You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Héctor Mora-Montes | -- | 2321 | 2022-12-27 18:30:36 | | | |
| 2 | Sirius Huang | Meta information modification | 2321 | 2022-12-28 01:38:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Pathogen–Host Interaction in Different Non-albicans Candida Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/39462 (accessed on 13 December 2025).
Gómez-Gaviria M, Ramírez-Sotelo U, Mora-Montes HM. Pathogen–Host Interaction in Different Non-albicans Candida Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/39462. Accessed December 13, 2025.
Gómez-Gaviria, Manuela, Uriel Ramírez-Sotelo, Héctor M. Mora-Montes. "Pathogen–Host Interaction in Different Non-albicans Candida Species" Encyclopedia, https://encyclopedia.pub/entry/39462 (accessed December 13, 2025).
Gómez-Gaviria, M., Ramírez-Sotelo, U., & Mora-Montes, H.M. (2022, December 27). Pathogen–Host Interaction in Different Non-albicans Candida Species. In Encyclopedia. https://encyclopedia.pub/entry/39462
Gómez-Gaviria, Manuela, et al. "Pathogen–Host Interaction in Different Non-albicans Candida Species." Encyclopedia. Web. 27 December, 2022.
Copy Citation
Fungal infections caused by Candida species have become a constant threat to public health, especially for immunocompromised patients, who are considered susceptible to this type of opportunistic infections. Candida albicans is known as the most common etiological agent of candidiasis; however, other species, such as Candida tropicalis, Candida parapsilosis, Nakaseomyces glabrata (previously known as Candida glabrata), Candida auris, Candida guilliermondii, and Pichia kudriavzevii (previously named as Candida krusei), have also gained great importance.
antifungal drugs
host–fungus interaction
antifungal immunity
1. Introduction
In the last 50 years, we have experienced great advances in healthcare services, which have improved life quality and expectancy. However, this has been accompanied by the increased risk to develop opportunistic infections, such as systemic candidiasis, one of the leading causes of infection-related morbidity and mortality [1]. There are more than 18 different Candida species that cause infections in humans, but at least six of these are associated with more than 95% of invasive diseases [2]. Currently, a major part of candidiasis is still owing to Candida albicans (63–70%) [3]; however, other Candida species, such as Candida tropicalis, Candida parapsilosis, Pichia kudriavzevii, Nakaseomyces glabrata, and Candida auris, among others, are collectively as important as C. albicans in the clinical setting and are known as non-albicans Candida species (NAC). Usually, these are found in the environment, skin, or as mucosal colonizers in humans [2][3][4].
C. tropicalis is widely distributed in nature, being a common colonizer of the human skin, oral cavity, and digestive tract. This yeast is an important opportunistic pathogen capable of causing nosocomial infections and it is the second most frequently isolated species after C. albicans [5][6]. An important aspect that contributes to invasive candidiasis is drug resistance, and in recent years, this biological trait has increased among the C. tropicalis clinical isolates [6][7][8]. The main reason for C. tropicalis’ increased drug resistance is due to mutations of the ergosterol synthase encoding gene ERG11 and overexpression of the transcriptional regulator encoded by UPC2 (Figure 1) [6][8].
