Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Kaleem | -- | 1255 | 2022-12-29 09:14:58 | | | |
| 2 | Catherine Yang | Meta information modification | 1255 | 2022-12-29 09:18:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kaleem, M.; Dalhat, M.H.; Azmi, L.; Asar, T.O.; Ahmad, W.; Alghanmi, M.; Almostadi, A.; Zughaibi, T.A.; Tabrez, S. Molecular Pathways Involved in the Regulation of BCBM. Encyclopedia. Available online: https://encyclopedia.pub/entry/39567 (accessed on 08 February 2026).
Kaleem M, Dalhat MH, Azmi L, Asar TO, Ahmad W, Alghanmi M, et al. Molecular Pathways Involved in the Regulation of BCBM. Encyclopedia. Available at: https://encyclopedia.pub/entry/39567. Accessed February 08, 2026.
Kaleem, Mohammed, Mahmood Hassan Dalhat, Lubna Azmi, Turky Omar Asar, Wasim Ahmad, Maimonah Alghanmi, Amal Almostadi, Torki A. Zughaibi, Shams Tabrez. "Molecular Pathways Involved in the Regulation of BCBM" Encyclopedia, https://encyclopedia.pub/entry/39567 (accessed February 08, 2026).
Kaleem, M., Dalhat, M.H., Azmi, L., Asar, T.O., Ahmad, W., Alghanmi, M., Almostadi, A., Zughaibi, T.A., & Tabrez, S. (2022, December 29). Molecular Pathways Involved in the Regulation of BCBM. In Encyclopedia. https://encyclopedia.pub/entry/39567
Kaleem, Mohammed, et al. "Molecular Pathways Involved in the Regulation of BCBM." Encyclopedia. Web. 29 December, 2022.
Copy Citation
Brain metastasis is one of the major reasons of death in breast cancer (BC) patients, significantly affecting the quality of life, physical activity, and interdependence on several individuals. The tendency to develop breast cancer brain metastases (BCBMs) differs by the BC subtype, varying from almost half with triple-negative breast cancer (TNBC) (HER2− ER− PR−), one-third with HER2+ (human epidermal growth factor receptor 2-positive, and around one-tenth with luminal subclass (ER+ (estrogen positive) or PR+ (progesterone positive)) breast cancer.
brain metastases
breast cancer
chemokine receptor
HER2
TNBC
1. Breast Cancer Brain Metastases (BCBM)
Breast cancer brain metastases (BCBM) are the second most frequent type of brain metastases and one of the most typical breast cancer metastases [1][2]. Scientific studies have identified that patients with breast cancer had a 5.1% rate of BCBM incidence. Moreover, among patients with any metastatic disease, 14.2% developed BCBM during the therapeutic phase of the illness [2]. Most BM occurs in patients with HER2-positive and ER-negative metastatic BC. Among these, HER2-positive BC patients have a higher rate of survival. Due to the therapeutic challenges, BCBM requires an integrated therapy approach for its management [3].
Three therapeutic approaches are available for treating metastatic brain tumors, including anticancer agents, surgery, and radiation therapy [2][4][5]. Metastasis represents the primary cause of death in BC patients suffering from BMs, which gradually progresses into a more advanced stage [6]. Metastasized breast cancers or circulating tumor cells evade the BBB, and upon reaching the specified cranial cells (astrocytes), they initiate tumorigenesis leading to tumor formation. The initial growth of BMs in the brain is associated with the entry of cancerous cells into the bloodstream and different locations of the brain, where they grow and multiply rapidly [5]. Hence, further insight into breast cancer brain metastasis mechanisms is expected to provide a mode of management or inhibition of these cancer types. Figure 1 depicts the formation of breast cancer brain tumors.
