
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Monica Sanchez-Roman | -- | 4899 | 2022-12-30 12:15:12 | | | |
| 2 | Rita Xu | -1 word(s) | 4898 | 2023-01-03 03:13:27 | | |
Video Upload Options
Microbially induced carbonate precipitation (MICP) is an important process in the synthesis of carbonate minerals, and thus, it is widely explored as a novel approach with potential for many technological applications. The processes and mechanisms involved in carbonate mineral formation in the presence of microbes are not yet fully understood. This research covers the current knowledge regarding the role of microbial cells and metabolic products (e.g., extracellular polymeric substances, proteins and amino acids) on the adsorption of divalent metals, adsorption of ionic species and as templates for crystal nucleation. Moreover, they can play a role in the mineral precipitation, size, morphology and lattice. By understanding how microbes and their metabolic products promote suitable physicochemical conditions (pH, Mg/Ca ratio and free carbonate ions) to induce carbonate nucleation and precipitation, the manipulation of the final mineral precipitates could be a reality for (geo)biotechnological approaches. The applications and implications of biogenic carbonates in areas such as geology and engineering are presented and discussed in this research, with a major focus on biotechnology.
1. Introduction
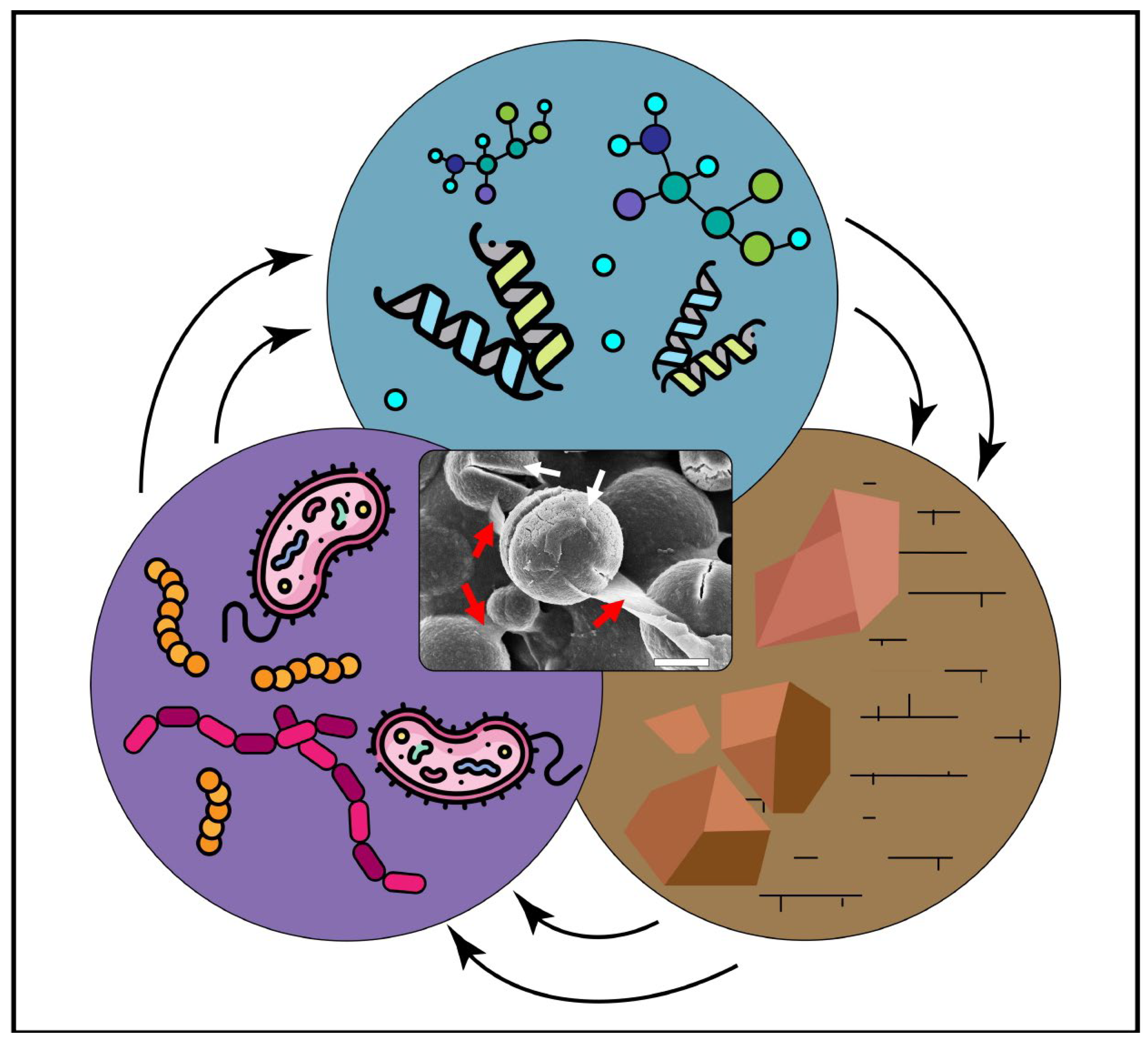
2. Biopolymers Associated with Carbonates
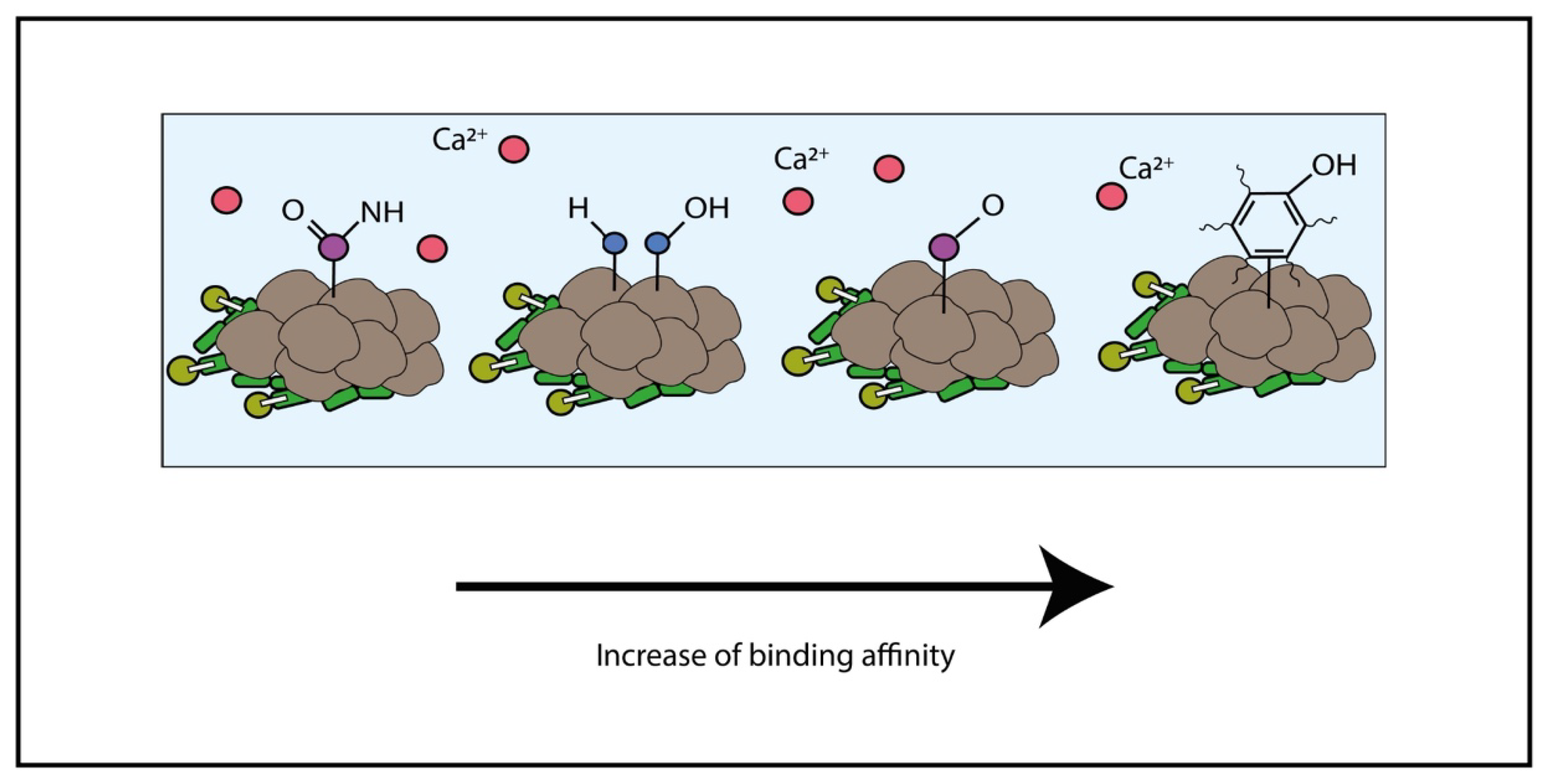
2.1. Adsorption and Linkage of Metal
2.2. Polymers Influencing Size, Morphology, Texture and Chemical Composition of Carbonate Minerals
2.3. Polymeric Substances Secreted by Microorganisms and Micritization, Lithification and Porosity Processes
3. Conclusions
Many different microorganisms and higher organisms can induce and determine MICP through the production of EPS, enzymes, amino acids and other organic metabolites that by their interactions determine the type and rate of the process. Many studies have focused on the molecular routes and metabolic pathways activated by these organisms that determine and regulate the precipitation process, suggesting that the final properties of the minerals obtained are genetically regulated and control of the process is achievable. Understanding the parameters for the synthesis of the organic molecules involved in MICP and EICP might shed light on developing fine-tune control of these processes for biotechnological applications. Carbonates are important molecules for carbon stock and for potentially regulating the current trends in CO2 accumulation in the atmosphere.
