Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Virendra Kumar Yadav | -- | 1420 | 2022-12-27 10:32:30 | | | |
| 2 | Catherine Yang | Meta information modification | 1420 | 2022-12-28 02:06:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Agarwal, N.; Solanki, V.S.; Gacem, A.; Hasan, M.A.; Pare, B.; Srivastava, A.; Singh, A.; Yadav, V.K.; Yadav, K.K.; Lee, C.; et al. Bacterial Laccases as Biocatalysts for Environmental Toxic Pollutants. Encyclopedia. Available online: https://encyclopedia.pub/entry/39443 (accessed on 07 February 2026).
Agarwal N, Solanki VS, Gacem A, Hasan MA, Pare B, Srivastava A, et al. Bacterial Laccases as Biocatalysts for Environmental Toxic Pollutants. Encyclopedia. Available at: https://encyclopedia.pub/entry/39443. Accessed February 07, 2026.
Agarwal, Neha, Vijendra Singh Solanki, Amel Gacem, Mohd Abul Hasan, Brijesh Pare, Amrita Srivastava, Anupama Singh, Virendra Kumar Yadav, Krishna Kumar Yadav, Chaigoo Lee, et al. "Bacterial Laccases as Biocatalysts for Environmental Toxic Pollutants" Encyclopedia, https://encyclopedia.pub/entry/39443 (accessed February 07, 2026).
Agarwal, N., Solanki, V.S., Gacem, A., Hasan, M.A., Pare, B., Srivastava, A., Singh, A., Yadav, V.K., Yadav, K.K., Lee, C., Lee, W., Chaiprapat, S., & Jeon, B. (2022, December 27). Bacterial Laccases as Biocatalysts for Environmental Toxic Pollutants. In Encyclopedia. https://encyclopedia.pub/entry/39443
Agarwal, Neha, et al. "Bacterial Laccases as Biocatalysts for Environmental Toxic Pollutants." Encyclopedia. Web. 27 December, 2022.
Copy Citation
Laccases are the potential enzymes for oxidoreductases (a broad group of enzymes that catalyze electron transfers from one molecule to another), which are widely distributed in nature in plants, bacteria, fungi, and insects. They are suitable for green catalysis, organic synthesis, and the biodegradation of environmental xenobiotics due to their high efficiency and sustainable applications. A wide variety of organic compounds can be oxidized by laccase, and they can be widely applied in the biodegradation of pollutants for detoxification of environments, such as delignification and pulp-bleaching, treatment of textile dyes, wastewater treatment, and treatment of other environmental xenobiotics.
biodegradation
laccases
oxidoreductases
green biocatalysts
1. Bacterial Laccases
1.1. Sources and Evolution
The bacterial laccase was first reported in Azospirillum lipoferum, which was isolated from the rhizosphere (plant root) [1]. Since then, most of the identified laccases have belonged to the Bacillus and Streptomyces genera, such as Bacillus subtilis, Bordetella campestris, Caulobacter crescentus, E. coli, Mycobacterium tuberculosum, Yersinia pestis, etc. [2][3]. Streptomyces laccases work on pigmentation, antibiotics, and morphogenesis, and they are helpful in lignin degradation [4]. Many species have also been reported for the detoxication of post-methanated distillery effluents and pulp–paper waste, which contains chlorolignin [5][6]. According to a recent study, Bacillus atrophaeus laccases’ genes were coded, which corresponds to a protein with 278 amino acids [7]. Many species of laccase-producing bacteria were reported in the last decade, including Bacillus [8], Pseudomonas species [9], the Geobacillus species [10], Marinomonas mediterrânea, and Pseudomonas putida [11]. The latest research isolated different strains of laccase-producing bacteria in waste released from the soap industry [12]. The researchers emphasized that the bacterial species may be of significant importance commercially in producing laccase during the scaling-up process at the bioreactor level.