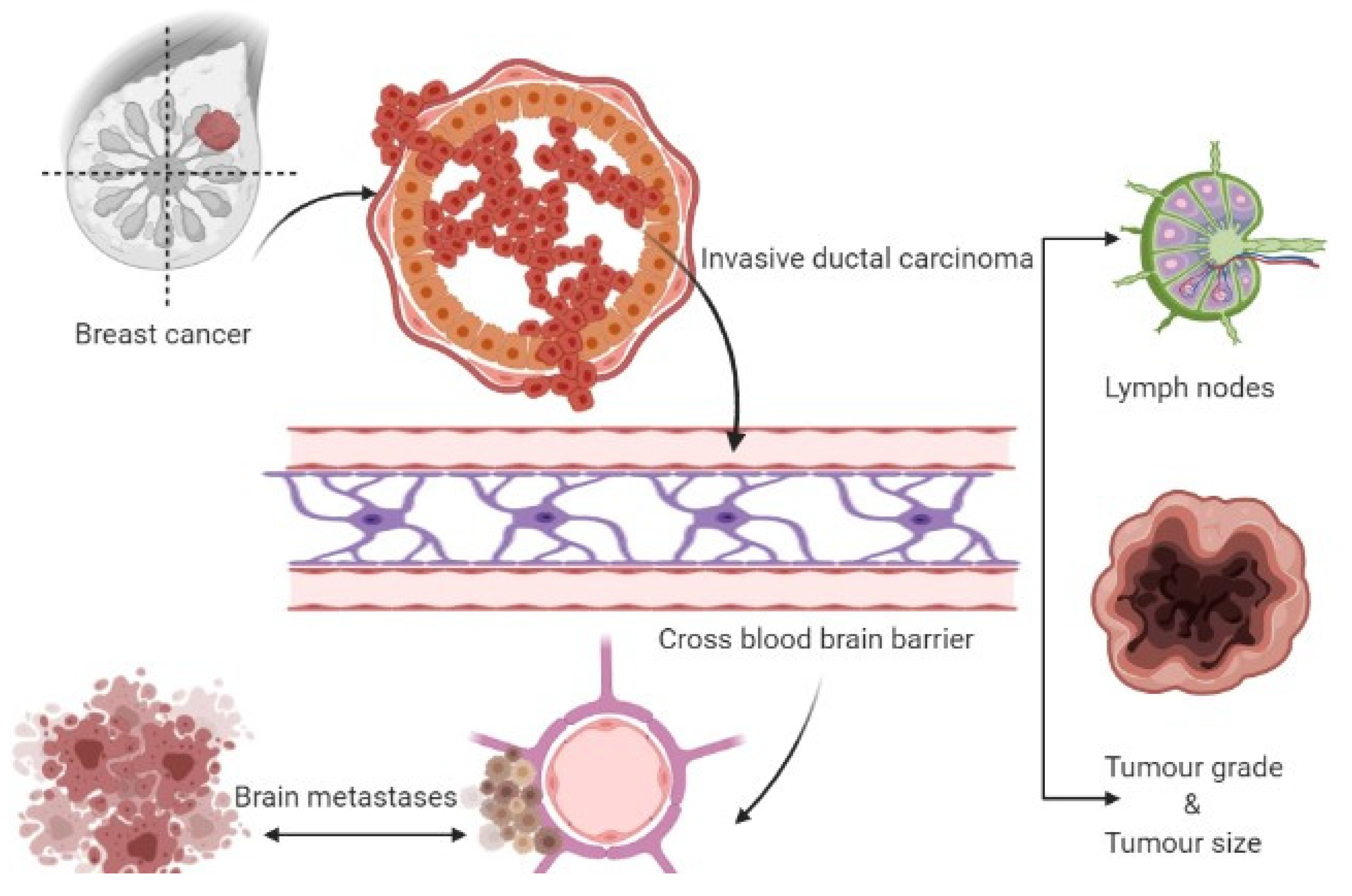
Figure 1. Formation of breast cancer-associated brain tumors.
