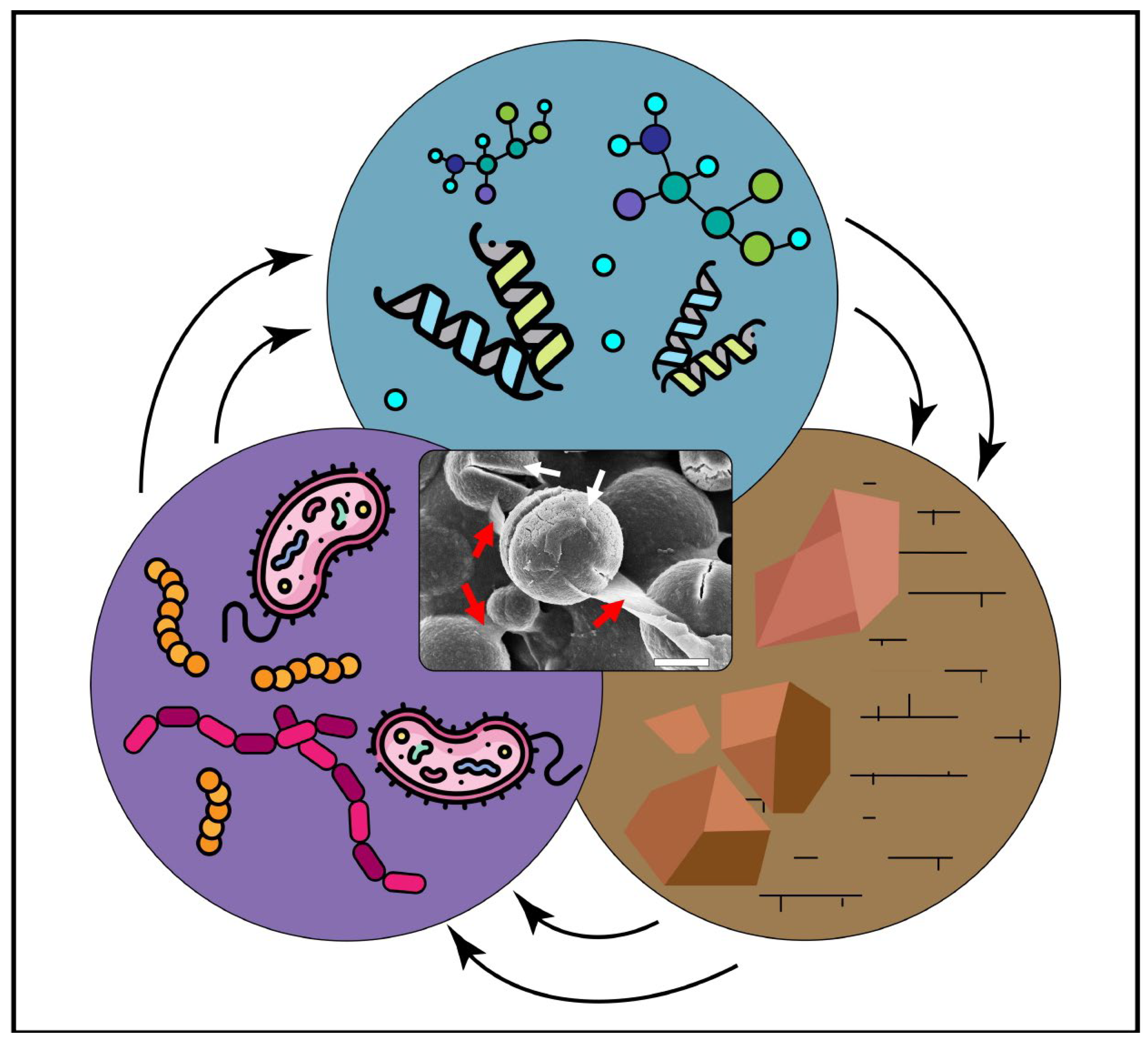
Microbially induced carbonate precipitation (MICP) is an important process in the synthesis of carbonate minerals, and thus, it is widely explored as a novel approach with potential for many technological applications. However, the processes and mechanisms involved in carbonate mineral formation in the presence of microbes are not yet fully understood. This review covers the current knowledge regarding the role of microbial cells and metabolic products (e.g., extracellular polymeric substances, proteins and amino acids) on the adsorption of divalent metals, adsorption of ionic species and as templates for crystal nucleation. Moreover, they can play a role in the mineral precipitation, size, morphology and lattice. By understanding how microbes and their metabolic products promote suitable physicochemical conditions (pH, Mg/Ca ratio and free carbonate ions) to induce carbonate nucleation and precipitation, the manipulation of the final mineral precipitates could be a reality for (geo)biotechnological approaches. The applications and implications of biogenic carbonates in areas such as geology and engineering are presented and discussed in this review, with a major focus on biotechnology.
Microbially induced carbonate precipitation (MICP) is an important process in the synthesis of carbonate minerals, and thus, it is widely explored as a novel approach with potential for many technological applications. The processes and mechanisms involved in carbonate mineral formation in the presence of microbes are not yet fully understood. This research covers the current knowledge regarding the role of microbial cells and metabolic products (e.g., extracellular polymeric substances, proteins and amino acids) on the adsorption of divalent metals, adsorption of ionic species and as templates for crystal nucleation. Moreover, they can play a role in the mineral precipitation, size, morphology and lattice. By understanding how microbes and their metabolic products promote suitable physicochemical conditions (pH, Mg/Ca ratio and free carbonate ions) to induce carbonate nucleation and precipitation, the manipulation of the final mineral precipitates could be a reality for (geo)biotechnological approaches. The applications and implications of biogenic carbonates in areas such as geology and engineering are presented and discussed in this research, with a major focus on biotechnology.