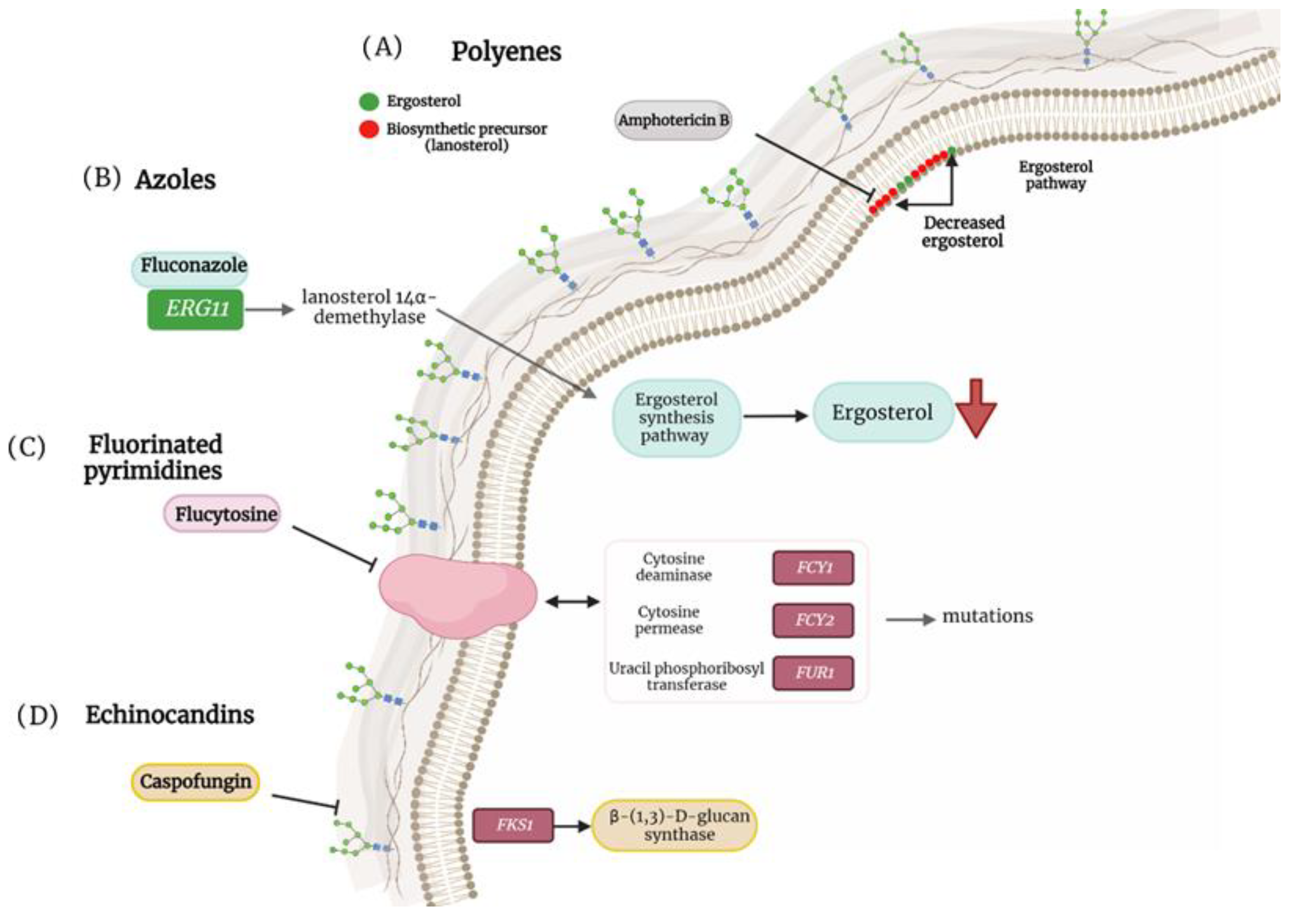
Figure 1. Antifungal resistance mechanisms in non-albicans Candida species. (A) Mutation in ergosterol biosynthesis that causes a decrease in the ergosterol content in the cell membrane and induces the replacement of biosynthetic precursors such as liquesterol and lanosterol. (B) Mutation in ERG11 that encodes the enzyme lanosterol 14α-demethylase, causing defects in ergosterol synthesis. (C) Mutation in cytosine deaminase (FCY1), cytosine permease (FCY2) and uracil phosphoribosyltransferase (FUR1) facilitating resistance to flucytosine. (D) Mutation in FKS1 that generates resistance to caspofungins.