2. TGFβ/SMAD Signaling Pathway
It is well-known that epithelial to mesenchymal transition (EMT) proteins such as Slug, Snail, and Zeb1 are expressed through transforming growth factor β (TGFβ), SMAD, and PI3K signaling pathways (Figure 2) [7]. The EMT proteins promote this transition by decreasing the expression of E-cadherin and increasing the expression of N-cadherin [8]. EMT plays a significant role in cancer by increasing invasiveness and metastasis, resulting in poor prognosis and survival [9]. The EMT proteins suppress the expression of CDH1 [10]. The CDH1 gene translates to E-cadherin, which plays a critical role in cell adhesion and is involved in cell attachment to other cells and the extracellular matrix (ECM) [11]. Without E-cadherin, the breast cancer cells are detached from the breast tissue, forming circulating tumor cells (CTCs) that can metastasize to other tissues, including the lungs and brain [12]. Under normal physiological conditions, the BBB selectively regulates materials that go into the brain compartment by preventing the paracellular diffusion of compounds. This causes an obstacle for breast CTCs to pass through the BBB; however, in BCBM, the breast CTCs diffuse through the endothelial cell junctions [12][13]. The endothelial cell junctions are the part of the BBB that is modified during BM formation. Slug, Snail, Zeb1, VEGFA, and CD44 contribute to BM formation by enhancing the trans-endothelial migration of tumor cells via downregulation of endothelial integrity, enabling the breast CTCs to pass the BBB [14] (Mittal, 2018). Targeting the SMAD protein in the TGFβ/SMAD signaling pathway has been suggested to attenuate brain metastasis in BCBM patients [15]. The lipoprotein receptor-related protein 1 (LRP-1) inhibitors (ANG1005 and GRN1005) bind to LRP-1, leading to LRP-1 receptor-mediated transcytosis or endocytosis across the BBB, resulting in tumor growth arrest and apoptosis [16][17].
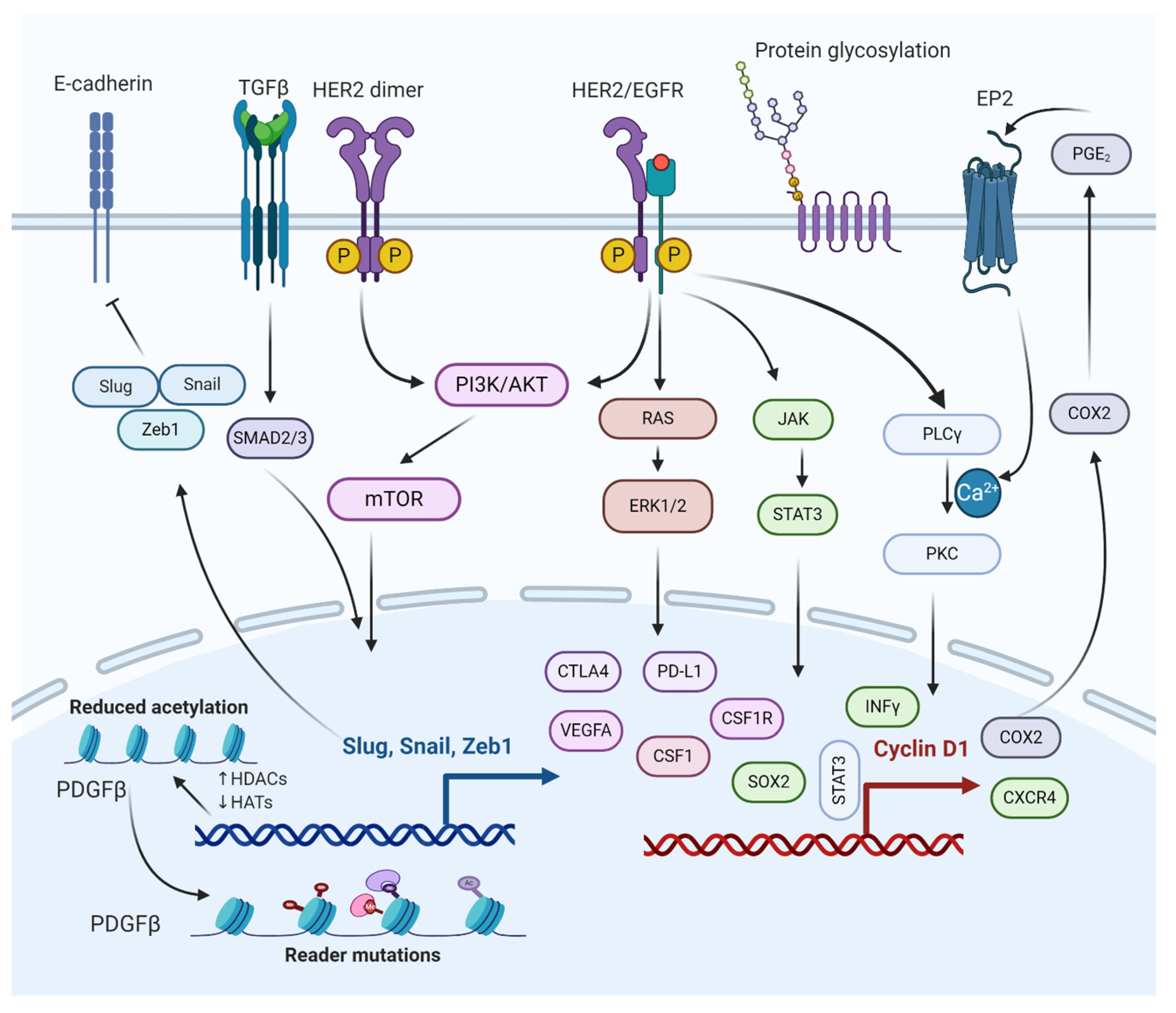
Figure 2. Molecular interactions of different pathways involved in the regulation of BCBM. The epithelial to mesenchymal transition (EMT) genes inhibit cadherin, promoting metastasis. PI3K/AKT/mTOR and RAS/RAF/ERK pathways activate cellular processes, such as cell proliferation, survival, migration, and angiogenesis. PI3K/AKT/mTOR and JAK/STAT pathways help BCBMs escape immunosurveillance. COX2 aids in prostaglandin synthesis and inflammation activation. Histone deacetylation of growth factors and protein glycosylation aid in bypassing the BBB.