1.2. Production Conditions, Properties, Substrates, and Mediators
Some species of bacteria, such as the Streptomyces sp., are known to produce extracellular laccases that are useful in micropollutant degradations [13]. One of the cheaper and more easily available substrates for laccase production is agricultural waste, such as rice bran, banana peel, and sawdust. The production of laccase is significantly affected by the optimum growth time, the intensity of light, the optimum pH and shaking conditions, and the amount of dissolved oxygen and organic salts, though different microorganisms require different amounts of time for an optimum yield of laccase [14] (Figure 1).
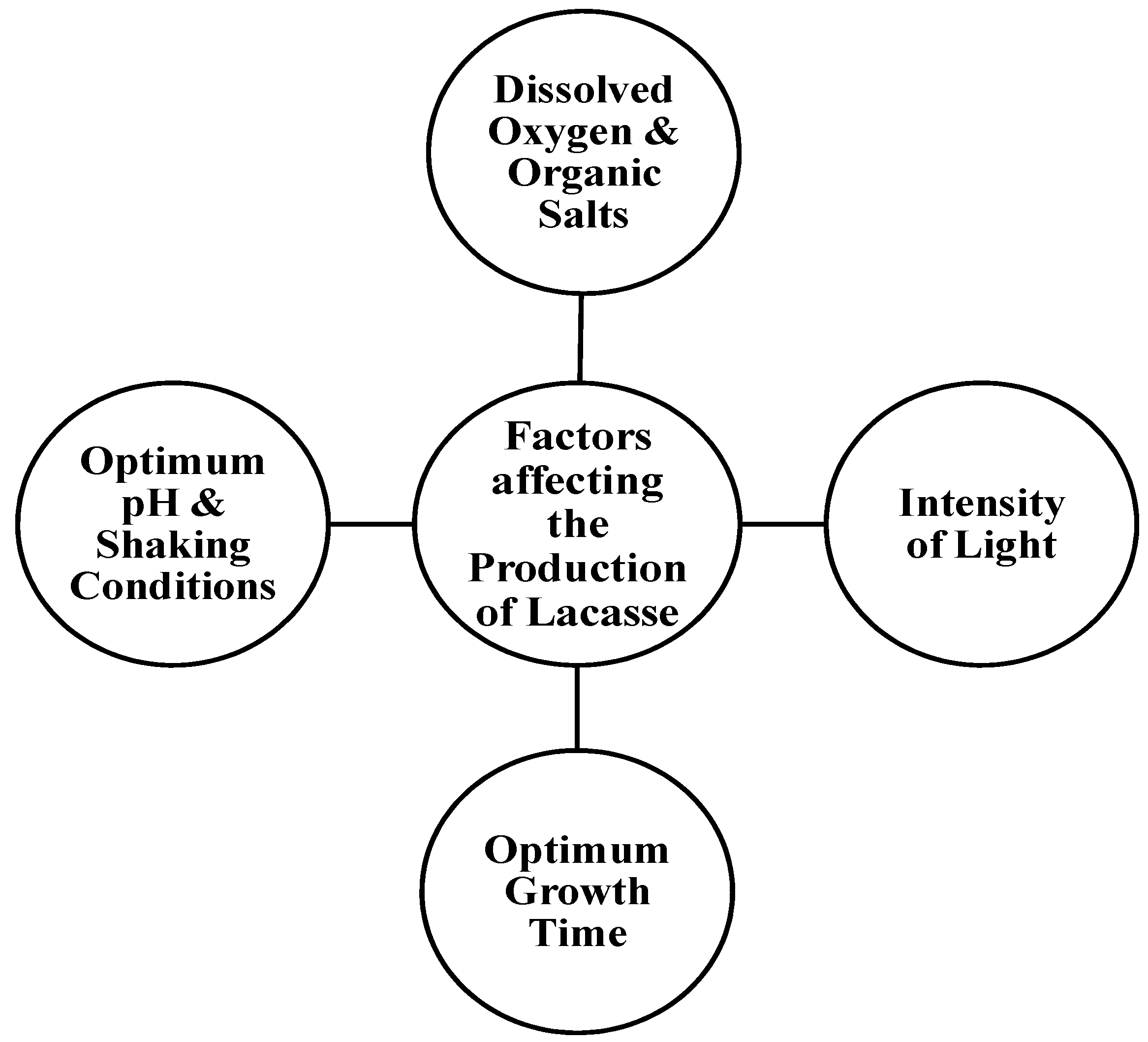
Figure 1. Factors affecting the production of Laccase.