C. parapsilosis is often the second or third most frequently isolated Candida species in intensive care units (ICUs) since it is capable to form biofilms on central venous catheters and other medically indwelling devices, thus menacing patients who have undergone invasive medical interventions. In addition, C. parapsilosis-caused infections are a significant problem among neonates [9][10]. Even though these infections generally result in lower morbidity and mortality rates than those caused by C. albicans, diverse clinical isolates of this species have been reported to be less susceptible to echinocandins, and in some regions, resistance to azole treatment has also been noted, which complicates the choice of antifungal drug therapy [10][11][12]. The echinocandin resistance mechanism in C. parapsilosis differs from the phenotypic changes seen in other Candida species because this fungal species has a natural polymorphism in FKS1, which leads to reduced in vitro echinocandin susceptibility (Figure 1) [9][13]. The mechanism for azole resistance is like that described for C. tropicalis [6][8].
N. glabrata is responsible for nosocomial infections, particularly in adult wards, and is characterized by single biofilm formation, which increases its pathogenesis [2][14][15]. In the United States, approximately one-third of candidiasis cases are caused by N. glabrata, causing hematogenous infections [16][17]. Like other NAC, drug-resistant strains of N. glabrata are of great concern, as they are being isolated with increasing frequency. For example, in some medical centers, up to 25% of N. glabrata isolates are resistant or moderately susceptible to echinocandins [17][18][19]. These data remark on the relevance of N. glabrata in the clinical setting. Antifungal drug resistance in this species is related to the over-expression of membrane transporters, point mutations in ERG11/CYP51, altered sterol import, and genome plasticity, with segmental rearrangements in the M and F chromosomes (Figure 1) [20].
On the other hand, P kudriavzevii is an important pathogen in cancer patients, causing both systemic and superficial infections [21]. This organism can cause bronchopneumonia, vasculitis, infections of the tonsils, arthritis, ulcers, and urinary tract, but it is a rare etiologic agent of vaginitis (it has only been isolated in 0.1% of cases) [21][22]. P. kudriavzevii has intrinsic resistance to fluconazole and variable resistance to other drugs used in its treatment, including voriconazole, itraconazole, posaconazole, anidulafungin, micafungin, 5-flucytosine, and amphotericin B [23][24][25]. Thus, this changing sensitivity to antifungal drugs makes this organism a potential threat to human health.
As known, Candida guilliermondii has become a relevant causative agent of candidiasis in recent years. According to a report by Chen et al., [26] the Meyerozyma guilliermondii complex is the second most common Candida species isolated from bloodstream infections in a Chinese hospital, and this is the first study that assessed the risk factors, clinical characteristics, and outcomes of candidemia caused by the M. guilliermondii complex in cancer patients who have undergone recent surgery. Similarly, this organism has been increasingly isolated in Japan, with a rise in frequency of detection of 14%-24% [27][28][29]. Strains with resistance to azoles, polyenes, and echinocandins have been reported [30]. The most common mechanisms of drug resistance in this species include increased activity of the efflux pump, alteration of the 14 α-demethylase, and point mutations in FKS1 (Figure 1) [30].
Just over a decade ago, in East Asia, the identification of a new species, C. auris, was reported, whose peculiarity was its resistance to fluconazole [31]. Currently, this species has been identified throughout the world, and its relevance relies on the fact that it is difficult to identify in the clinical laboratory, leading to erroneous diagnosis and failure in the treatments, a fact that favors the development of resistance acquisition to multiple drugs [32]. Similar to other Candida species, in C. auris the molecular mechanisms of resistance to azoles include the overexpression and mutations in the ERG11 gene (which codes for lanosterol demethylase), and alterations in the sterol synthesis pathway that involves the replacement of ergosterol by other sterols, among others (Figure 1) [32][33]. Mutations in this and other genes, such as FKS1, result in elevated minimal inhibitory concentration (MIC) ranges for echinocandins and have been linked to treatment failure (Figure 1) [32][33]. C. auris has been classified as a fungal pathogen of concern, which is often associated with nosocomial infections and is considered a threat to human health throughout the world due to its ability to spread efficiently from person to person and cause deadly diseases [34][35][36].
2. Pathogen–Host Interaction in Different NAC Species
The control of infections caused by the different NAC species, and other fungi, is based on the correct activation of innate and adaptive immune responses [10]. The first step during this interaction is the recognition of the fungal cell wall components. This is a key structure that fulfills very specific functions within the cell, it is responsible for communication with the extracellular environment, and provides strength and protection against host immunity [37][38]. The cell wall has pathogen-associated molecular patterns (PAMPs), which are recognized by pattern recognition receptors (PRRs) located mostly on the cell surface of immune cells [39]. The cell wall PAMPs of the different NAC species are chitin, β-1,3-glucans, β-1, 6-glucans, and N-linked and O-linked mannans [38][39].