- carbonates
- microorganisms
- bacteria
- biomineralization
1. Introduction
2. Biopolymers Associated with Carbonates
2.1. Adsorption and Linkage of Metal
The EPS are generally made of high molecular weight molecules with charged functional groups and possess both adsorptive and adhesive functions. Thus, EPS can serve as a source of natural ligands that provide binding sites for other charged groups, including metals [45]. The EPS present a high ability for metal binding generally higher than mineral sorbents [46]. The ability and properties of microbial EPS have been studied and characterized [32]. The EPS and negatively loaded proteins in the cellular membranes can attract metal cations (e.g., Ca2+, Mg2+ and Fe2+), increasing the ion concentration in the surrounding environment of the microbial cell. Such process may lead to ion supersaturation in the cell surrounding, which enhances the changes in the carbonate mineral nucleation and precipitation. However, as Ca2+ can be attracted by electrostatic interactions, many other divalent metal cations could be attracted following the same physicochemical reasoning, such as Cd2+, Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, Pb2+ and Sr2+. Once other metals are attached to the cell walls, or the proteinaceous S-layers and the conditions are favorable, such metal cations can slip in the previously formed crystalline structure following the ionic exchange with Ca2+. Such metal–mineral–microbe interactions can reshape the chemical nature of the carbonates though biomineralization to molecules other than calcium carbonates, such as strontium carbonates, magnesium carbonates or iron carbonates [7][39][47][48][49][50][51][52][53][54][55][56]. Many studies have concluded the main role of EPS in concentrating these divalent ions [7][48][53][54][55][57][58][59][60][61][62]. In an experiment that tried to characterize the differences in the interactions between biofilms and metal ions, when the biofilms (EPS) were deposited in (i) commercial plastics or (ii) periphyton (a natural substrate of EPS in freshwater environments), it was shown that EPS organic components, such as amines and phenolic compounds, could attract and bind metal ions of Cu(II) and Cd(II) very quickly. Additionally, amidic and aliphatic components of EPS showed a preferential binding to heavy metals [63]. The implications of the metal-binding properties of bacterial EPS and its possible role in the bioaccumulation of pollutants in the marine food chain were under study after the isolation and purification of EPS synthesized by species of Marinobacter species [64]. It was demonstrated such a type of EPS bind more efficiently Cu2+ than Pb2+ per mg of EPS, preferentially at a neutral pH. It was also shown that the adsorption increased with higher concentrations of the metals to bind, but certain factors such as increasing concentrations of NaCl decreased adsorption rates, as simplified in Figure 2.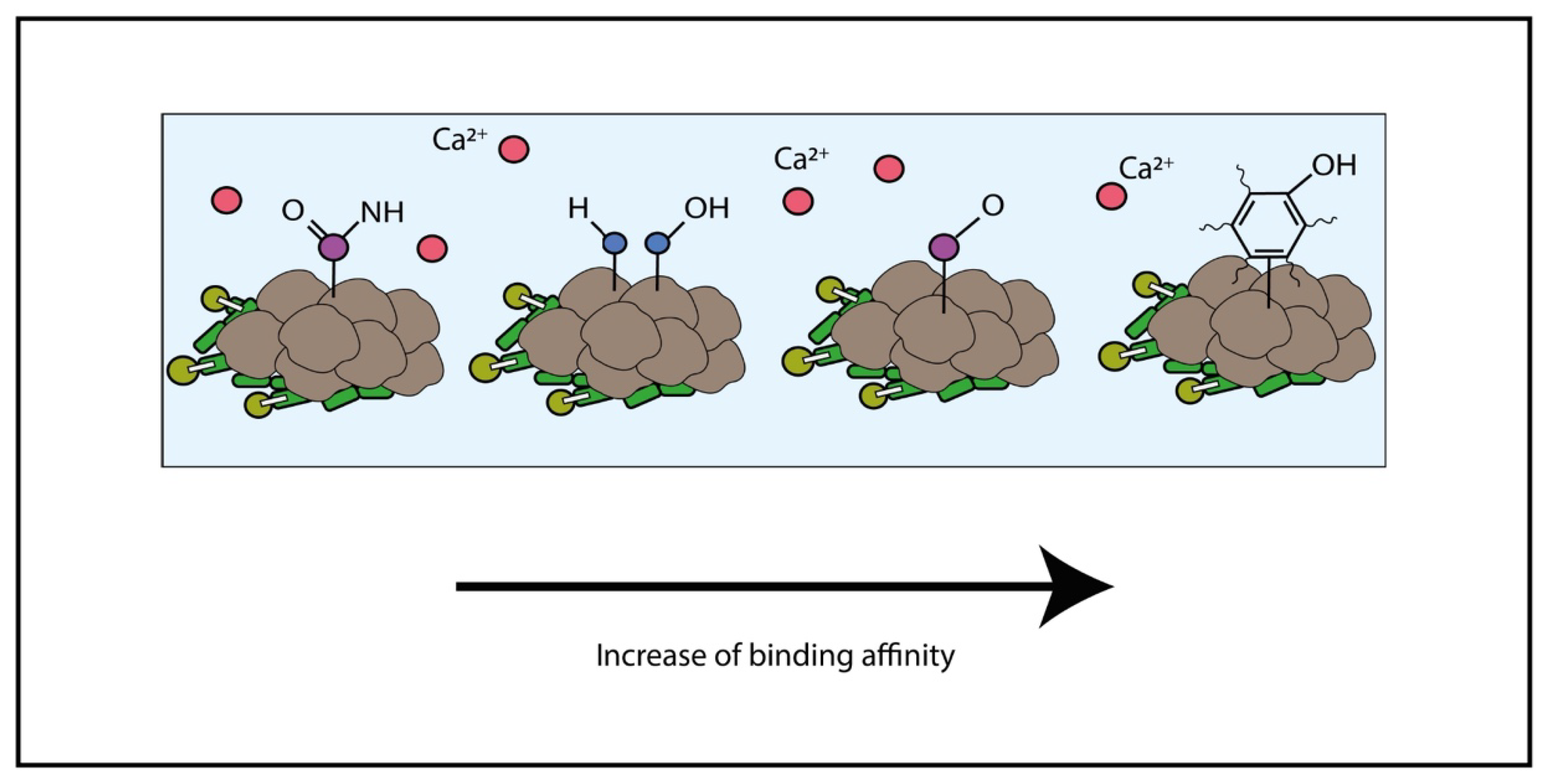
2.2. Polymers Influencing Size, Morphology, Texture and Chemical Composition of Carbonate Minerals
Since microbes are involved in biomineralization processes, much effort has been put into trying to understand how they can actually influence the final mineral formed. There is some evidence that superior organisms and microorganisms are able to tightly control biomineralization processes during MICP. Bioprecipitation starts with the formation of nanoclusters and nanoparticle precursors. Different organisms such as mollusks can orchestrate the type of calcium carbonate polymorph that will result from the biomineralization. According to the free energy and enthalpy values of the different polymorphs of certain minerals such as carbonates, the differences are so small that reversions to the stability state can occur [67]. Taking this into account, a possible strategy followed by the microorganisms to control the final polymorph might be related to the control of the initial size of the crystals [67]. This step could be biotechnologically manipulated by monitoring the ionic strength, supplying controlled concentrations of reactants, and by adding certain organic and inorganic molecules to the growth medium. Therefore, obtaining the desired polymorph with relatively uniform size distributions could be feasible. Calcium carbonate polymorphs constitute a special case: vaterite is less dense than calcite, and aragonite is denser than calcite. Vaterite and aragonite are formed as metastable phases in the biomineralization processes, being the most stable polymorph calcite. The addition of certain impurities, such as Mg2+, to the mineral seed surfaces could lead to changes in the superficial energy and finally influence the polymorph formed. Considering that the three main polymorphs of calcium carbonate minerals have very similar free energy, the addition of certain impurities can hamper their stability and control the kinetic and thermodynamics of the crystallization and which phase crystallizes in the end [67]. It has also been demonstrated that alcohols can affect the crystal morphologies, polymorphs [68][69] and Mg composition [70]. The presence of microorganisms may deeply influence the mineral crystal size, morphology and type of polymorph. Liu et al. (2021) [71] obtained and characterized crystals of amorphous calcium carbonate (ACC) and vaterite carbonate polymorphs synthesized by Bacillus subtilis in the presence of organic matter, nanometric crystals of different shapes and elemental compositions nucleated in an organic matrix. The influence of the Ca2+ concentration positively affected the biomineralization rate, and a Ca2+ concentration above 0.8% affected the quantity, morphology and structure of CaCO3 crystals. The EPS might potentiate the formation of vaterite, regarding the formation of inorganic–organic complexes. On the contrary, proteins released in the medium resulted in inhibition of the stabilization of the unstable/metastable polymorphs into calcite. Braissant et al. (2003) [72] demonstrated the role of EPS and L-amino acids present in the medium determining the size and shape of minerals precipitated by Xanthobacter autotrophicus. Changes in the concentrations and composition of EPS lead to precipitated calcite and vaterite crystals with different spatial configurations. Chekroun et al. (2004) [73] showed that the presence of calcite and vaterite crystals with different morphologies were associated to the presence of living or dead cells and that the organic molecules promoted calcium carbonate growth. Coccolithophores and their ability to create small crystals of calcite (coccoliths) in marine habitats have also been related to MICP processes. In the model species Pleurochrysis carterae, a unicellular microalgae, it