A wide range of molecules can be oxidized by laccases, and more than a hundred compounds have been identified as substrates for laccases; however, it is difficult to oxidize all types of substrates directly by laccases due to their large sizes, which obstruct their penetration into the active site of the enzyme, and their high redox potentials. To remove this difficulty, many chemical mediators that are suitably oxidized by laccase are used, and, eventually, the oxidized forms are able to interact with the substrate with high redox potential. Bacterial laccases comprise enzymes with low redox potential, from 0.4 to 0.5 V, which can withstand more difficult conditions than fungal laccases [6].
Laccase is a type of enzyme that is substrate-specific, which oxidizes a wide range of substrates, acts as a biocatalyst in the synthesis of organic compounds, and stops reactions of many aromatic organic contaminants. The degradation of highly toxic contaminants leads to a green and eco-friendly environment, and the organic synthesis via the production of nonhazardous by-products leads to bioremediation [14][15]. Substrates such as 2,2 ′-azino-bis (3-ethylbenzothiazoline6-sulfonic acid), 2,6-dimethylphenol, syringaldazine, guaiacol, etc. are extensively used and are the most commonly used substrates for enzyme assays. The binding of the substrate with protein by using syringaldazine is shown in Figure 2.
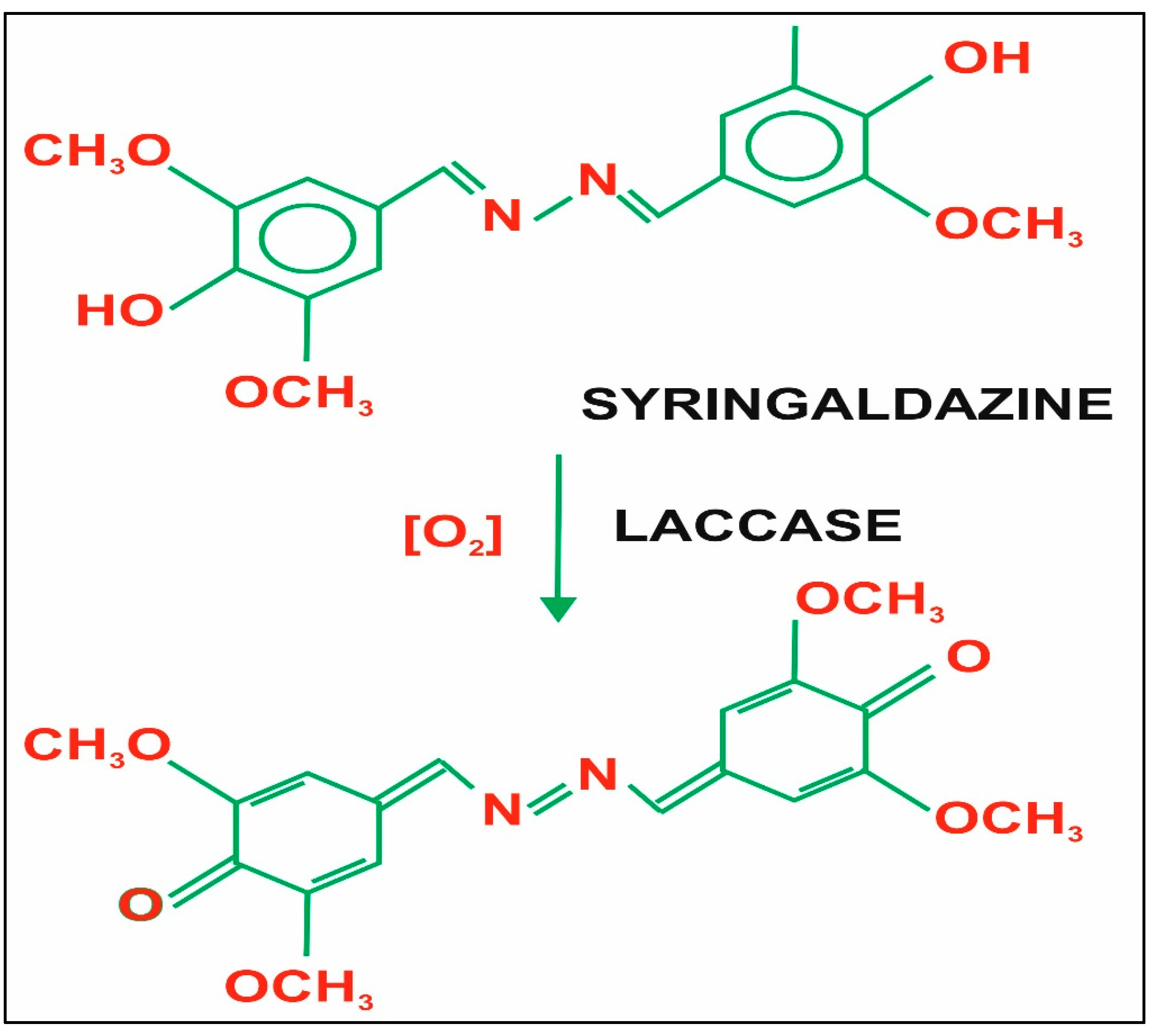
Figure 2. Structure of the substrate syringaldazine [16].