Moreover, carbonates are very useful minerals in several engineering, medical engineering and geological applications. Although many questions remain to be answered regarding the role of microorganisms in the production and turnover of carbonates, such as the kind of microorganisms able to induce the process and their ecology and interactions; the role of the environmental factors involved; or the role and mechanisms of the biological molecules (EPS, proteins, enzymes, organic cofactors, organic acids, etc.) involved, current research has shaped the biologically mediated precipitation and rearrangements of carbonates as a world-scale process that has very great interest for the protection of the environment, regulation of the climate and application in many (geo)biotechnological problems that may contribute to the understanding of the past of our planet and the search for life on other planets.
References
- Walker, L.J.; Wilkinson, B.H.; Ivany, L.C. Continental Drift and Phanerozoic Carbonate Accumulation in Shallow-Shelf and Deep-Marine Settings. J. Geol. 2002, 110, 75–87.
- Reddy, M.S. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314.
- Swart, P.K.; Eberli, G.P.; McKenzie, J.A. Perspectives in Carbonate Geology: A Tribute to the Career of Robert Nathan Ginsburg; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-4443-1205-8.
- Levi, Y.; Albeck, S.; Brack, A.; Weiner, S.; Addadi, L. Control Over Aragonite Crystal Nucleation and Growth: An In Vitro Study of Biomineralization. Chem.-Eur. J. 1998, 4, 389–396.
- Wang, X.; Kong, R.; Pan, X.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Role of Ovalbumin in the Stabilization of Metastable Vaterite in Calcium Carbonate Biomineralization. J. Phys. Chem. B 2009, 113, 8975–8982.
- Spadafora, A.; Perri, E.; Mckenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40.
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; McKenzie, J.A. Microbial Dolomite Precipitation under Aerobic Conditions: Results from Brejo do Espinho Lagoon (Brazil) and Culture Experiments. In Perspectives in Carbonate Geology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 167–178. ISBN 978-1-4443-1206-5.
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787.
- Da Silva, S.; Bernet, N.; Delgenès, J.P.; Moletta, R. Effect of culture conditions on the formation of struvite by Myxococcus xanthus. Chemosphere 2000, 40, 1289–1296.
- Rivadeneyra, M.A.; Pérez-García, I.; Ramos-Cormenzana, A. Struvite precipitation by soil and fresh water bacteria. Curr. Microbiol. 1992, 24, 343–347.
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230.
- Zeth, K.; Hoiczyk, E.; Okuda, M. Ferroxidase-Mediated Iron Oxide Biomineralization: Novel Pathways to Multifunctional Nanoparticles. Trends Biochem. Sci. 2016, 41, 190–203.
- Staicu, L.C.; Wojtowicz, P.J.; Pósfai, M.; Pekker, P.; Gorecki, A.; Jordan, F.L.; Barton, L.L. PbS biomineralization using cysteine: Bacillus cereus and the sulfur rush. FEMS Microbiol. Ecol. 2020, 96, fiaa151.
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Biomineralization: From Nature to Application, Volume 4; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-98631-8.
- Liu, B.; Cao, Y.; Huang, Z.; Duan, Y.; Che, S. Silica Biomineralization via the Self-Assembly of Helical Biomolecules. Adv. Mater. 2015, 27, 479–497.