was shown that the interaction of the coccoliths with vertical gel-like structures inside the Golgi contributed to the final morphology of the mineral [74]. This interaction was combined with the 3D structural action of the coccolith lipidic membrane. Specific macromolecules can control the crystal phase or even shape the lattice structure that forms the crystal texture, size and orientation of single-crystal domains and the macroscopic shape of the crystals [23][75][76][77]. The influence of certain biopolymers in the crystallization of calcium carbonates was tested [78], determining that the influence of nongelling biopolymers (xantan and gellan) and stack-line rhombohedra aggregates were formed, while in the presence of gelling biopolymers (pectin, sodium alginate and κ-carrageenan) influenced rosette-like aggregates. The concentration of these biopolymers also affected the propensity to nucleation. Additionally, microbial EPS composed of acid polysaccharides has been demonstrated as influential in the nucleation and precipitation of amorphous phases (ACC) that precede the formation of more thermodynamically stable phases (e.g., calcite or high Mg-calcite) [79]. Eukaryotic lysozyme can play an important role in calcification in avian eggshells [80]. Experimental approaches in determining the influence of this enzyme in the morphology and size of carbonate crystals [80] demonstrating that, under non-biotic conditions, hexagonal platelets of vaterite and spherical vaterite aggregates were the main components of the precipitate, being crystal faces well expressed in the hexagonal direction. In contrast, in the presence of lysozyme, calcite was favored to precipitate after dissolution-crystallization starting from vaterite, in the same conditions and after the same time reaction, and this effect was dependent on lysozyme concentration. Thus, lysozyme might accelerate the precipitation process and influence the final polymorph. Hernández-Hernández et al. (2008) [81], investigating the effect of globular proteins, such as lysozyme, myoglobin, alpha-lactalbumin and ribonuclease-A, on CaCO3 precipitation, found that the superficial charge and type and quantity of ionizable charged amino acids enhanced the biomineralization process and proposed the following reasons for such an effect: (i) the presence of different charges on the protein surface led to different preferential adsorption of these proteins to specific crystal faces, decreasing the mineral formation rate; (ii) the effects of the morphology of the calcite growth varied depending on the nature of the charges; (iii) all the proteins favored precipitation of calcite among the different types of polymorphs; (iv) acidic proteins at low concentrations inhibited the precipitation of vaterite and calcite. It was proposed that acidic proteins tend to act as nucleation sites for calcite because of the strong attraction of Ca2+ ions to the surface. However, in the case of alpha-lactalbumin, its high affinity acted as a chelate, sequestering the calcium ions and, thus, reducing the precipitation. Interestingly, lysozyme, resulting from such a study, enhanced the precipitation rate even though it was positively charged. In some recent studies, the control of MICP by ureolytic-driven activities has been investigated [82][83]. The expression of a green fluorescent protein (GFP) was used as a genetic marker of MICP to compare the biomineralization performances of Sporosarcina pasteurii, with low vs. high ureolytic activity rates [82]. In another recent work [83], it was demonstrated for the first time that the morphology and nanomechanical properties of CaCO3 can be adapted if the metabolic activities and precipitation kinetics of certain ureolytic microorganisms are modulated or controlled via genetic engineering. It was hypothesized that microorganisms with low urease activity rates would produce larger calcite crystals. Two genetically engineered strains of E. coli were compared with natural S. pasteurii strains, well known for their MICP capacity. The results highlighted that the modified strains were able to precipitate the calcite crystals, firstly performing a quick and almost complete depletion of calcium ions present in the medium and, at the same time, an increased average size of the crystal. This higher concentration of calcite did not affect the viability of the strains. These initial studies demonstrated that the interactions of microorganisms and organic compounds, including EPS and enzymes, are important for defining the rates and properties of MICP and provided the proof of concept that the modulation of the final characteristics of the precipitated minerals is possible by manipulating microorganisms even by genetic modifications.2.3. Polymeric Substances Secreted by Microorganisms and Micritization, Lithification and Porosity Processes
Microbial geoactive interaction with the environment has been widely studied for understanding the biotransformation of elements, biogeochemical cycling and metal and mineral transformations [84][85] as well as from an application perspective for enhancing and controlling biomining or bioleaching processes [39]. Because of the variety of properties displayed on their charged surfaces and their versatile metabolic activities, microorganisms can induce profound changes in the metal composition in their environment, or they can enhance or mitigate the toxicity of such metals in the environment as well as modulate mineral precipitation and formation, dissolution or deterioration [86][87]. Metals and/or minerals are directly involved as passive and/or active promoters of the microbial metabolism [39], also in relation to the metal speciation, the changes in which can cause an increase or decrease in the metal mobility [88]. Metal speciation is directly influenced by microorganisms that use different processes to modulate the chemical state of the minerals, including direct redox transformations, protein–ligand binding reactions, organic- and inorganic-mediated precipitation, active transport, externalization and intracellular compartmentalization. The metal-binding abilities that present the negatively charged proteins are particularly enriched at the cell wall level and especially at membrane levels, either on the external cellular membrane of those delimiting organelles or cell compartments inside the cell [43][44]. Among the different types of minerals, carbonates are strongly influenced by microorganisms for their turnover, precipitation and solubilization. A quite large range of microorganisms, including eukaryotic algae, fungi and bacteria, can have important roles in the precipitation of carbonates, such as aragonite (CaCO3), dolomite [Ca, Mg(CO3)2], calcite (CaCO3) and