Substrates with a large redox potential, such as azo dyes, anthraquinolic dyes, etc., are not degraded or oxidized by laccase, directly. These kinds of substrates require an electron shuttle mediator between the laccase and themselves [17]. Basically, the mediators are those low molecular weight laccase substrates whose enzymatic oxidation produces stable intermediates with high oxidation potential. The first kind of such a synthetic mediator was 2,2′-azino-bis (3-ethylbenzothiazoline6-sulfonic acid), which is used to function as a laccase substrate mediator with enhanced enzyme action [18].
In liquid media, the growth of bacteria is usually faster than that of fungi, which favors scaling-up processes for the production of laccase [19]. In different bacteria, different inorganic metals and detergents affect the yield of laccase distinctly. In many cases, inorganic metals (Mg+2, Hg+2, and Zn+2) highly inhibit the activity by changing the protein conformation, indicating that the enzymatic yield of laccase was not dependent on positively charged metals [20]. According to one hypothesis, Hg+2 decreases the yield of laccase, which shows the important role of a thiol-possessing amino acid in laccase activity [21][22].
2. Applications of Laccases
Laccases as biocatalysts are gaining popularity in different fields from the application point of view. Laccases are potential green, biological tools that work efficiently in the air and release water as the only by-product. Hence, laccases, especially bacterial and fungal laccases, have wide applications when applied in different areas (Figure 3).
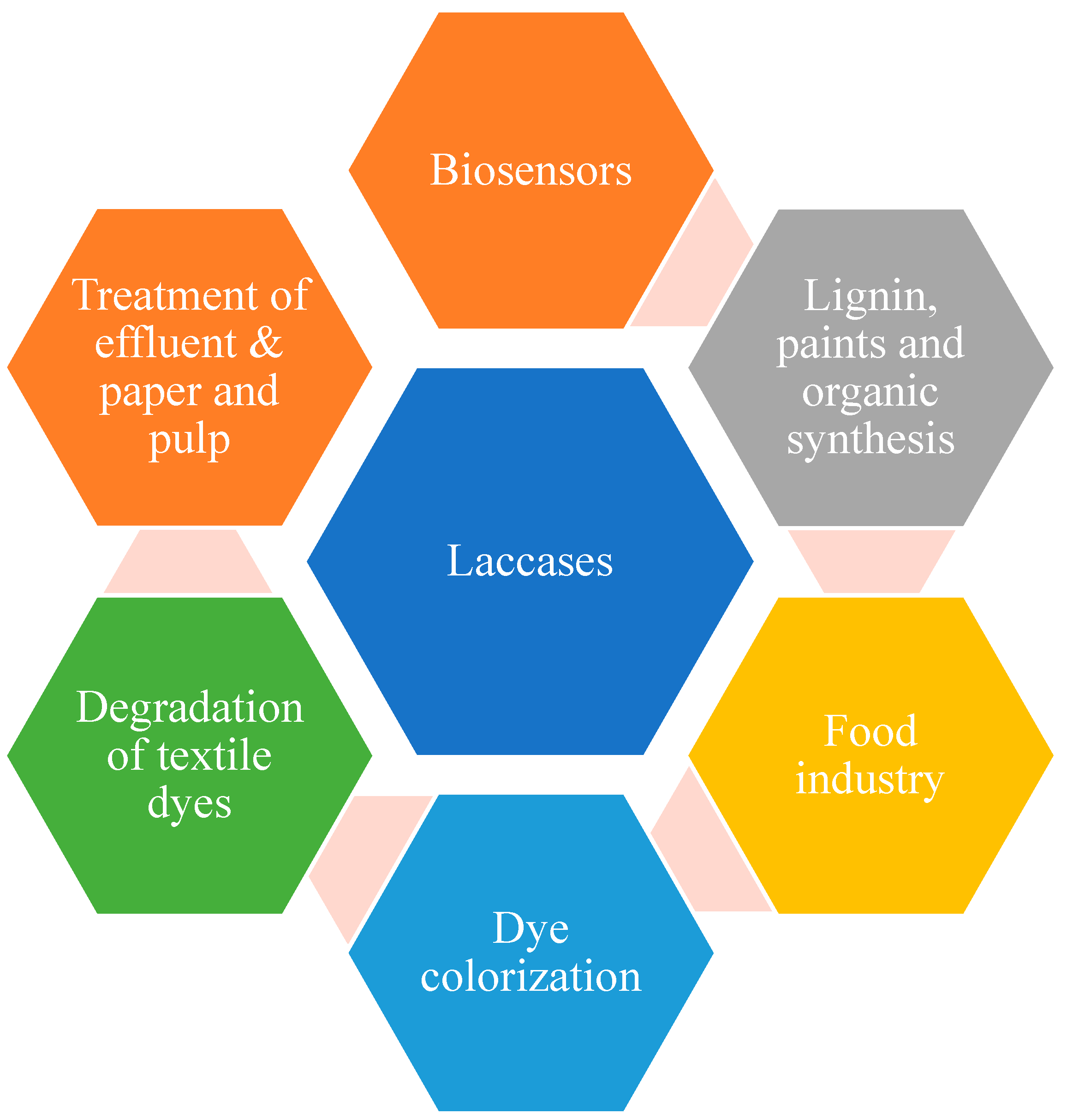