3. PI3K/mTOR Signaling Pathway
The phosphoinositide 3-kinase (PI3K) signaling pathway is a central pathway involved in cellular processes such as cell survival, cell proliferation, cell metabolism, and angiogenesis (Figure 2) [18][19][20]. It also plays a significant role in BCBM with approximately 77% of patients having been noted to have an activated PI3K signaling pathway [21][22]. The activation of the PI3K signaling pathway is associated with increased expression of metastatic and immunosuppressive genes, which include CTLA4, PD-L1, CSF1R, and CSF1 in the tumor microenvironment of metastasized brain cells [5][23]. The loss of function of phosphatase and tensin homolog (PTEN), a tumor suppressor and a negative regulator of PI3K signaling, is detected in 25–71% of BCBM patients, with the highest percentage in TNBC cases [24]. Overexpression of PTEN in astrocytes suppresses invasiveness and cell migration, suggesting PTEN as a promising therapeutic target for BCBM treatment [22]. The mTOR is a serine/threonine protein kinase, a downstream protein of PI3K, and Akt plays a significant role in several cancer types [19][25]. Simultaneous mTOR and PI3K protein inhibition have been reported to attenuate BCBM [26]. Everolimus and Buparlisib (BKM120), mTOR and PI3K inhibitors, are used to treat BCBM in combination with other anticancer drugs such as trastuzumab and vinorelbine [27].
4. HER2/Epidermal Growth Factor Receptor (EGFR) Signaling Pathway
EGFR is a transmembrane protein that activates the EGFR signaling pathway through homo/hetero-dimerization and auto-phosphorylation in response to ligand binding [28]. EGFR forms heterodimer with HER2, activating the PI3K/AKT signaling cascade. HER2+ breast cancer is susceptible to brain metastasis due to its link with PI3K signaling pathway (Figure 2) [29]. The HER2 protein dimerizes with another similar protein called HER3, triggering cell proliferation and survival. One study based on immune-histochemistry reported that HER3 is over-expressed in around 60% of BCBM patients [30].