Mg-calcite as well as vaterite (CaCO3) and ACC [47][48][61]. Once carbonate precipitates are formed, several geochemical and geological processes modify the initial crystalline structure in order to stabilize the mineral at the nano- and micrometric-scale, in a process known as ‘micritization’. Micritization, known to occur in marine shallow waters, can be defined as a diagenetic process in which the original fabric of carbonate grains is gradually altered to cryptocrystalline textures by the repeated cyclical boring and filling of boreholes with micritic precipitates catalyzed by the interaction of abiotic and microbially mediated processes [89][90]. It was thought that biotic processes at the nanometric and micrometric scale follow the same path as the abiotic process according to nucleation theory; the formation of the mineral occurs by the attraction of ions to affine molecules in a supersaturated solution, and the consequent formation of chemical boundaries initiate the physical shaping of a nucleus, also with pH and temperature optimal conditions [90]. Due to the fact of electrostatic interactions, the initial nucleus would start attracting more and more ions from the solution, and the precipitation and growth of crystals would eventually occur [91]. The selection of specific isomorphs is determined by the superficial free energy followed by the orientation of the initial nucleus in space. The following interactions between mineral particles and the variable physicochemical changes in the near environment would further modify the metastable isomorphs in order to reach the most stable mineral phase to be stabilized at the minimum free energy (e.g., in case of carbonates, from vaterite, aragonite or low Mg-calcite to calcite or high Mg-calcite). Nonetheless, several studies have suggested that such a process is a simplified view of what is indeed occurring during biomineralization and that additional more complex steps are needed for achieving the final crystal structure. For example, it has been observed in the biotic processes of teeth formation in some organisms that an amorphous carbonate phase, such as ACC, first occurs as a precursor to the final mineral, not necessarily through a nucleation process [92][93][94]. In such a type of mineral formation pathway, the initial nucleus leads to an amorphous momentaneous stable phase. Geological records found in the Wolfenden tufa (British Columbia) suggest that the precipitation of nanocrystalline calcite was preceded by the precipitation of ACC and an intermediate monohydrocalcite (MHC). This finding was astonishing, since ACC, as a first stage to nucleation and precipitation of calcite, is not usually observed in nature. The ACC effect in nucleation and precipitation of aragonite has been mostly described in cold water environments [95]. This record also provides insight into the nucleation and depositional effects of microbial biofilm (EPS), resulting from the bacterial metabolism, that provided nucleation sites because of the acid polysaccharides found in the matrix may stabilize the formation of the ACC [79]. Although amorphous precursors have been considered and studied in biomineralization processes, the formation of an ionic nucleus cannot be excluded from the process of mineral synthesis. Despite analogies with the abiotic mineralization process, minerals resulting from biotically mediated processes exhibit higher complexity because of the many different interactions with the organic matrix and biological compounds, which influence the final size, shape, polymorph or physicochemical properties of the minerals that, as a whole, can have rather different properties from those produced abiotically [96]. Endolithic microorganisms capable of boring minerals have been associated to the micritization, lithification and coastal erosion processes in marine environments [97]. Such endoliths encompass a large taxonomically and evolutionary nonhomogeneous group of microorganisms, including photosynthetic cyanophytes, eukaryotic green/red algae and heterotrophic fungi, that actively penetrate carbonate substrates and actively rebuild the minerals. These groups of endolithic microorganisms are widespread in different geological areas throughout the Phanerozoic record [97]. A mechanism proposed for the precipitation of calcium carbonates is mediated by P-type ATPases, which modulate Ca2+ concentrations by exchanging these ions against a concentration gradient for obtaining energy [98]. The ultimate reason(s) to perform such boring activity is debated, and the acquisition of nutrients, the competition for resources, the protection from physically extreme conditions such as UV radiation, escaping from predation or the prevention of detachment from rocks because of water currents have been proposed, among others [99]. For instance, in microbial mats it has been shown that EPS remains attached to the CaCO3 formed as a consequence of the microbial metabolism, and, thus, it could be expected that its presence might influence the late diagenetic processes [100][101][102][103][104]. Another relevant example of the contribution of microorganisms to porosity, lithification and micritization processes is found in microbialites. Microbialites are extremely diverse benthic sedimentary deposits made of carbonate mud-sized crystals (with a particle diameter of less than 5 µm) formed by the mediation of microorganisms [105]. The diversity of microbialites, which can vary depending on the type of paleoenvironment, can be explained by the enormous heterogeneity of microorganisms forming the ancient microbial mats or microbial communities where these sediments were formed. Thus, the “trapping and binding” activities mediated by the microbial cell walls and EPS, which can interact with metals and ions and provide the physicochemical conditions to determine the mineral precipitation, vary with the microbial types and the occurring environment and led to the formation of different types of microbialites, such as oncoids, thrombolites, skeletal stromatolites, agglutinated stromatolites, micritic-evaporitic stromatolites and fenestral laminities [106][107]. For instance, in the case of the oncoids, the most common microbialites and the conditions for their formation in most freshwater habitats are related to the high saturation of carbonate ions, which combined with the metabolic activity of cyanobacteria led to the precipitation of the calcium carbonates [108]. Nonetheless, the grainy faces observed in this type of microbialites, combined with the continuous laminae and truncations in the oncoid cortices, suggest that they accumulated in waved or hectic environments, but the appearance of discontinuous laminae and stromatolitic overgrowths might