Figure 3. Applications of laccases.
2.1. Detoxification and Bioremediation of Industrial Effluents
Bacterial laccases have the capability of oxidizing all kinds of substrates, whether toxic or non-toxic. Various effluents from industries such as the pulp–paper, textile, pharmaceutical, distillery, and food industries are also treated by bacterial laccases. In the pulp–paper industry, laccase has become known for the removal of black liquor and the wastewater produced from pulp–paper mills [23]. Laccases work on phenolic lignin fragments in which the substrate reacts with the lignin polymer, resulting in the degradation of the lignin. Though decolorization by chemical bleaching is very effective, these methods have serious drawbacks due to the release of toxic byproducts. Delignification by laccase systems is a better method for reducing toxicity, and it has been adapted to current pulp production lines as a green and sustainable alternative.
2.2. Textile Dye Degradation and Decolorization
Chemicals of diverse compositions, ranging from organic to polymer products, are used in the textile industry. The chemical structure of dyes makes them fade-resistant on exposure to chemicals, heat, light, and water, and synthetic dyes hardly decolorize due to their synthetic nature. Many dyes are prepared from aromatic organic compounds, such as benzidine, which are highly carcinogenic [23]. Consequently, the textile industry’s effluents, when disposed of in water, reduce light penetration into the water and strongly affect the photosynthetic process of green aquatic plants [24].