The most studied fungus–host interaction is that of C. albicans, and it is thought that this interaction may be similar in NAC species [40]. Recently, several works have reported the immune recognition of different Candida species of medical importance [38][41][42]. Immune cells, such as neutrophils and murine phagocytic cells, can distinguish between different Candida species. In the case of neutrophils, these show reduced uptake against P. kudriavzevii; however, murine phagocytic cells have a greater ability to kill C. guilliermondii and C. krusei when compared to C. albicans [43]. In the same line, C. tropicalis is more susceptible to damage by neutrophils than C. albicans [44]. Moreover, it has been reported that the interaction of C. parapsilosis, C. tropicalis, P. kudriavzevii, C. albicans, N. glabrata, and C. guilliermondii with human peripheral blood mononuclear cells (PBMCs) is species-specific [38][45].
Analysis of C. tropicalis, C. guilliermondii, and P. kudriavzevii cell walls showed that the composition between the three species is similar [38]; however, this is not necessarily an indicator of a similar interaction profile with components of innate immunity. As proof of this, it was found that C. guilliermondii cells have lower levels of β-1,3-glucan, which stimulates low cytokine levels when this polysaccharide is exposed on the surface [38]. Yeast cells of C. tropicalis, C. guilliermondii, and P. kudriavzevii can stimulate higher cytokines levels than C. albicans when interacting with human PBMCs and human monocyte-derived macrophages [38]. C. parapsilosis shows structural similarities to the C. albicans cell wall; however, the arrangement of the components within the wall is different, which has an impact on the ability to activate PBMCs [46].
C. parapsilosis is one of the NAC species for which more information has been generated in recent years, including its interaction with the host [10]. For this species, it is known that the Toll-like receptors (TLRs) TLR2, TLR4, and TLR6 are involved in its recognition by gingival epithelial cells, human PBMCs and macrophages [42][46][47][48]. In addition, galectin-3, mannose receptor, and dectin-1 are suggested to be receptors required for responses induced by C. parapsilosis [46][49][50]. In recent reports, it has been shown that C. parapsilosis stimulates stronger cytokine production than other species, such as C. albicans, because of greater exposure of β-1,3-glucan at the cell surface [46]. The disruption of C. parapsilosis OCH1, an important gene in the synthesis of the N-linked mannan’s outer chain [42], led to changes in the fungal interaction with human PBMCs, stimulating greater levels of IL-1β, in a dectin-1- and TLR4-dependent way [42].
The N. glabrata immune recognition has also been studied, and it is known that null mutants with defects in different components of the cell wall, such as β-1,3-glucan or chitin, stimulate a stronger inflammatory response in macrophages [41]. Little is known about the PRRs responsible for N. glabrata recognition by macrophages, but dectin-2 is important for host defense against this fungus [51]. A downstream analysis of the PRR signaling pathways determined that the fungus does not induce phosphorylation of the MAP kinases Erk1/2, SAPK/JNK, and p38. However, Syk tyrosine kinase, which signals downstream of C-type lectin receptors (dectin-1 and dectin-2), was activated upon infection of macrophages by N. glabrata [41][52]. This organism can survive and replicate in macrophages, which would offer it some advantages, such as immune evasion [53].
A component that plays an important role during the immune recognition of some Candida species is phosphomannan [54]. To determine the importance of this wall component in the C. tropicalis–host interaction, an mnn4∆ null mutant was generated [55]. It was found that cell wall phosphomannans are not required for the stimulation of pro- and anti-inflammatory cytokine production by PBMCs [55]. Assays carried out with human monocyte-derived macrophages showed that the mnn4Δ null mutant strain was poorly phagocytosed by these cells [55]. These results are in line with those obtained in C. albicans, where the loss of phosphomannan reduced yeast phagocytosis by approximately 50% [56]. However, C. tropicalis cells are more phagocytosed than C. albicans cells in a dectin-1-dependent mechanism [55].