indicate that, in some periods, the hydrodynamic forces were not enough to overturn the oncoids [109][110]. Such observations highlight the influence of environmental conditions on the formation of microbialites. Reid et al. (2000) [111] defined a series of mechanisms implicated in the formation of modern and possibly ancient stromatolites by an interplay between the stages of sedimentation and periods of lithification associated with a succession of microbial communities. The lithification stages were suggested to be dependent on two main microbial processes: (i) heterotrophic bacterial respiration and (ii) photosynthetic activity by present cyanobacteria. Thus, the laminated structures observed in stromatolites could be related to a micritization process in surface biofilms occurring during discontinuities in sedimentation processes [111]. The same authors proposed that microboring and precipitation are related to the presence of thicker layers of fused grains that might result from the degradation of the polymer in boreholes. Such observations provide new insight into the role of microbes and lithification for the formation of the stromatolites in the Precambrian [112]. Microbial mats trap and bind particles, as well they induce mineral precipitation, both processes that contribute to the lithification of the mat. When mats are lithified, they start being known as microbialites or stromatolites, which are fossilized [113]. This is why microbial mats are considered living analogues and potential precursors of stromatolites, which were among the first biological structures capable of MICP established on the ancient Earth [114]. By reconstructing the chemical metabolic signatures performed by different microorganisms fossilized in these stromatolites, it would be possible to learn more about how the ancient atmosphere or ancient seas were composed. Moreover, stromatolites contain the geochemical key to ourthe understanding of the evolution of microbial life on Earth [62]. As a remarkable example, the characterization of the cyanobacteria Geitlerinema sp., as one of the dominant microorganisms found in microbial mats and analogous stromatolites, has led to the suggestion that these microorganisms greatly contributed to the oxygenation of the ancient Earth’s atmosphere [62]. Before this event took place, some hints in fossilized stromatolites have proved that arsenic was a chemical choice for ancient photosynthesizers and respirators. Experiments and measures of a purple microbial mat in Laguna la Brava, in the Atacama Desert in Chile, showed high levels of arsenic, sulfur and lithium but not oxygen [115]. The experiments performed with microorganisms isolated from the microbial mat led to the conclusion of the existence of an arsenic cycle linking photosynthesis and respiration carried out by purple sulfur bacteria and arsenate-reducing bacteria in what is called arsenic-cycling mats. Since the Atacama Desert shares so many similarities with extraterrestrial bodies such as Mars, the understanding of current and ancient life in this ecosystem might shed light onto life on other planets [116]. The approaches used on living microbial mats can be adapted for understanding more the composition of ancient microbial mats. These methods mainly consist of the analysis of fossilized proteins embedded in ancient carbonates [117][118][119] and even polysaccharides well preserved into the mineral structures [120], hints that could lead to a better comprehension of the formation processes of modern and ancient microbialites. Further research on the taxonomy of microorganisms involved in the diagenetic processes will lead to a better understanding of the role of organics secreted by microorganisms and how their properties and chemistry may influence mineral precipitation, growth and evolving changes in carbonate structure that are observed in many rock samples from different geographical locations around the globe. Not limited to that, these studies may increase ourthe knowledge regarding ourthe ancient past.3. Conclusions
Many different microorganisms and higher organisms can induce and determine MICP through the production of EPS, enzymes, amino acids and other organic metabolites that by their interactions determine the type and rate of the process. Many studies have focused on the molecular routes and metabolic pathways activated by these organisms that determine and regulate the precipitation process, suggesting that the final properties of the minerals obtained are genetically regulated and control of the process is achievable. Understanding the parameters for the synthesis of the organic molecules involved in MICP and EICP might shed light on developing fine-tune control of these processes for biotechnological applications. Carbonates are important molecules for carbon stock and for potentially regulating the current trends in CO2 accumulation in the atmosphere.
Moreover, carbonates are very useful minerals in several engineering, medical engineering and geological applications. Although many questions remain to be answered regarding the role of microorganisms in the production and turnover of carbonates, such as the kind of microorganisms able to induce the process and their ecology and interactions; the role of the environmental factors involved; or the role and mechanisms of the biological molecules (EPS, proteins, enzymes, organic cofactors, organic acids, etc.) involved, current research has shaped the biologically mediated precipitation and rearrangements of carbonates as a world-scale process that has very great interest for the protection of the environment, regulation of the climate and application in many (geo)biotechnological problems that may contribute to the understanding of the past of our planet and the search for life on other planets.
References
- Walker, L.J.; Wilkinson, B.H.; Ivany, L.C. Continental Drift and Phanerozoic Carbonate Accumulation in Shallow-Shelf and Deep-Marine Settings. J. Geol. 2002, 110, 75–87.
- Reddy, M.S. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314.
- Swart, P.K.; Eberli, G.P.; McKenzie, J.A. Perspectives in Carbonate Geology: A Tribute to the Career of Robert Nathan Ginsburg; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-4443-1205-8.
- Levi, Y.; Albeck, S.; Brack, A.; Weiner, S.; Addadi, L. Control Over Aragonite Crystal Nucleation and Growth: An In Vitro Study of Biomineralization. Chem.-Eur. J. 1998, 4, 389–396.
- Wang, X.; Kong, R.; Pan, X.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Role of Ovalbumin in the Stabilization of Metastable Vaterite in Calcium Carbonate Biomineralization. J. Phys. Chem. B 2009, 113, 8975–8982.
- Spadafora, A.; Perri, E.; Mckenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40.