HER2 signaling is a master regulator of many pro-inflammatory, proliferative, and pro-metastatic pathways, the most notable of which is the cyclo-oxygenase 2 (COX2) [31][32]. The HER2/EGFR signaling pathway is directly or indirectly associated with COX2 upregulation, which has shown to induce specified brain metastasis. Because BCBM patients have high expression of both HER2 and EGFR, several drugs (some approved and others in clinical trials) are used to target various stages of the HER2/EGFR signaling pathway [33][34]. Some of the drugs that target HER2/EGFR are Lapatinib (targets HER2 receptor), Trastuzumab (targets HER2 receptor), KD019 (targets HER2, Src, and EGFR), ARRY-380 (targets HER2 receptor), HKI-272 (targets HER1, HER2, and HER4 receptors), Afatinib (targets EGFR1, EGFR2, and EGFR4 receptors), and tucatinib (targets HER2 receptor). It is worth mentioning that tucatinib, a tyrosine kinase inhibitor, combined with trastuzumab and capecitabine, was approved by the USFDA on April 17, 2020, as a BCBM treatment regimen [27][35][36].
5. JAK/Signal Transducer and Activator of Transcription 3 (STAT3) Signaling Pathway
The JAK-STAT pathway is known for regulating the expression of growth factors and cytokines [37]. Some of the genes involved in the JAK-STAT pathway include PD-L1, VEGFA, and CTLA4, which play a crucial role in the survival of BCBM by escaping from immunosurveillance (Figure 2) [38]. STAT3 is critical for astrocytic scar formation and is involved in axon regeneration [39] (Anderson et al., 2016). Most astrocytes in BMs are expressed as an activated form of STAT3, the phosphorylated STAT3 (pSTAT3) [40] (Priego et al., 2018). The pSTAT3+ cancerous astrocytes bypass immunosurveillance by expressing escape-promoting genes, such as PD-L1, CTLA4, VEGFA, and TIMP-1 [41]. Therefore, STAT3 in BMs could be a potential therapeutic target for BCBM treatment. Nivolumab, an approved anticancer drug, targets PD-1 in BCBM, thereby preventing the binding of PD-L1 to PD-1. Similarly, using nivolumab and other treatment regimens helps cancer immunotherapy [42][43].
References
- Friedrich, M.; Swords, D.; Terjung Thill, M.; Baum, S.; Bischoff, J. Breast cancer and metastases of the central nervous system. Eur. J. Gynaecol. Oncol. 2017, 38, 653–656.
- Watase, C.; Shiino, S.; Shimoi, T.; Noguchi, E.; Kaneda, T.; Yamamoto, Y.; Yonemori, K.; Takayama, S.; Suto, A. Breast cancer brain metastasis—Overview of disease state, treatment options and future perspectives. Cancers 2021, 13, 1078.
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment strategies for breast cancer brain metastases. Br. J. Cancer 2021, 124, 142–155.
- Bollig-Fischer, A.; Michelhaugh, S.; Ali-Fehmi, R.; Mittal, S. The molecular genomics of metastatic brain tumours. OA Mol. Oncol. 2013, 1, 6.
- Franchino, F.; Rudà, R.; Soffietti, R. Mechanisms and therapy for cancer metastasis to the brain. Front. Oncol. 2018, 8, 161.
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126.
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.J.; ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260.
- Chow, A.; Arteaga, C.L.; Wang, S.E. When tumor suppressor TGFβ meets the HER2 (ERBB2) oncogene. J. Mammary Gland. Biol. Neoplasia 2011, 16, 81–88.