The mentioned cell–cell interactions play a relevant role in fungal immune recognition; however, there are humoral factors that are responsible for defending the host against NAC species. The complement system is known as a humoral factor of innate immunity against various pathogens. In species such as N. glabrata, C. parapsilosis, and C. tropicalis, the binding of complement proteins has been documented [40][57].
Even though there is considerable progress in understanding C. auris’s biological and clinical aspects, its interaction with the host’s immune system is just beginning to be investigated [58]. Previous work has shown that C. auris can evade the immune response generated by neutrophils [59]. These immune cells have an important role in the control of fungal infections such as candidiasis. These cells can kill the pathogen through the release of extracellular traps known as NETs [60][61]. It is known that after 4 h of interaction between C. albicans and human neutrophils there is an inhibition of cell growth; however, this is not observed when interacting with C. auris [61]. Human neutrophils cannot effectively kill C. auris, and cell recruitment is poor [59]. This type of immune evasion would have many consequences for those patients who have invasive candidiasis caused by this pathogen [59].
Analysis of cytokine production by human PBMCs established that C. auris and C. albicans could hardly stimulate TNFα, IL-6, IL-1β, and IL-10 [38]. However, when heat-inactivated cells from the two species were used to stimulate cytokines, higher and similar levels of them were observed [38][46]. In addition, the C. auris uptake by human monocyte-derived macrophages is lower when compared to that observed with C. tropicalis, C. guilliermondii, and P. kudriavzevii [38].
In vivo and in vitro studies carried out with the C. auris clinical isolate BJCA001 demonstrated that once the infection with the fungus is carried out in immunocompetent C57BL/6 female mice, the yeasts can remain in the host and evade the mechanisms of defense [62]. When the fungal load in infected organs was analyzed, increased tissue colonization was observed; however, no morphological changes, such as pseudohyphae or mycelium, were documented [62]. Although there was an increase in colonization, the inflammation and tissue damage suffered by the mice proved to be less severe than the infection caused by C. albicans [62]. In line with these observations, interactions with bone-marrow-derived murine macrophages showed a significant increment in the levels of IL-1β, IL-6, TNF-α, CXCL1, and CXCL2 when interacting with C. albicans but not when the experiments were performed with C. auris, suggesting that the latter is a lesser potent inducer of the MAPK signaling pathway [62]. This reduced proinflammatory response could be related to changes in the β-1,3-glucans masking [62]
Finally, although the knowledge on immunity against different NAC species is an evolving and growing area, it is evident that the immune cell–fungus interaction differs among NAC species.
References
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413.
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.J.S.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24.
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The Inhibition of Non-albicans Candida species and uncommon yeast pathogens by selected essential oils and their major compounds. Molecules 2021, 26, 4937.
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 2017, 1508, 17–65.
- Zuza-Alves, D.L.; Silva-Rocha, W.P.; Chaves, G.M. An update on Candida tropicalis based on basic and clinical approaches. Front. Microbiol. 2017, 8, 1927.
- Wang, D.; An, N.; Yang, Y.; Yang, X.; Fan, Y.; Feng, J. Candida tropicalis distribution and drug resistance is correlated with ERG11 and UPC2 expression. Antimicrob. Resist. Infect. Control. 2021, 10, 54.
- Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Antifungal susceptibility patterns of a global collection of fungal isolates: Results of the SENTRY Antifungal Surveillance Program (2013). Diagn. Microbiol. Infect. Dis. 2016, 85, 200–204.
- Fan, X.; Xiao, M.; Liao, K.; Kudinha, T.; Wang, H.; Zhang, L.; Hou, X.; Kong, F.; Xu, Y.C. Notable increasing trend in azole non-susceptible Candida tropicalis causing invasive candidiasis in China (august 2009 to july 2014): Molecular epidemiology and clinical Azole consumption. Front. Microbiol. 2017, 8, 464.
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798.