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; McKenzie, J.A. Microbial Dolomite Precipitation under Aerobic Conditions: Results from Brejo do Espinho Lagoon (Brazil) and Culture Experiments. In Perspectives in Carbonate Geology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 167–178. ISBN 978-1-4443-1206-5.
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787.
- Da Silva, S.; Bernet, N.; Delgenès, J.P.; Moletta, R. Effect of culture conditions on the formation of struvite by Myxococcus xanthus. Chemosphere 2000, 40, 1289–1296.
- Rivadeneyra, M.A.; Pérez-García, I.; Ramos-Cormenzana, A. Struvite precipitation by soil and fresh water bacteria. Curr. Microbiol. 1992, 24, 343–347.
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230.
- Zeth, K.; Hoiczyk, E.; Okuda, M. Ferroxidase-Mediated Iron Oxide Biomineralization: Novel Pathways to Multifunctional Nanoparticles. Trends Biochem. Sci. 2016, 41, 190–203.
- Staicu, L.C.; Wojtowicz, P.J.; Pósfai, M.; Pekker, P.; Gorecki, A.; Jordan, F.L.; Barton, L.L. PbS biomineralization using cysteine: Bacillus cereus and the sulfur rush. FEMS Microbiol. Ecol. 2020, 96, fiaa151.
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Biomineralization: From Nature to Application, Volume 4; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-98631-8.
- Liu, B.; Cao, Y.; Huang, Z.; Duan, Y.; Che, S. Silica Biomineralization via the Self-Assembly of Helical Biomolecules. Adv. Mater. 2015, 27, 479–497.
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth-Sci. Rev. 1998, 45, 45–60.
- Ehrlich, H.L. Microbes as Geologic Agents: Their Role in Mineral Formation. Geomicrobiol. J. 1999, 16, 135–153.
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46.
- Krumbein, W.E. Photolithotropic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (gulf of Aqaba, Sinai). Geomicrobiol. J. 1979, 1, 139–203.
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23.
- Riding, R. Cyanophyte calcification and changes in ocean chemistry. Nature 1982, 299, 814–815.
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162.
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of Aragonite or Calcite Polymorphism by Mollusk Shell Macromolecules. Science 1996, 271, 67–69.
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem.—A Eur. J. 2006, 12, 980–987.
- Tambutté, S.; Holcomb, M.; Ferrier-Pagès, C.; Reynaud, S.; Tambutté, É.; Zoccola, D.; Allemand, D. Coral biomineralization: From the gene to the environment. J. Exp. Mar. Biol. Ecol. 2011, 408, 58–78.
- Li, H.; Sun, C.-Y.; Fang, Y.; Carlson, C.M.; Xu, H.; Ješovnik, A.; Sosa-Calvo, J.; Zarnowski, R.; Bechtel, H.A.; Fournelle, J.H.; et al. Biomineral armor in leaf-cutter ants. Nat. Commun. 2020, 11, 5792.
- Hild, S.; Marti, O.; Ziegler, A. Spatial distribution of calcite and amorphous calcium carbonate in the cuticle of the terrestrial crustaceans Porcellio scaber and Armadillidium vulgare. J. Struct. Biol. 2008, 163, 100–108.
- Wilt, F.H.; Killian, C.E.; Livingston, B.T. Development of calcareous skeletal elements in invertebrates. Differentiation 2003, 71, 237–250.
- Ozawa, H.; Hoshi, K.; Amizuka, N. Current Concepts of Bone Biomineralization. J. Oral Biosci. 2008, 50, 1–14.
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov. Mater. 2020, 1, 2.
- Weiner, S. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29.
- Bolhuis, H.; Stal, L.J. Analysis of bacterial and archaeal diversity in coastal microbial mats using massive parallel 16S rRNA gene tag sequencing. ISME J. 2011, 5, 1701–1712.
- Baumgartner, L.K.; Reid, R.P.; Dupraz, C.; Decho, A.W.; Buckley, D.H.; Spear, J.R.; Przekop, K.M.; Visscher, P.T. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries. Sediment. Geol. 2006, 185, 131–145.
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760.
- Gilbert, P.U.P.A.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 2022, 8, eabl9653.
- Gilbert, P.U.P.A.; Porter, S.M.; Sun, C.-Y.; Xiao, S.; Gibson, B.M.; Shenkar, N.; Knoll, A.H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665.
- Sun, X.; Miao, L.; Yuan, J.; Wang, H.; Wu, L. Application of enzymatic calcification for dust control and rainfall erosion resistance improvement. Sci. Total Environ. 2021, 759, 143468.
- Wang, Z.; Zhang, N.; Cai, G.; Jin, Y.; Ding, N.; Shen, D. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesources Geotechnol. 2017, 35, 1135–1146.
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021, 166, 107856.
- Miftah, A.; Khodadadi Tirkolaei, H.; Bilsel, H. Biocementation of Calcareous Beach Sand Using Enzymatic Calcium Carbonate Precipitation. Crystals 2020, 10, 888.
- Wilson, J.L. Limestone and dolomite reservoirs. Pet. Geol.(Engl. Transl.) 1980, 2.
- Norris, S.E. Characteristics of Limestone and Dolomite Aquifers in Western Ohio. J. AWWA 1957, 49, 464–468.
- Burchette, T.P. Carbonate rocks and petroleum reservoirs: A geological perspective from the industry. Geol. Soc. Lond. Spec. Publ. 2012, 370, 17–37.
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; World Scientific: Singapore, 2014; ISBN 978-1-78326-330-1.
- Barnes, H. Oceanography And Marine Biology; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-1-4822-6728-0.
- Quigley, M.S.; Santschi, P.H.; Hung, C.-C.; Guo, L.; Honeyman, B.D. Importance of acid polysaccharides for 234Th complexation to marine organic matter. Limnol. Oceanogr. 2002, 47, 367–377.
- Sánchez-Román, M.; Rivadeneyra, M.A.; Vasconcelos, C.; McKenzie, J.A. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 2007, 61, 273–284.
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150.
- Zhao, Y.; Yao, J.; Yuan, Z.; Wang, T.; Zhang, Y.; Wang, F. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation. Environ. Sci. Pollut. Res. 2017, 24, 372–380.
- Mitchell, A.C.; Ferris, F.G. Effect of Strontium Contaminants upon the Size and Solubility of Calcite Crystals Precipitated by the Bacterial Hydrolysis of Urea. Environ. Sci. Technol. 2006, 40, 1008–1014.