The contaminated water may be carcinogenic and pose threats to the environment and marine life due to the presence of degraded dye products, metals, halogens, etc. [25][26][27]. The laccases are considered promising solutions for chemically diversified dyes, including synthetic dyes [28][29]. The released reactive dyestuff can be bleached quickly by laccase as a part of the washing solution, which results in less processing time, cost, energy, and volume of water required for the desired quality of textile [30]. Laccases have been widely studied for the degradation of azo dyes [31][32]. The decolorization of some synthetic dyes, such as methyl orange, Congo red, methylene, and toluidine blue, etc., and the industrial effluents were achieved by the bacterial species S. maltophilia AAP56 [33].
2.3. Bioremediation of Food Industry Wastewater Effluents
The wastewater effluents released from the food industry contain a remarkable number of aromatic compounds, especially phenols, which have toxic effects on health [34]. According to a study, approximately 40–90% of phenolic compounds are removed in a co-immobilized form by 95% of the laccase units in a bioreactor [35]. Organic gel-trapped laccase removes organic aromatic compounds from aqueous suspensions, and the enzyme is reused without any efficiency loss [36]. Dark brown wastewater released from beer factories has a high concentration of polyphenols in bioremediation via C. gallica [37]. Sugarcane factories release vinasse as a by-product in the production of ethanol, which contains toxic organic matter and is also treated by the laccase from T. versicolor [32][38].
2.4. Other Applications
Many reports have shown that xenobiotics can also be degraded by laccases. According to a recent report, the bacterial laccase CueO’s mutations of chemical plant sludge displayed that the mutants G276R, G276N, G276Y, and G276K can oxidize the carcinogen benzo[ɑ]pyrene very efficiently [39]. The degradation of Tyramine (a toxic compound in food) by laccases can resolve the problems generated in food. In addition to the applications discussed above, laccases are also used in the production of polymers [40], indo-dye synthesis [41], biosensors, and bioremediation [42][43]. Laccases are green catalytic enzymes with great potential for the biodegradation of environmental xenobiotics. They have great potential for biotechnological applications, such as biosensors, biopulping, biobleaching, organic synthesis, biofuels, antimicrobial applications, etc. Laccases are currently being represented as the latest topic of research for the biodegradation of xenobiotic compounds, pharmaceutical products, and different dyes in an eco-friendly manner [44][45][46][47][48][49][50].
References
- Mukhopadhyay, A.; Dasgupta, A.K.; Chakrabarti, K. Thermostability, ph stability and dye degrading activity of a bacterial laccase are enhanced in the presence of Cu2o nanoparticles. Bioresour. Technol. 2013, 127, 25–36.
- Givaudan, A.; Effosse, A.; Faure, D.; Potier, P.; Bouillant, M.-L.; Bally, R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol. Lett. 1993, 108, 205–210.
- Enguita, F.J.; Martins, L.O.; Henriques, A.O.; Carrondo, M.A. Crystal structure of a bacterial endospore coat component: A laccase with enhanced thermostability properties. J. Biol. Chem. 2003, 278, 19416–19425.
- Bains, J.; Capalash, N.; Sharma, P. Laccase from a non-melanogenic, alkalotolerant γ-proteobacterium JB isolated from industrial wastewater drained soil. Biotechnol. Lett. 2003, 25, 1155–1159.
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966.
- Yadav, S.; Chandra, R. Biodegradation of organic compounds of molasses melanoidin (MM) from biomethanated distillery spent wash (BMDS) during the decolourisation by a potential bacterial consortium. Biodegradation 2012, 23, 609–620.
- Chandra, R.; Singh, R. Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem. Eng. J. 2012, 61, 49–58.
- Narnoliya, L.K.; Agarwal, N.; Patel, S.N.; Singh, S.P. Kinetic characterization of laccase from Bacillus atrophaeus, and its potential in juice clarification in free and immobilized forms. J. Microbiol. 2019, 57, 900–909.
- Singh, D.; Sharma, K.K.; Jacob, S.; Gakhar, S.K. Molecular docking of laccase protein from Bacillus safensis DSKK5 isolated from earthworm gut: A novel method to study dye decolorization potential. Water Air Soil Pollut. 2014, 225, 2175.
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial laccase: Recent update on production, properties and industrial applications. 3 Biotech 2017, 7, 323.