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth-Sci. Rev. 1998, 45, 45–60.
- Ehrlich, H.L. Microbes as Geologic Agents: Their Role in Mineral Formation. Geomicrobiol. J. 1999, 16, 135–153.
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46.
- Krumbein, W.E. Photolithotropic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (gulf of Aqaba, Sinai). Geomicrobiol. J. 1979, 1, 139–203.
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23.
- Riding, R. Cyanophyte calcification and changes in ocean chemistry. Nature 1982, 299, 814–815.
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162.
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of Aragonite or Calcite Polymorphism by Mollusk Shell Macromolecules. Science 1996, 271, 67–69.
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem.—A Eur. J. 2006, 12, 980–987.
- Tambutté, S.; Holcomb, M.; Ferrier-Pagès, C.; Reynaud, S.; Tambutté, É.; Zoccola, D.; Allemand, D. Coral biomineralization: From the gene to the environment. J. Exp. Mar. Biol. Ecol. 2011, 408, 58–78.
- Li, H.; Sun, C.-Y.; Fang, Y.; Carlson, C.M.; Xu, H.; Ješovnik, A.; Sosa-Calvo, J.; Zarnowski, R.; Bechtel, H.A.; Fournelle, J.H.; et al. Biomineral armor in leaf-cutter ants. Nat. Commun. 2020, 11, 5792.
- Hild, S.; Marti, O.; Ziegler, A. Spatial distribution of calcite and amorphous calcium carbonate in the cuticle of the terrestrial crustaceans Porcellio scaber and Armadillidium vulgare. J. Struct. Biol. 2008, 163, 100–108.
- Wilt, F.H.; Killian, C.E.; Livingston, B.T. Development of calcareous skeletal elements in invertebrates. Differentiation 2003, 71, 237–250.
- Ozawa, H.; Hoshi, K.; Amizuka, N. Current Concepts of Bone Biomineralization. J. Oral Biosci. 2008, 50, 1–14.
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov. Mater. 2020, 1, 2.
- Weiner, S. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29.
- Bolhuis, H.; Stal, L.J. Analysis of bacterial and archaeal diversity in coastal microbial mats using massive parallel 16S rRNA gene tag sequencing. ISME J. 2011, 5, 1701–1712.
- Baumgartner, L.K.; Reid, R.P.; Dupraz, C.; Decho, A.W.; Buckley, D.H.; Spear, J.R.; Przekop, K.M.; Visscher, P.T. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries. Sediment. Geol. 2006, 185, 131–145.
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760.
- Gilbert, P.U.P.A.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 2022, 8, eabl9653.
- Gilbert, P.U.P.A.; Porter, S.M.; Sun, C.-Y.; Xiao, S.; Gibson, B.M.; Shenkar, N.; Knoll, A.H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665.
- Sun, X.; Miao, L.; Yuan, J.; Wang, H.; Wu, L. Application of enzymatic calcification for dust control and rainfall erosion resistance improvement. Sci. Total Environ. 2021, 759, 143468.
- Wang, Z.; Zhang, N.; Cai, G.; Jin, Y.; Ding, N.; Shen, D. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesources Geotechnol. 2017, 35, 1135–1146.
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021, 166, 107856.
- Miftah, A.; Khodadadi Tirkolaei, H.; Bilsel, H. Biocementation of Calcareous Beach Sand Using Enzymatic Calcium Carbonate Precipitation. Crystals 2020, 10, 888.
- Wilson, J.L. Limestone and dolomite reservoirs. Pet. Geol.(Engl. Transl.) 1980, 2.
- Norris, S.E. Characteristics of Limestone and Dolomite Aquifers in Western Ohio. J. AWWA 1957, 49, 464–468.
- Burchette, T.P. Carbonate rocks and petroleum reservoirs: A geological perspective from the industry. Geol. Soc. Lond. Spec. Publ. 2012, 370, 17–37.
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; World Scientific: Singapore, 2014; ISBN 978-1-78326-330-1.
- Barnes, H. Oceanography And Marine Biology; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-1-4822-6728-0.
- Quigley, M.S.; Santschi, P.H.; Hung, C.-C.; Guo, L.; Honeyman, B.D. Importance of acid polysaccharides for 234Th complexation to marine organic matter. Limnol. Oceanogr. 2002, 47, 367–377.