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210.
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Rivadeneyra, M.A.; Vasconcelos, C. Presence of sulfate does not inhibit low-temperature dolomite precipitation. Earth Planet. Sci. Lett. 2009, 285, 131–139.
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Romanek, C.S.; Sánchez-Navas, A.; Vasconcelos, C. Experimentally determined biomediated Sr partition coefficient for dolomite: Significance and implication for natural dolomite. Geochim. Cosmochim. Acta 2011, 75, 887–904.
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; Martin-Uriz, P.S.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4, 4767.
- Sánchez-Román, M.; Puente-Sánchez, F.; Parro, V.; Amils, R. Nucleation of Fe-rich phosphates and carbonates on microbial cells and exopolymeric substances. Front. Microbiol. 2015, 6, 1024.
- Geesey, G.G.; Jang, L.; Jolley, J.G.; Hankins, M.R.; Iwaoka, T.; Griffiths, P.R. Binding of Metal Ions by Extracellular Polymers of Biofilm Bacteria. Water Sci. Technol. 1988, 20, 161–165.
- Wen, Y.; Sánchez-Román, M.; Li, Y.; Wang, C.; Han, Z.; Zhang, L.; Gao, Y. Nucleation and stabilization of Eocene dolomite in evaporative lacustrine deposits from central Tibetan plateau. Sedimentology 2020, 67, 3333–3354.
- Liu, Y.; Lam, M.C.; Fang, H.H. Adsorption of heavy metals by EPS of activated sludge. Water Sci. Technol. 2001, 43, 59–66.
- Sheng, G.-P.; Xu, J.; Luo, H.-W.; Li, W.-W.; Li, W.-H.; Yu, H.-Q.; Xie, Z.; Wei, S.-Q.; Hu, F.-C. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013, 47, 607–614.
- Jang, A.; Kim, S.M.; Kim, S.Y.; Lee, S.G.; Kim, I.S. Effect of heavy metals (Cu, Pb, and Ni) on the compositions of EPS in biofilms. Water Sci. Technol. 2001, 43, 41–48.
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A.; Zenobi, R.; Rivadeneyra, M.A. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882.
- Popall, R.M.; Bolhuis, H.; Muyzer, G.; Sánchez-Román, M. Stromatolites as Biosignatures of Atmospheric Oxygenation: Carbonate Biomineralization and UV-C Resilience in a Geitlerinema sp.—Dominated Culture. Front. Microbiol. 2020, 11, 948.
- Wang, L.; Chen, W.; Song, X.; Li, Y.; Zhang, W.; Zhang, H.; Niu, L. Cultivation substrata differentiate the properties of river biofilm EPS and their binding of heavy metals: A spectroscopic insight. Environ. Res. 2020, 182, 109052.
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56.
- Beveridge, T.J. Ultrastructure, Chemistry, and Function of the Bacterial Wall. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 72, pp. 229–317.
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88.
- Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101.
- Jin, D.; Wang, F.; Yue, L. Phase and morphology evolution of vaterite crystals in water/ethanol binary solvent. Cryst. Res. Technol. 2011, 46, 140–144.
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water–Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853.
- Fang, Y.; Zhang, F.; Farfan, G.A.; Xu, H. Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications. ACS Omega 2022, 7, 281–292.
- Liu, R.; Huang, S.; Zhang, X.; Song, Y.; He, G.; Wang, Z.; Lian, B. Bio-mineralisation, characterization, and stability of calcium carbonate containing organic matter. RSC Adv. 2021, 11, 14415–14425.
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially Induced Mineralization of Calcium Carbonate in Terrestrial Environments: The Role of Exopolysaccharides and Amino Acids. J. Sediment. Res. 2003, 73, 485–490.
- Chekroun, K.B.; Rodríguez-Navarro, C.; González-Muñoz, M.T.; Arias, J.M.; Cultrone, G.; Rodríguez-Gallego, M. Precipitation and Growth Morphology of Calcium Carbonate Induced by Myxococcus Xanthus: Implications for Recognition of Bacterial Carbonates. J. Sediment. Res. 2004, 74, 868–876.
- Kadan, Y.; Tollervey, F.; Varsano, N.; Mahamid, J.; Gal, A. Intracellular nanoscale architecture as a master regulator of calcium carbonate crystallization in marine microalgae. Proc. Natl. Acad. Sci. USA 2021, 118, e2025670118.
- Aizenberg, J.; Addadi, L.; Weiner, S.; Lambert, G. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226.
- Aizenberg, J.; Hanson, J.; Ilan, M.; Leiserowitz, L.; Koetzle, T.F.; Addadi, L.; Weiner, S. Morphogenesis of calcitic sponge spicules: A role for specialized proteins interacting with growing crystals. FASEB J. 1995, 9, 262–268.
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 1996, 381, 56–58.
- Butler, M.F.; Glaser, N.; Weaver, A.C.; Kirkland, M.; Heppenstall-Butler, M. Calcium Carbonate Crystallization in the Presence of Biopolymers. Cryst. Growth Des. 2006, 6, 781–794.
- Broughton, P.L. Microbial EPS-mediated amorphous calcium carbonate–monohydrocalcite–calcite transformations during early tufa deposition. Depos. Rec. 2022, 1–28.
- Wang, X.; Sun, H.; Xia, Y.; Chen, C.; Xu, H.; Shan, H.; Lu, J.R. Lysozyme mediated calcium carbonate mineralization. J. Colloid Interface Sci. 2009, 332, 96–103.
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-Lopez, C.; Nys, Y.; García-Ruiz, J.M. Influence of Model Globular Proteins with Different Isoelectric Points on the Precipitation of Calcium Carbonate. Cryst. Growth Des. 2008, 8, 1495–1502.
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299.
- Heveran, C.M.; Liang, L.; Nagarajan, A.; Hubler, M.H.; Gill, R.; Cameron, J.C.; Cook, S.M.; Srubar, W.V. Engineered Ureolytic Microorganisms Can Tailor the Morphology and Nanomechanical Properties of Microbial-Precipitated Calcium Carbonate. Sci. Rep. 2019, 9, 14721.
- Gadd, G.-M.; Burford, E.-P.; Fomina, M. Biogeochemical Activities of Microorganisms in Mineral Transformations: Consequences for Metal and Nutrient Mobility. J. Microbiol. Biotechnol. 2003, 13, 323–331.
