.; Wei, H.J.; Zhang, Y.; Wang, B.L. Slaughter Test on the Male Hybrid (F1) Crossed Xifeng Sika Deer with Wapiti and the Maternal Stage (Xifeng). J. Econ. Anim. 2006, 9, 12–14.
- Yu, X.Z. Comparative Observations on the Karyotype of North-eastern Red Deer and North-eastern Sika Deer and Karyotype Analysis of Their Hybrid offsprings of Five Hybridized Combinations. Acta Genet. Sin. 1986, 2, 125–131.
- Ma, K.; Shi, L.M.; Yu, X.Z.; Wang, E.K.; Sun, B.Q. Analysis of Synaptonemal Complexes in Spermatocytes of the Hybrid F1 Between Red Deer and Sika Deer. Acta Genet. Sin. 1988, 3, 197–200.
- Fan, H.H.; Wang, T.J.; Li, Y. Development and validation of a 1 K sika deer (Cervus nippon) SNP Chip. BMC Genom. Data 2021, 22, 35.
- Hsiao, C.; Lin, H.H.; Kang, S.R. Development of 16 novel EST-SSR markers for species identification and cross-genus amplification in sambar, sika, and red deer. PLoS ONE 2022, 17, e0265311.
- Balloux, F.; Moulin, N.L. The estimation of population differentiation with microsatellite markers. Mol. Ecol. 2002, 11, 155–165.
- Thiébaut, C.D.; Le, C.S. Isolation of 11 microsatellite markers in Crepidula convexa (Gastropoda, Calyptraeideae) for parentage analyses. Mol. Ecol. Resour. 2009, 9, 917–920.
- Yang, W.; Zheng, J.; Jia, B.Y. Isolation of novel microsatellite markers and their application for genetic diversity and parentage analyses in sika deer. Gene 2018, 643, 68–73.
- Xia, Y.; Qu, H.; Lu, B.; Zhang, Q.; Li, H. Molecular cloning and expression analysis of annexin A2 gene in sika deer antler tip. Asian Australas. J. Anim. Sci. 2018, 31, 467.
- Hu, P.F.; Wang, T.J.; Liu, H.M.; Xu, J.; Wang, L.; Zhao, P.; Xing, X. Full-length transcriptome and microRNA sequencing reveal the specific gene-regulation network of velvet antler in sika deer with extremely different velvet antler weight. Mol. Genet. Genom. 2019, 294, 431–443.
- Yang, F.-F.; Huo, L.-J.; Yang, L.-G.; Riaz, H.; Xiong, L.-R.; Chen, J.-G.; Zhang, S.-J.; Xiong, J.-J. Association between melatonin receptor 1A (MTNR1A) gene single-nucleotide polymorphisms and the velvet antler yield of Sika deer. Mol. Biol. Rep. 2014, 41, 1901–1906.
- Jia, B.Y.; Wang, G.; Zheng, J.J.; Yang, W.; Chang, S.; Zhang, J.; Liu, Y.; Li, Q.; Ge, C.; Chen, G.; et al. Development of novel EST microsatellite markers for genetic diversity analysis and correlation analysis of velvet antler growth characteristics in Sika deer. Hereditas 2020, 157, 1–14.
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829.
- Xing, X.M.; Ai, C.; Wang, T.J.; Li, Y.; Liu, H.; Hu, P.; Wang, G.; Liu, H.; Wang, H.; Zhang, R.; et al. The First High-Quality Reference Genome of Sika Deer Provides Insights for High-Tannin Adaptation. Genom. Proteom. Bioinform. 2022; accepted.
- Hu, P.F.; Xu, J.P.; Ai, C.; Xing, X.M.; Hongliang, W.; Yimeng, D.; Xuezhe, C.; Fuhe, Y.; Xiumei, X. Screening weight related genes of velvet antlers by whole genome re-sequencing. Hereditas 2017, 39, 1090–1101.
- Hu, P.F.; Deng, Y.; Ba, H.X.; Li, C. Association analysis of thirty-one single nucleotide polymorphisms with antler weight in sika deer. Anim. Genet. 2020, 51, 990–991.
- Ba, H.X.; Cai, Z.; Gao, H.; Qin, T.; Liu, W.; Xie, L.; Zhang, Y.; Jing, B.; Wang, D.; Li, C. Chromosome-level genome assembly of Tarim red deer, Cervus elaphus yarkandensis. Sci. Data 2020, 7, 187.