- Sánchez-Román, M.; Rivadeneyra, M.A.; Vasconcelos, C.; McKenzie, J.A. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 2007, 61, 273–284.
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150.
- Zhao, Y.; Yao, J.; Yuan, Z.; Wang, T.; Zhang, Y.; Wang, F. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation. Environ. Sci. Pollut. Res. 2017, 24, 372–380.
- Mitchell, A.C.; Ferris, F.G. Effect of Strontium Contaminants upon the Size and Solubility of Calcite Crystals Precipitated by the Bacterial Hydrolysis of Urea. Environ. Sci. Technol. 2006, 40, 1008–1014.
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210.
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Rivadeneyra, M.A.; Vasconcelos, C. Presence of sulfate does not inhibit low-temperature dolomite precipitation. Earth Planet. Sci. Lett. 2009, 285, 131–139.
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Romanek, C.S.; Sánchez-Navas, A.; Vasconcelos, C. Experimentally determined biomediated Sr partition coefficient for dolomite: Significance and implication for natural dolomite. Geochim. Cosmochim. Acta 2011, 75, 887–904.
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; Martin-Uriz, P.S.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4, 4767.
- Sánchez-Román, M.; Puente-Sánchez, F.; Parro, V.; Amils, R. Nucleation of Fe-rich phosphates and carbonates on microbial cells and exopolymeric substances. Front. Microbiol. 2015, 6, 1024.
- Geesey, G.G.; Jang, L.; Jolley, J.G.; Hankins, M.R.; Iwaoka, T.; Griffiths, P.R. Binding of Metal Ions by Extracellular Polymers of Biofilm Bacteria. Water Sci. Technol. 1988, 20, 161–165.
- Wen, Y.; Sánchez-Román, M.; Li, Y.; Wang, C.; Han, Z.; Zhang, L.; Gao, Y. Nucleation and stabilization of Eocene dolomite in evaporative lacustrine deposits from central Tibetan plateau. Sedimentology 2020, 67, 3333–3354.
- Liu, Y.; Lam, M.C.; Fang, H.H. Adsorption of heavy metals by EPS of activated sludge. Water Sci. Technol. 2001, 43, 59–66.
- Sheng, G.-P.; Xu, J.; Luo, H.-W.; Li, W.-W.; Li, W.-H.; Yu, H.-Q.; Xie, Z.; Wei, S.-Q.; Hu, F.-C. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013, 47, 607–614.
- Jang, A.; Kim, S.M.; Kim, S.Y.; Lee, S.G.; Kim, I.S. Effect of heavy metals (Cu, Pb, and Ni) on the compositions of EPS in biofilms. Water Sci. Technol. 2001, 43, 41–48.
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A.; Zenobi, R.; Rivadeneyra, M.A. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882.
- Popall, R.M.; Bolhuis, H.; Muyzer, G.; Sánchez-Román, M. Stromatolites as Biosignatures of Atmospheric Oxygenation: Carbonate Biomineralization and UV-C Resilience in a Geitlerinema sp.—Dominated Culture. Front. Microbiol. 2020, 11, 948.
- Wang, L.; Chen, W.; Song, X.; Li, Y.; Zhang, W.; Zhang, H.; Niu, L. Cultivation substrata differentiate the properties of river biofilm EPS and their binding of heavy metals: A spectroscopic insight. Environ. Res. 2020, 182, 109052.
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56.
- Beveridge, T.J. Ultrastructure, Chemistry, and Function of the Bacterial Wall. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 72, pp. 229–317.
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88.
- Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101.
- Jin, D.; Wang, F.; Yue, L. Phase and morphology evolution of vaterite crystals in water/ethanol binary solvent. Cryst. Res. Technol. 2011, 46, 140–144.
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water–Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853.
- Fang, Y.; Zhang, F.; Farfan, G.A.; Xu, H. Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications. ACS Omega 2022, 7, 281–292.
- Liu, R.; Huang, S.; Zhang, X.; Song, Y.; He, G.; Wang, Z.; Lian, B. Bio-mineralisation, characterization, and stability of calcium carbonate containing organic matter. RSC Adv. 2021, 11, 14415–14425.
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially Induced Mineralization of Calcium Carbonate in Terrestrial Environments: The Role of Exopolysaccharides and Amino Acids. J. Sediment. Res. 2003, 73, 485–490.