- Toth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vagvolgyi, C.; Gacser, A. Candida parapsilosis: From genes to the bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18.
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: Results from laboratory-based sentinel surveillance in South Africa. J. Antimicrob. Chemother. 2016, 71, 1994–2004.
- Pinhati, H.M.; Casulari, L.A.; Souza, A.C.; Siqueira, R.A.; Damasceno, C.M.; Colombo, A.L. Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect. Dis. 2016, 16, 433.
- Papp, C.; Kocsis, K.; Tóth, R.; Bodai, L.; Willis, J.R.; Ksiezopolska, E.; Lozoya-Pérez, N.E.; Vágvölgyi, C.; Mora Montes, H.; Gabaldón, T.; et al. Echinocandin-induced microevolution of Candida parapsilosis influences virulence and abiotic stress tolerance. mSphere 2018, 3, e00547-18.
- Li, Q.; Liu, J.; Chen, M.; Ma, K.; Wang, T.; Wu, D.; Yan, G.; Wang, C.; Shao, J. Abundance interaction in Candida albicans and Candida glabrata mixed biofilms under diverse conditions. Med. Mycol. 2021, 59, 158–167.
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305.
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361.
- Filler, E.E.; Liu, Y.; Solis, N.V.; Wang, L.; Diaz, L.F.; Edwards, J.E., Jr.; Filler, S.G.; Yeaman, M.R. Identification of Candida glabrata transcriptional regulators that govern stress resistance and virulence. Infect. Immun. 2021, 89, e00146-20.
- Cleveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: Results from population-based surveillance. PLoS ONE 2015, 10, e0120452.
- Vallabhaneni, S.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Schaffner, W.; Beldavs, Z.G.; Derado, G.; Pham, C.D.; Lockhart, S.R.; Smith, R.M. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: Data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect. Dis. 2015, 2, ofv163.
- Martínez-Herrera, E.; Frías-De-León, M.G.; Hernández-Castro, R.; García-Salazar, E.; Arenas, R.; Ocharan-Hernández, E.; Rodríguez-Cerdeira, C. Antifungal resistance in clinical isolates of Candida glabrata in Ibero-America. J. Fungi 2021, 8, 14.
- Gómez-Gaviria, M.; Mora-Montes, H.M. Current aspects in the biology, pathogeny, and treatment of Candida krusei, a neglected fungal pathogen. Infect. Drug Resist. 2020, 13, 1673–1689.
- Fan, S.R.; Liu, X.P.; Li, J.W. Clinical characteristics of vulvovaginal candidiasis and antifungal susceptibilities of Candida species isolates among patients in southern China from 2003 to 2006. J. Obstet. Gynaecol. Res. 2008, 34, 561–566.
- Nguyen, K.T.; Ta, P.; Hoang, B.T.; Cheng, S.; Hao, B.; Nguyen, M.H.; Clancy, C.J. Anidulafungin is fungicidal and exerts a variety of postantifungal effects against Candida albicans, C. glabrata, C. parapsilosis, and C. krusei isolates. Antimicrob. Agents Chemother. 2009, 53, 3347–3352.
- Forastiero, A.; Garcia-Gil, V.; Rivero-Menendez, O.; Garcia-Rubio, R.; Monteiro, M.C.; Alastruey-Izquierdo, A.; Jordan, R.; Agorio, I.; Mellado, E. Rapid development of Candida krusei echinocandin resistance during caspofungin therapy. Antimicrob. Agents Chemother. 2015, 59, 6975–6982.
- Gong, J.; Xiao, M.; Wang, H.; Kudinha, T.; Wang, Y.; Zhao, F.; Wu, W.; He, L.; Xu, Y.C.; Zhang, J. Genetic differentiation, diversity, and drug susceptibility of Candida krusei. Front. Microbiol. 2018, 9, 2717.
- Chen, J.; Tian, S.; Li, F.; Sun, G.; Yun, K.; Cheng, S.; Chu, Y. Clinical characteristics and outcomes of candidemia caused by Meyerozyma guilliermondii complex in cancer patients undergoing surgery. Mycopathologia 2020, 185, 975–982.