- Grusch, M.; Petz, M.; Metzner, T.; Ozturk, D.; Schneller, D.; Mikulits, W. The crosstalk of RAS with the TGF-β family during carcinoma progression and its implications for targeted cancer therapy. Curr. Cancer Drug Targets 2010, 10, 849–857.
- Demirkan, B. The Roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in Breast Cancer Bone Metastasis: Potential Targets for Prevention and Treatment. J. Clin. Med. 2013, 2, 264–282.
- Liu, F.; Gu, L.N.; Shan, B.E.; Geng, C.Z.; Sang, M.X. Biomarkers for EMT and MET in breast cancer: An update (review). Oncol. Lett. 2016, 12, 4869–4876.
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra48.
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Müller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 8.
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412.
- Krizbai, I.A.; Gasparics, Á.; Nagyőszi, P.; Fazakas, C.; Molnár, J.; Wilhelm, I.; Bencs, R.; Rosivall, L.; Sebe, A. Endothelial-mesenchymal transition of brain endothelial cells: Possible role during metastatic extravasation. PLoS ONE 2015, 10, e0119655.
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576.
- Li, F.; Tang, S.C. Targeting metastatic breast cancer with ANG1005, a novel peptide-paclitaxel conjugate that crosses the blood-brain-barrier (BBB). Genes Dis. 2017, 4, 1–3.
- Islam, B.U.; Khan, M.S.; Husain, F.M.; Rehman, M.T.; Zughaibi, T.A.; Abuzenadah, A.M.; Urooj, M.; Kamal, M.A.; Tabrez, S. mTOR Targeted Cancer Chemoprevention by Flavonoids. Curr. Med. Chem. 2020, 8, 8068–8082.
- ul Islam, B.; Suhail, M.; Khan, M.S.; Ahmad, A.; Zughaibi, T.A.; Husain, F.M.; Rehman, M.T.; Tabrez, S. Flavonoids and PI3K/Akt/mTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment. Curr. Med. Chem. 2021, 28, 8083–8097.
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting pi3k/akt/mtor pathway by different flavonoids: A cancer chemopreventive approach. Int. J. Mol. Sci. 2021, 22, 12455.
- Blazquez, R.; Wlochowitz, D.; Wolff, A.; Seitz, S.; Wachter, A.; Perera-Bel, J.; Bleckmann, A.; Beißbarth, T.; Salinas, G.; Riemenschneider, M.J.; et al. PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. Glia 2018, 66, 2438–2455.
- Hohensee, I.; Chuang, H.-N.N.; Grottke, A.; Werner, S.; Schulte, A.; Horn, S.; Lamszus, K.; Bartkowiak, K.; Witzel, I.; Westphal, M.; et al. PTEN mediates the cross talk between breast and glial cells in brain metastases leading to rapid disease progression. Oncotarget 2017, 8, 6155–6168.
- Corti, C.; Antonarelli, G.; Criscitiello, C.; Lin, N.U.; Carey, L.A.; Cortés, J.; Poortmans, P.; Curigliano, G. Targeting brain metastases in breast cancer. Cancer Treat. Rev. 2022, 103, 102324.
- Schmit, F.; Utermark, T.; Zhang, S.; Wang, Q.; Von, T.; Roberts, T.M.; Zhao, J.J. PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context. Proc. Natl. Acad. Sci. USA 2014, 111, 6395–6400.
- Ni, J.; Ramkissoon, S.H.; Xie, S.; Goel, S.; Stover, D.G.; Guo, H.; Luu, V.; Marco, E.; Ramkissoon, L.A.; Kang, Y.J.; et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat. Med. 2016, 22, 723–726.
- Elmenier, F.M.; Lasheen, D.S.; Abouzid, K.A.M. Phosphatidylinositol 3 kinase (PI3K) inhibitors as new weapon to combat cancer. Eur. J. Med. Chem. 2019, 183, 111718.