- Violante, A.; Huang, P.M.; Gadd, G.M. Biophysico-Chemical Processes of Heavy Metals and Metalloids in Soil Environments; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-17547-7.
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305.
- Gadd, G.M. Metals and microorganism: A problem of definition. FEMS Microbiol. Lett. 1992, 100, 197–203.
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643.
- Reid, R.P.; Macintyre, I.G. Microboring Versus Recrystallization: Further Insight into the Micritization Process. J. Sediment. Res. 2000, 70, 24–28.
- Kobluk, D.R.; Risk, M.J. Micritization and Carbonate-Grain Binding by Endolithic Algae1. AAPG Bull. 1977, 61, 1069–1082.
- Boulos, R.A.; Zhang, F.; Tjandra, E.S.; Martin, A.D.; Spagnoli, D.; Raston, C.L. Spinning up the polymorphs of calcium carbonate. Sci. Rep. 2014, 4, 3616.
- Politi, Y.; Levi-Kalisman, Y.; Raz, S.; Wilt, F.; Addadi, L.; Weiner, S.; Sagi, I. Structural Characterization of the Transient Amorphous Calcium Carbonate Precursor Phase in Sea Urchin Embryos. Adv. Funct. Mater. 2006, 16, 1289–1298.
- Loste, E.; Meldrum, F.C. Control of calcium carbonate morphology by transformation of an amorphous precursor in a constrained volume. Chem. Commun. 2001, 10, 901–902.
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970.
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilbert, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, E7670–E7678.
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68.
- Golubic, S.; Perkins, R.D.; Lukas, K.J. Boring Microorganisms and Microborings in Carbonate Substrates. In The Study of Trace Fossils: A Synthesis of Principles, Problems, and Procedures in Ichnology; Frey, R.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 229–259. ISBN 978-3-642-65923-2.
- Garcia-Pichel, F.; Ramírez-Reinat, E.; Gao, Q. Microbial excavation of solid carbonates powered by P-type ATPase-mediated transcellular Ca2+ transport. Proc. Natl. Acad. Sci. USA 2010, 107, 21749–21754.
- Cockell, C.S.; Herrera, A. Why are some microorganisms boring? Trends Microbiol. 2008, 16, 101–106.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636.
- Fong, J.N.C.; Yildiz, F.H. Biofilm Matrix Proteins. Microbiol. Spectr. 2015, 3, 201–222.
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288.
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238.
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary Deposits of Benthic Microbial Communities. PALAIOS 1987, 2, 241–254.
- Suarez-Gonzalez, P.; Benito, M.I.; Quijada, I.E.; Mas, R.; Campos-Soto, S. ‘Trapping and binding’: A review of the factors controlling the development of fossil agglutinated microbialites and their distribution in space and time. Earth-Sci. Rev. 2019, 194, 182–215.
- Suarez-Gonzalez, P.; Quijada, I.E.; Benito, M.I.; Mas, R. Sedimentology of Ancient Coastal Wetlands: Insights From A Cretaceous Multifaceted Depositional System. J. Sediment. Res. 2015, 85, 95–117.
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-Induced Biofilm Calcification and Calcium Concentrations in Phanerozoic Oceans. Science 2001, 292, 1701–1704.
- Campos-Soto, S.; Benito, M.I.; Mas, R.; Caus, E.; Cobos, A.; Suárez-González, P.; Quijada, I.E. Revisiting the Late Jurassic-Early Cretaceous of the NW South Iberian Basin: New ages and sedimentary environments. J. Iber. Geol. 2016, 42, 69–94.
- Lanés, S.; Palma, R.M. Environmental implications of oncoids and associated sediments from the Remoredo Formation (Lower Jurassic) Mendoza, Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 140, 357–366.
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992.
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian Carbonates: Evolutionary Mileposts or Environmental Dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358.
- Eymard, I.; Alvarez, M. del P.; Bilmes, A.; Vasconcelos, C.; Ariztegui, D. Tracking Organomineralization Processes from Living Microbial Mats to Fossil Microbialites. Minerals 2020, 10, 605.
- Margulis, L.; Barghoorn, E.S.; Ashendorf, D.; Banerjee, S.; Chase, D.; Francis, S.; Giovannoni, S.; Stolz, J. The microbial community in the layered sediments at Laguna Figueroa, Baja California, Mexico: Does it have Precambrian analogues? Precambrian Res. 1980, 11, 93–123.
- Oremland, R.S.; Saltikov, C.W.; Wolfe-Simon, F.; Stolz, J.F. Arsenic in the Evolution of Earth and Extraterrestrial Ecosystems. Geomicrobiol. J. 2009, 26, 522–536.
- Parro, V.; de Diego-Castilla, G.; Moreno-Paz, M.; Blanco, Y.; Cruz-Gil, P.; Rodríguez-Manfredi, J.A.; Fernández-Remolar, D.; Gómez, F.; Gómez, M.J.; Rivas, L.A.; et al. A Microbial Oasis in the Hypersaline Atacama Subsurface Discovered by a Life Detector Chip: Implications for the Search for Life on Mars. Astrobiology 2011, 11, 969–996.
- Demarchi, B. Amino Acids and Proteins in Fossil Biominerals: An Introduction for Archaeologists and Palaeontologists; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-119-08951-3.
- Saitta, E.T.; Vinther, J.; Crisp, M.K.; Abbott, G.D.; Kaye, T.G.; Pittman, M.; Bull, I.; Fletcher, I.; Chen, X.; Collins, M.J.; et al. Non-Avian Dinosaur Eggshell Calcite Contains Ancient, Endogenous Amino Acids. bioRxiv 2020. bioRxiv:2020.06.02.129999.
- Hendy, J.; Welker, F.; Demarchi, B.; Speller, C.; Warinner, C.; Collins, M.J. A guide to ancient protein studies. Nat. Ecol. Evol. 2018, 2, 791–799.
- Benzerara, K.; Menguy, N.; López-García, P.; Yoon, T.-H.; Kazmierczak, J.; Tyliszczak, T.; Guyot, F.; Brown, G.E. Nanoscale detection of organic signatures in carbonate microbialites. Proc. Natl. Acad. Sci. USA 2006, 103, 9440–9445.