- Chekroun, K.B.; Rodríguez-Navarro, C.; González-Muñoz, M.T.; Arias, J.M.; Cultrone, G.; Rodríguez-Gallego, M. Precipitation and Growth Morphology of Calcium Carbonate Induced by Myxococcus Xanthus: Implications for Recognition of Bacterial Carbonates. J. Sediment. Res. 2004, 74, 868–876.
- Kadan, Y.; Tollervey, F.; Varsano, N.; Mahamid, J.; Gal, A. Intracellular nanoscale architecture as a master regulator of calcium carbonate crystallization in marine microalgae. Proc. Natl. Acad. Sci. USA 2021, 118, e2025670118.
- Aizenberg, J.; Addadi, L.; Weiner, S.; Lambert, G. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226.
- Aizenberg, J.; Hanson, J.; Ilan, M.; Leiserowitz, L.; Koetzle, T.F.; Addadi, L.; Weiner, S. Morphogenesis of calcitic sponge spicules: A role for specialized proteins interacting with growing crystals. FASEB J. 1995, 9, 262–268.
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 1996, 381, 56–58.
- Butler, M.F.; Glaser, N.; Weaver, A.C.; Kirkland, M.; Heppenstall-Butler, M. Calcium Carbonate Crystallization in the Presence of Biopolymers. Cryst. Growth Des. 2006, 6, 781–794.
- Broughton, P.L. Microbial EPS-mediated amorphous calcium carbonate–monohydrocalcite–calcite transformations during early tufa deposition. Depos. Rec. 2022, 1–28.
- Wang, X.; Sun, H.; Xia, Y.; Chen, C.; Xu, H.; Shan, H.; Lu, J.R. Lysozyme mediated calcium carbonate mineralization. J. Colloid Interface Sci. 2009, 332, 96–103.
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-Lopez, C.; Nys, Y.; García-Ruiz, J.M. Influence of Model Globular Proteins with Different Isoelectric Points on the Precipitation of Calcium Carbonate. Cryst. Growth Des. 2008, 8, 1495–1502.
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299.
- Heveran, C.M.; Liang, L.; Nagarajan, A.; Hubler, M.H.; Gill, R.; Cameron, J.C.; Cook, S.M.; Srubar, W.V. Engineered Ureolytic Microorganisms Can Tailor the Morphology and Nanomechanical Properties of Microbial-Precipitated Calcium Carbonate. Sci. Rep. 2019, 9, 14721.
- Gadd, G.-M.; Burford, E.-P.; Fomina, M. Biogeochemical Activities of Microorganisms in Mineral Transformations: Consequences for Metal and Nutrient Mobility. J. Microbiol. Biotechnol. 2003, 13, 323–331.
- Violante, A.; Huang, P.M.; Gadd, G.M. Biophysico-Chemical Processes of Heavy Metals and Metalloids in Soil Environments; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-17547-7.
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305.
- Gadd, G.M. Metals and microorganism: A problem of definition. FEMS Microbiol. Lett. 1992, 100, 197–203.
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643.
- Reid, R.P.; Macintyre, I.G. Microboring Versus Recrystallization: Further Insight into the Micritization Process. J. Sediment. Res. 2000, 70, 24–28.
- Kobluk, D.R.; Risk, M.J. Micritization and Carbonate-Grain Binding by Endolithic Algae1. AAPG Bull. 1977, 61, 1069–1082.
- Boulos, R.A.; Zhang, F.; Tjandra, E.S.; Martin, A.D.; Spagnoli, D.; Raston, C.L. Spinning up the polymorphs of calcium carbonate. Sci. Rep. 2014, 4, 3616.
- Politi, Y.; Levi-Kalisman, Y.; Raz, S.; Wilt, F.; Addadi, L.; Weiner, S.; Sagi, I. Structural Characterization of the Transient Amorphous Calcium Carbonate Precursor Phase in Sea Urchin Embryos. Adv. Funct. Mater. 2006, 16, 1289–1298.
- Loste, E.; Meldrum, F.C. Control of calcium carbonate morphology by transformation of an amorphous precursor in a constrained volume. Chem. Commun. 2001, 10, 901–902.
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970.
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilbert, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, E7670–E7678.
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68.
- Golubic, S.; Perkins, R.D.; Lukas, K.J. Boring Microorganisms and Microborings in Carbonate Substrates. In The Study of Trace Fossils: A Synthesis of Principles, Problems, and Procedures in Ichnology; Frey, R.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 229–259. ISBN 978-3-642-65923-2.