- Jeon, S.J.; Park, J.H. Refolding, characterization, and dye decolorization ability of a highly thermostable laccase from Geobacillus sp. JS12. Protein Expr. Purif. 2020, 173, 105646.
- Road, H. Production and purification strategies for laccase. Int. J. Pharm. Sci. Res. 2020, 11, 2617–2625.
- Karuna, D.; Poonam, S. Production, partial purification and characterization of laccase from rhizospheric bacteria Pseudomonas putida strain LUA15.1. Res. J. Biotechnol. 2020, 15, 144–152.
- Deepa, T.; Gangwane, A.K.; Sayyed, R.Z.; Jadhav, H.P. Optimization and scale-up of laccase production by Bacillus sp. BAB-4151 isolated from the waste of the soap industry. Environ. Sustain. 2020, 3, 471–479.
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830.
- Guan, Z.B.; Luo, Q.; Wang, H.R.; Chen, Y.; Liao, X.R. Bacterial laccases: Promising biological green tools for industrial applications. Cell. Mol. Life Sci. 2018, 75, 3569–3592.
- Rezaei, S.; Shahverdi, A.R.; Faramarzi, M.A. Isolation, one-step affinity purification, and characterization of a polyextremotolerantlaccase from the halophilic bacterium Aquisalibacillus elongatus and its application in the delignification of sugar beet pulp. Bioresour. Technol. 2017, 230, 67–75.
- Diamantidis, G.; Effosse, A.; Potier, P.; Bally, R. Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol. Biochem. 2000, 32, 919–927.
- Kalme, S.; Jadhav, S.; Jadhav, M.; Govindwar, S. Textile dye degrading laccase from Pseudomonas desmolyticum NCIM 2112. Enzym. Microb. Technol. 2008, 44, 65–71.
- Trott, O.; Olson, A.J. Auto Dock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461.
- Arora, D.; Sharma, R. Ligninolytic fungal laccases and their biotechnological applications. Appl. Biochem. Biotechnol. 2010, 160, 1760–1788.
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467.
- Bisht, S.; Pandey, P.; Bhargava, B.; Sharma, S.; Sharma, K.D. Bioremediation of Polyaromatic Hydrocarbons (PAHs) using rhixosphere technology. Braz. J. Microbiol. 2015, 46, 7–21.
- Atlas, R.M. Microbial Degradation of Petroleum Hydrocarbons: An Environmental Perspective. Microbiol. Rev. 1981, 45, 180–209.
- Couto, S.R. Dye removal by immobilised fungi. Biotechnol. Adv. 2009, 27, 227–235.
- Baughman, G.L.; Perenich, T.A. Fate of dyes in aquatic systems: I solubility and partitioning of some hydrophobic dyes and related compounds. Environ. Toxicol. Chem. 1988, 7, 183–199.
- Guan, Z.B.; Shui, Y.; Song, C.M.; Zhang, N.; Cai, Y.J.; Liao, X.R. Efcient secretory production of CotA-laccase and its application in the decolorization and detoxifcation of industrial textile wastewater. Environ. Sci. Pollut. Res. 2015, 22, 9515–9523.
- Khlif, R.; Belbahri, L.; Woodward, S.; Ellouz, M.; Dhouib, A.; Sayadi, S.; Mechichi, T. Decolourization and detoxifcation of textile industry wastewater by the laccase-mediator system. J. Hazard. Mater. 2010, 175, 802–808.
- Singh, G.; Bhalla, A.; Kaur, P.; Capalash, N.; Sharma, P. Laccase from prokaryotes: A new source for an old enzyme. Rev. Environ. Sci. Biotechnol. 2011, 10, 309–326.
- Hou, H.; Zhou, J.; Wang, C.; Yan, B. Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem. 2004, 39, 1415–1419.
- Dominguez, A.; Couto, S.R.; Sanoroman, M.A. Dye decolourisation by Trametes histuta immobilized into alginate beads. World J. Biotechnol. 2005, 21, 405–409.