- Riecke, K.; Müller, V.; Weide, R.; Schmidt, M.; Park-simon, T.W.; Möbus, V.; Mundhenke, C.; Polasik, A.; Lübbe, K.; Hesse, T.; et al. Predicting prognosis of breast cancer patients with brain metastases in the BMBC registry—Comparison of three different GPA prognostic scores. Cancers 2021, 13, 844.
- Kodack, D.P.; Askoxylakis, V.; Ferraro, G.B.; Sheng, Q.; Badeaux, M.; Goel, S.; Qi, X.; Shankaraiah, R.; Cao, Z.A.; Ramjiawan, R.R.; et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci. Transl. Med. 2017, 9, eaal4682.
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res Treat. 2020, 52, 1059–1066.
- Da Silva, L.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010, 12, R46.
- Giri, D.K.; Ali-Seyed, M.; Li, L.-Y.; Lee, D.-F.; Ling, P.; Bartholomeusz, G.; Wang, S.-C.; Hung, M.-C. Endosomal Transport of ErbB-2: Mechanism for Nuclear Entry of the Cell Surface Receptor. Mol. Cell. Biol. 2005, 25, 11005–11018.
- Wang, S.-C.C.; Lien, H.-C.C.; Xia, W.; Chen, I.-F.F.; Lo, H.-W.W.; Wang, Z.; Ali-Seyed, M.; Lee, D.-F.F.; Bartholomeusz, G.; Ou-Yang, F.; et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer cell 2004, 6, 251–261.
- Bryan, S.; Witzel, I.; Borgmann, K.; Oliveira-Ferrer, L. Molecular mechanisms associated with brain metastases in her2-positive and triple negative breast cancers. Cancers 2021, 13, 4137.
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Miller, L.; Metheny-Barlow, L.; Lo, H.W. EGFR and HER2 signaling in breast cancer brain metastasis. Front. Biosci. (Elite Ed.) 2016, 8, 245–263.
- Balch, S.M.; Vaz-Luis, I.; Li, T.; Tayob, N.; Jain, E.; Helvie, K.; Buendia-Buendia, J.E.; Shannon, E.; Isakoff, S.J.; Tung, N.M.; et al. A phase II study of efficacy, toxicity, and the potential impact of genomic alterations on response to eribulin mesylate in combination with trastuzumab and pertuzumab in women with human epidermal growth factor receptor 2 (HER2)+ metastatic breast cancer. Breast Cancer Res. Treat. 2021, 189, 411–423.
- Batra, A.; Kong, S.; Cheung, W.Y. Eligibility of real-world patients with metastatic breast cancer for clinical trials. Breast 2020, 54, 171–178.
- Lo, H.-W.W.; Hsu, S.-C.C.; Xia, W.; Cao, X.; Shih, J.-Y.Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.-C.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67, 9066–9076.
- Heiland, D.H.; Ravi, V.M.; Behringer, S.P.; Frenking, J.H.; Wurm, J.; Joseph, K.; Garrelfs, N.W.C.; Strähle, J.; Heynckes, S.; Grauvogel, J.; et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 2019, 10, 2541.
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation AIDS central nervous system axon regeneration. Nature 2016, 532, 195–200.
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martinez-Saez, E.; y Cajal, S.R.; Megías, D. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035.
- Kim, S.-J.; Kim, J.-S.; Park, E.S.; Lee, J.-S.; Lin, Q.; Langley, R.R.; Maya, M.; He, J.; Kim, S.-W.; Weihua, Z.; et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 2011, 13, 286–298.
- Crinò, L.; Bronte, G.; Bidoli, P.; Cravero, P.; Minenza, E.; Cortesi, E.; Garassino, M.C.; Proto, C.; Cappuzzo, F.; Grossi, F.; et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019, 129, 35–40.
- Lorger, M.; Andreou, T.; Fife, C.; James, F. Immune Checkpoint Blockade—How Does It Work in Brain Metastases? Front. Mol. Neurosci. 2019, 12, 282.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Nitric Oxide: Physiology, Pharmacology, and Therapeutic Applications
Revisions:
2 times
(View History)
Update Date:
29 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