- Garcia-Pichel, F.; Ramírez-Reinat, E.; Gao, Q. Microbial excavation of solid carbonates powered by P-type ATPase-mediated transcellular Ca2+ transport. Proc. Natl. Acad. Sci. USA 2010, 107, 21749–21754.
- Cockell, C.S.; Herrera, A. Why are some microorganisms boring? Trends Microbiol. 2008, 16, 101–106.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636.
- Fong, J.N.C.; Yildiz, F.H. Biofilm Matrix Proteins. Microbiol. Spectr. 2015, 3, 201–222.
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288.
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238.
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary Deposits of Benthic Microbial Communities. PALAIOS 1987, 2, 241–254.
- Suarez-Gonzalez, P.; Benito, M.I.; Quijada, I.E.; Mas, R.; Campos-Soto, S. ‘Trapping and binding’: A review of the factors controlling the development of fossil agglutinated microbialites and their distribution in space and time. Earth-Sci. Rev. 2019, 194, 182–215.
- Suarez-Gonzalez, P.; Quijada, I.E.; Benito, M.I.; Mas, R. Sedimentology of Ancient Coastal Wetlands: Insights From A Cretaceous Multifaceted Depositional System. J. Sediment. Res. 2015, 85, 95–117.
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-Induced Biofilm Calcification and Calcium Concentrations in Phanerozoic Oceans. Science 2001, 292, 1701–1704.
- Campos-Soto, S.; Benito, M.I.; Mas, R.; Caus, E.; Cobos, A.; Suárez-González, P.; Quijada, I.E. Revisiting the Late Jurassic-Early Cretaceous of the NW South Iberian Basin: New ages and sedimentary environments. J. Iber. Geol. 2016, 42, 69–94.
- Lanés, S.; Palma, R.M. Environmental implications of oncoids and associated sediments from the Remoredo Formation (Lower Jurassic) Mendoza, Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 140, 357–366.
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992.
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian Carbonates: Evolutionary Mileposts or Environmental Dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358.
- Eymard, I.; Alvarez, M. del P.; Bilmes, A.; Vasconcelos, C.; Ariztegui, D. Tracking Organomineralization Processes from Living Microbial Mats to Fossil Microbialites. Minerals 2020, 10, 605.
- Margulis, L.; Barghoorn, E.S.; Ashendorf, D.; Banerjee, S.; Chase, D.; Francis, S.; Giovannoni, S.; Stolz, J. The microbial community in the layered sediments at Laguna Figueroa, Baja California, Mexico: Does it have Precambrian analogues? Precambrian Res. 1980, 11, 93–123.
- Oremland, R.S.; Saltikov, C.W.; Wolfe-Simon, F.; Stolz, J.F. Arsenic in the Evolution of Earth and Extraterrestrial Ecosystems. Geomicrobiol. J. 2009, 26, 522–536.
- Parro, V.; de Diego-Castilla, G.; Moreno-Paz, M.; Blanco, Y.; Cruz-Gil, P.; Rodríguez-Manfredi, J.A.; Fernández-Remolar, D.; Gómez, F.; Gómez, M.J.; Rivas, L.A.; et al. A Microbial Oasis in the Hypersaline Atacama Subsurface Discovered by a Life Detector Chip: Implications for the Search for Life on Mars. Astrobiology 2011, 11, 969–996.
- Demarchi, B. Amino Acids and Proteins in Fossil Biominerals: An Introduction for Archaeologists and Palaeontologists; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-119-08951-3.
- Saitta, E.T.; Vinther, J.; Crisp, M.K.; Abbott, G.D.; Kaye, T.G.; Pittman, M.; Bull, I.; Fletcher, I.; Chen, X.; Collins, M.J.; et al. Non-Avian Dinosaur Eggshell Calcite Contains Ancient, Endogenous Amino Acids. bioRxiv 2020. bioRxiv:2020.06.02.129999.
- Hendy, J.; Welker, F.; Demarchi, B.; Speller, C.; Warinner, C.; Collins, M.J. A guide to ancient protein studies. Nat. Ecol. Evol. 2018, 2, 791–799.
- Benzerara, K.; Menguy, N.; López-García, P.; Yoon, T.-H.; Kazmierczak, J.; Tyliszczak, T.; Guyot, F.; Brown, G.E. Nanoscale detection of organic signatures in carbonate microbialites. Proc. Natl. Acad. Sci. USA 2006, 103, 9440–9445.




