Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Brain metastasis is one of the major reasons of death in breast cancer (BC) patients, significantly affecting the quality of life, physical activity, and interdependence on several individuals. The tendency to develop breast cancer brain metastases (BCBMs) differs by the BC subtype, varying from almost half with triple-negative breast cancer (TNBC) (HER2− ER− PR−), one-third with HER2+ (human epidermal growth factor receptor 2-positive, and around one-tenth with luminal subclass (ER+ (estrogen positive) or PR+ (progesterone positive)) breast cancer.
- brain metastases
- breast cancer
- chemokine receptor
- HER2
- TNBC
1. Breast Cancer Brain Metastases (BCBM)
Breast cancer brain metastases (BCBM) are the second most frequent type of brain metastases and one of the most typical breast cancer metastases [31,32]. Scientific studies have identified that patients with breast cancer had a 5.1% rate of BCBM incidence. Moreover, among patients with any metastatic disease, 14.2% developed BCBM during the therapeutic phase of the illness [32]. Most BM occurs in patients with HER2-positive and ER-negative metastatic BC. Among these, HER2-positive BC patients have a higher rate of survival. Due to the therapeutic challenges, BCBM requires an integrated therapy approach for its management [33].
Three therapeutic approaches are available for treating metastatic brain tumors, including anticancer agents, surgery, and radiation therapy [19,21,32]. Metastasis represents the primary cause of death in BC patients suffering from BMs, which gradually progresses into a more advanced stage [34]. Metastasized breast cancers or circulating tumor cells evade the BBB, and upon reaching the specified cranial cells (astrocytes), they initiate tumorigenesis leading to tumor formation. The initial growth of BMs in the brain is associated with the entry of cancerous cells into the bloodstream and different locations of the brain, where they grow and multiply rapidly [21]. Hence, further insight into breast cancer brain metastasis mechanisms is expected to provide a mode of management or inhibition of these cancer types. Figure 1 depicts the formation of breast cancer brain tumors.
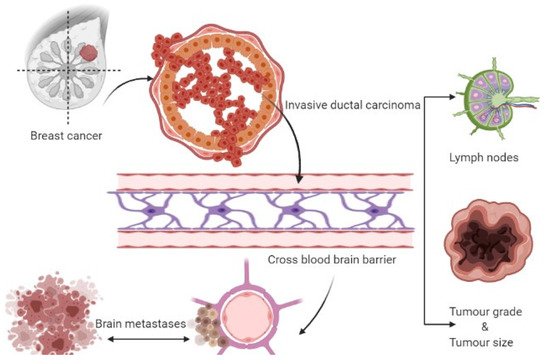
Figure 1. Formation of breast cancer-associated brain tumors.
2. TGFβ/SMAD Signaling Pathway
It is well-known that epithelial to mesenchymal transition (EMT) proteins such as Slug, Snail, and Zeb1 are expressed through transforming growth factor β (TGFβ), SMAD, and PI3K signaling pathways (Figure 3) [73]. The EMT proteins promote this transition by decreasing the expression of E-cadherin and increasing the expression of N-cadherin [74]. EMT plays a significant role in cancer by increasing invasiveness and metastasis, resulting in poor prognosis and survival [75]. The EMT proteins suppress the expression of CDH1 [76]. The CDH1 gene translates to E-cadherin, which plays a critical role in cell adhesion and is involved in cell attachment to other cells and the extracellular matrix (ECM) [77]. Without E-cadherin, the breast cancer cells are detached from the breast tissue, forming circulating tumor cells (CTCs) that can metastasize to other tissues, including the lungs and brain [24]. Under normal physiological conditions, the BBB selectively regulates materials that go into the brain compartment by preventing the paracellular diffusion of compounds. This causes an obstacle for breast CTCs to pass through the BBB; however, in BCBM, the breast CTCs diffuse through the endothelial cell junctions [24,78]. The endothelial cell junctions are the part of the BBB that is modified during BM formation. Slug, Snail, Zeb1, VEGFA, and CD44 contribute to BM formation by enhancing the trans-endothelial migration of tumor cells via downregulation of endothelial integrity, enabling the breast CTCs to pass the BBB [79] (Mittal, 2018). Targeting the SMAD protein in the TGFβ/SMAD signaling pathway has been suggested to attenuate brain metastasis in BCBM patients [80]. The lipoprotein receptor-related protein 1 (LRP-1) inhibitors (ANG1005 and GRN1005) bind to LRP-1, leading to LRP-1 receptor-mediated transcytosis or endocytosis across the BBB, resulting in tumor growth arrest and apoptosis [81,82].