- Juang, R.S.; Tseng, R.L.; Wu, F.C.; Lin, S.J. Use of chitin and chitosan in lobster shell wastes for color removal from aqueous solutions. J. Environ. Sci. Eng. 1996, 31, 325–338.
- Chivukula, M.; Renganathan, V. Phenolic Azo Dye Oxidation by Laccase from Pyricularia oryzae. Appl. Environ. Microbiol. 1995, 61, 4374–4377.
- Blanquez, P.; Casas, N.; Font, X.; Gabarrell, X.; Sarr’a, M.; Caminal, G.; Vicent, T. Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res. 2004, 38, 2166–2172.
- Dube, E.; Shareck, F.; Hurtubise, Y.; Daneault, C.; Beauregard, M. Homologous cloning, expression and charatterization of a laccase from Streptomyces coelicolor and enzymatic decolorization of an indigo dye. Appl. Microbiol. Biotechnol. 2008, 79, 597–603.
- Osma, J.F.; Toca-Herrera, J.L.; RodrÂguez-Couto, S. Uses of laccases in the food industry. Enzym. Res. 2010, 2010, 918761.
- Krastanov, A. Removal of phenols from mixtures by coimmobilized laccase-tyrosinase and Polyclar adsorption. J. Ind. Microbiol. Biotechnol. 2000, 24, 383–388.
- Lante, A.; Crapisi, A.; Krastanov, A.; Spettoli, P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochem. 2000, 36, 51–58.
- Yadav, V.K.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Algahtani, A.; et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials 2022, 15, 953.
- Brenna, O.; Bianchi, E. Immobilized laccase for phenolic removal in must and wine. Biotechnol. Lett. 1994, 16, 35–40.
- Yue, Q.; Yang, Y.; Zhao, J.; Zhang, L.; Xu, L.; Chu, X.; Liu, X.; Tian, J.; Wu, N. Identification of bacterial laccase cueO mutation from the metagenome of chemical plant sludge. Bioresour. Bioprocess 2017, 4, 48–56.
- Lončar, N.; Božić, N.; Vujčić, Z. Expression and characterization of a thermostable organic solvent-tolerant laccase from Bacillus licheniformis ATCC 9945a. J. Mol. Catal. B Enzym. 2016, 134, 390–395.
- Sousa, A.C.; Piedade, M.F.M.D.; Martins, L.O.; Robalo, M.P.A. Eco-friendly synthesis of indo dyes mediated by a bacterial laccase. Green Chem. 2016, 18, 6063–6070.
- Jia, L.; Fei, R.; Zhang, X.; Tang, H.; Hu, Y. Sustainable endospore-based microreactor system for antioxidant capacity assay. Anal. Chem. 2014, 86, 11578–11585.
- Zhiming, Z.; Longjian, T.; Zheng, L.; Lina, J.; Xinya, Z.; Miaomiao, X.; Yonggang, H. Whole-cell method for phenol detection based on the color reaction of phenol with 4-aminoantipyrine catalyzed by CotA laccase on endospore surfaces. Biosens. Bioelectron. 2015, 69, 162–166.
- Dana, M.; Khaniki, G.B.; Mokhtarieh, A.A.; Davarpanah, S.J. Biotechnological and Industrial Applications of Laccase: A Review. J. Appl. Biotechnol. Rep. 2017, 4, 675–679.
- Arregui, L.; Ayala, M.; Gomez Gil, X.; Gutierrez Soto, G.; Hernandez Luna, C.E.; de los Santos, M.H.; Levin, L.; Rojo Dominguez, A.; Romero Martinez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell. Fact. 2019, 18, 200.
- Debnath, R.; Saha, T. An insight into the production strategies and applications of the ligninolytic enzyme laccase from bacteria and fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645.
- Patel, H.; Yadav, V.K.; Yadav, K.K.; Choudhary, N.; Kalasariya, H.; Alam, M.M.; Gacem, A.; Amanullah, M.; Ibrahium, H.A.; Park, J.-W.; et al. A Recent and Systemic Approach Towards Microbial Biodegradation of Dyes from Textile Industries. Water 2022, 14, 3163.
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-u.; Ahn, Y.; Son, C.T.; et al. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
28 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