- Hirano, R.; Sakamoto, Y.; Kudo, K.; Ohnishi, M. Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: A single hospital experience. Infect. Drug Resist. 2015, 8, 199–205.
- Hirano, R.; Sakamoto, Y.; Kitazawa, J.; Yamamoto, S.; Kayaba, H. Epidemiology, practice patterns, and prognostic factors for candidemia; and characteristics of fourteen patients with breakthrough Candida bloodstream infections: A single tertiary hospital experience in Japan. Infect. Drug Resist. 2018, 11, 821–833.
- Kimura, M.; Araoka, H.; Yamamoto, H.; Asano-Mori, Y.; Nakamura, S.; Yamagoe, S.; Ohno, H.; Miyazaki, Y.; Abe, M.; Yuasa, M.; et al. Clinical and microbiological characteristics of breakthrough candidemia in allogeneic hematopoietic stem cell transplant recipients in a japanese hospital. Antimicrob. Agents Chemother. 2017, 61, e01791-16.
- Savini, V.; Catavitello, C.; Onofrillo, D.; Masciarelli, G.; Astolfi, D.; Balbinot, A.; Febbo, F.; D’Amario, C.; D’Antonio, D. What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses 2011, 54, 434–441.
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44.
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2018, 56, e01588-17.
- Sharma, Y.; Khan, L.A.; Manzoor, N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J. Mycol. Med. 2016, 26, 244–254.
- Horton, M.V.; Johnson, C.J.; Zarnowski, R.; Andes, B.D.; Schoen, T.J.; Kernien, J.F.; Lowman, D.; Kruppa, M.D.; Ma, Z.; Williams, D.L.; et al. Candida auris cell wall mannosylation contributes to neutrophil evasion through pathways divergent from Candida albicans and Candida glabrata. mSphere 2021, 6, e0040621.
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181.
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 35.
- Díaz-Jiménez, D.F.; Pérez-García, L.A.; Martínez-Álvarez, J.A.; Mora-Montes, H.M. Role of the fungal cell wall in pathogenesis and antifungal resistance. Curr. Fungal Infect. Rep. 2012, 6, 275–282.
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; García-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, N.E.; Estrada-Mata, E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794.
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78.
- Mendoza-Reyes, D.F.; Gómez-Gaviria, M.; Mora-Montes, H.M. Candida lusitaniae: Biology, pathogenicity, virulence factors, diagnosis, and treatment. Infect. Drug Resist. 2022, 15, 5121–5135.
- Kasper, L.; Seider, K.; Hube, B. Intracellular survival of Candida glabrata in macrophages: Immune evasion and persistence. FEMS Yeast Res. 2015, 15, fov042.
- Pérez-García, L.A.; Csonka, K.; Flores-Carreón, A.; Estrada-Mata, E.; Mellado-Mojica, E.; Németh, T.; López-Ramírez, L.A.; Toth, R.; López, M.G.; Vizler, C.; et al. Role of protein glycosylation in Candida parapsilosis cell wall integrity and host interaction. Front. Microbiol. 2016, 7, 306.
- Vecchiarelli, A.; Bistoni, F.; Cenci, E.; Perito, S.; Cassone, A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia 1985, 23, 377–387.
- Roilides, E.; Holmes, A.; Blake, C.; Pizzo, P.A.; Walsh, T.J. Effects of granulocyte colony-stimulating factor and interferon-γ on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J. Leuk. Biol. 1995, 57, 651–656.
- Høgåsen, A.K.; Abrahamsen, T.G.; Gaustad, P. Various Candida and Torulopsis species differ in their ability to induce the production of C3, factor B and granulocyte-macrophage colony-stimulating factor (GM-CSF) in human monocyte cultures. J. Med. Microbiol. 1995, 42, 291–298.
- Estrada-Mata, E.; Navarro-Arias, M.J.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Csonka, K.; Gacser, A.; Mora-Montes, H.M. Members of the Candida parapsilosis complex and Candida albicans are differentially recognized by human peripheral blood mononuclear cells. Front. Microbiol. 2015, 6, 1527.