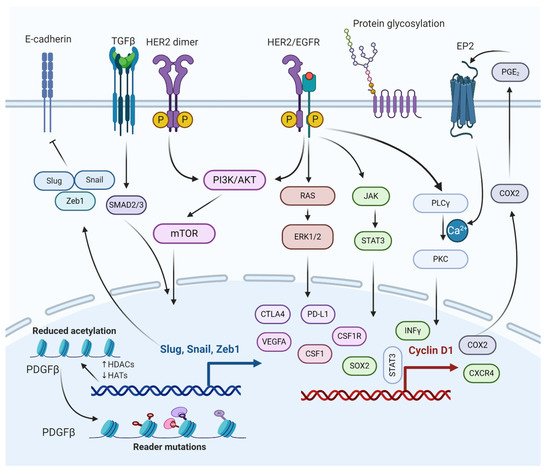
Figure 3. Molecular interactions of different pathways involved in the regulation of BCBM. The epithelial to mesenchymal transition (EMT) genes inhibit cadherin, promoting metastasis. PI3K/AKT/mTOR and RAS/RAF/ERK pathways activate cellular processes, such as cell proliferation, survival, migration, and angiogenesis. PI3K/AKT/mTOR and JAK/STAT pathways help BCBMs escape immunosurveillance. COX2 aids in prostaglandin synthesis and inflammation activation. Histone deacetylation of growth factors and protein glycosylation aid in bypassing the BBB.
3. PI3K/mTOR Signaling Pathway
The phosphoinositide 3-kinase (PI3K) signaling pathway is a central pathway involved in cellular processes such as cell survival, cell proliferation, cell metabolism, and angiogenesis (Figure 3) [83,84,85]. It also plays a significant role in BCBM with approximately 77% of patients having been noted to have an activated PI3K signaling pathway [86,87]. The activation of the PI3K signaling pathway is associated with increased expression of metastatic and immunosuppressive genes, which include CTLA4, PD-L1, CSF1R, and CSF1 in the tumor microenvironment of metastasized brain cells [21,88]. The loss of function of phosphatase and tensin homolog (PTEN), a tumor suppressor and a negative regulator of PI3K signaling, is detected in 25–71% of BCBM patients, with the highest percentage in TNBC cases [89]. Overexpression of PTEN in astrocytes suppresses invasiveness and cell migration, suggesting PTEN as a promising therapeutic target for BCBM treatment [87]. The mTOR is a serine/threonine protein kinase, a downstream protein of PI3K, and Akt plays a significant role in several cancer types [84,90]. Simultaneous mTOR and PI3K protein inhibition have been reported to attenuate BCBM [91]. Everolimus and Buparlisib (BKM120), mTOR and PI3K inhibitors, are used to treat BCBM in combination with other anticancer drugs such as trastuzumab and vinorelbine [38].
4. HER2/Epidermal Growth Factor Receptor (EGFR) Signaling Pathway
EGFR is a transmembrane protein that activates the EGFR signaling pathway through homo/hetero-dimerization and auto-phosphorylation in response to ligand binding [92]. EGFR forms heterodimer with HER2, activating the PI3K/AKT signaling cascade. HER2+ breast cancer is susceptible to brain metastasis due to its link with PI3K signaling pathway (Figure 3) [93]. The HER2 protein dimerizes with another similar protein called HER3, triggering cell proliferation and survival. One study based on immune-histochemistry reported that HER3 is over-expressed in around 60% of BCBM patients [94].
HER2 signaling is a master regulator of many pro-inflammatory, proliferative, and pro-metastatic pathways, the most notable of which is the cyclo-oxygenase 2 (COX2) [95,96]. The HER2/EGFR signaling pathway is directly or indirectly associated with COX2 upregulation, which has shown to induce specified brain metastasis. Because BCBM patients have high expression of both HER2 and EGFR, several drugs (some approved and others in clinical trials) are used to target various stages of the HER2/EGFR signaling pathway [97,98]. Some of the drugs that target HER2/EGFR are Lapatinib (targets HER2 receptor), Trastuzumab (targets HER2 receptor), KD019 (targets HER2, Src, and EGFR), ARRY-380 (targets HER2 receptor), HKI-272 (targets HER1, HER2, and HER4 receptors), Afatinib (targets EGFR1, EGFR2, and EGFR4 receptors), and tucatinib (targets HER2 receptor). It is worth mentioning that tucatinib, a tyrosine kinase inhibitor, combined with trastuzumab and capecitabine, was approved by the USFDA on April 17, 2020, as a BCBM treatment regimen [38,99,100].
5. JAK/Signal Transducer and Activator of Transcription 3 (STAT3) Signaling Pathway
The JAK-STAT pathway is known for regulating the expression of growth factors and cytokines [101]. Some of the genes involved in the JAK-STAT pathway include PD-L1, VEGFA, and CTLA4, which play a crucial role in the survival of BCBM by escaping from immunosurveillance (Figure 3) [102]. STAT3 is critical for astrocytic scar formation and is involved in axon regeneration [103] (Anderson et al., 2016). Most astrocytes in BMs are expressed as an activated form of STAT3, the phosphorylated STAT3 (pSTAT3) [104] (Priego et al., 2018). The pSTAT3+ cancerous astrocytes bypass immunosurveillance by expressing escape-promoting genes, such as PD-L1, CTLA4, VEGFA, and TIMP-1 [105]. Therefore, STAT3 in BMs could be a potential therapeutic target for BCBM treatment. Nivolumab, an approved anticancer drug, targets PD-1 in BCBM, thereby preventing the binding of PD-L1 to PD-1. Similarly, using nivolumab and other treatment regimens helps cancer immunotherapy [106,107].
This entry is adapted from the peer-reviewed paper 10.3390/ijms231911687
This entry is offline, you can click here to edit this entry!