- Bahri, R.; Curt, S.; Saidane-Mosbahi, D.; Rouabhia, M. Normal human gingival epithelial cells sense C. parapsilosis by toll-like receptors and module its pathogenesis through antimicrobial peptides and proinflammatory cytokines. Mediat. Inflamm. 2010, 2010, 940383.
- Tóth, A.; Zajta, E.; Csonka, K.; Vágvölgyi, C.; Netea, M.G.; Gácser, A. Specific pathways mediating inflammasome activation by Candida parapsilosis. Sci. Rep. 2017, 7, 43129.
- Linden, J.R.; Kunkel, D.; Laforce-Nesbitt, S.S.; Bliss, J.M. The role of galectin-3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils. Cell. Microbiol. 2013, 15, 1127–1142.
- Tóth, A.; Csonka, K.; Jacobs, C.; Vágvölgyi, C.; Nosanchuk, J.D.; Netea, M.G.; Gácser, A. Candida albicans and Candida parapsilosis induce different T-cell responses in human peripheral blood mononuclear cells. J. Infect. Dis. 2013, 208, 690–698.
- Ifrim, D.C.; Bain, J.M.; Reid, D.M.; Oosting, M.; Verschueren, I.; Gow, N.A.; van Krieken, J.H.; Brown, G.D.; Kullberg, B.J.; Joosten, L.A.; et al. Role of dectin-2 for host defense against systemic infection with Candida glabrata. Infect. Immun. 2014, 82, 1064–1073.
- Drummond, R.A.; Saijo, S.; Iwakura, Y.; Brown, G.D. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 2011, 41, 276–281.
- Seider, K.; Brunke, S.; Schild, L.; Jablonowski, N.; Wilson, D.; Majer, O.; Barz, D.; Haas, A.; Kuchler, K.; Schaller, M.; et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J. Immunol. 2011, 187, 3072–3086.
- Jigami, Y.; Odani, T. Mannosylphosphate transfer to yeast mannan. Biochim. Biophys. Acta 1999, 1426, 335–345.
- Hernández-Chávez, M.J.; Franco, B.; Clavijo-Giraldo, D.M.; Hernández, N.V.; Estrada-Mata, E.; Mora-Montes, H.M. Role of protein phosphomannosylation in the Candida tropicalis-macrophage interaction. FEMS Yeast Res. 2018, 18, foy053.
- González-Hernández, R.J.; Jin, K.; Hernández-Chávez, M.J.; Díaz-Jiménez, D.F.; Trujillo-Esquivel, E.; Clavijo-Giraldo, D.M.; Tamez-Castrellón, A.K.; Franco, B.; Gow, N.A.R.; Mora-Montes, H.M. Phosphomannosylation and the functional analysis of the extended Candida albicans MNN4-like gene family. Front. Microbiol. 2017, 8, 2156.
- Meri, T.; Hartmann, A.; Lenk, D.; Eck, R.; Würzner, R.; Hellwage, J.; Meri, S.; Zipfel, P.F. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect. Immun. 2002, 70, 5185–5192.
- Bruno, M.; Kersten, S.; Bain, J.M.; Jaeger, M.; Rosati, D.; Kruppa, M.D.; Lowman, D.W.; Rice, P.J.; Graves, B.; Ma, Z.; et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat. Microbiol. 2020, 5, 1516–1531.
- Johnson, C.J.; Davis, J.M.; Huttenlocher, A.; Kernien, J.F.; Nett, J.E. Emerging fungal pathogen Candida auris evades neutrophil attack. mBio 2018, 9, e01403-18.
- Mansour, M.K.; Levitz, S.M. Interactions of fungi with phagocytes. Curr. Opin. Microbiol. 2002, 5, 359–365.
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676.
- Wang, Y.; Zou, Y.; Chen, X.; Li, H.; Yin, Z.; Zhang, B.; Xu, Y.; Zhang, Y.; Zhang, R.; Huang, X.; et al. Innate immune responses against the fungal pathogen Candida auris. Nat. Commun. 2022, 13, 3553.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
28 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




