Laccases are the potential enzymes for oxidoreductases (a broad group of enzymes that catalyze electron transfers from one molecule to another), which are widely distributed in nature in plants, bacteria, fungi, and insects. They are suitable for green catalysis, organic synthesis, and the biodegradation of environmental xenobiotics due to their high efficiency and sustainable applications. A wide variety of organic compounds can be oxidized by laccase, and they can be widely applied in the biodegradation of pollutants for detoxification of environments, such as delignification and pulp-bleaching, treatment of textile dyes, wastewater treatment, and treatment of other environmental xenobiotics.
1. Bacterial Laccases
1.1. Sources and Evolution
The bacterial laccase was first reported in
Azospirillum lipoferum, which was isolated from the rhizosphere (plant root) [
25]. Since then, most of the identified laccases have belonged to the Bacillus and Streptomyces genera, such as
Bacillus subtilis,
Bordetella campestris,
Caulobacter crescentus,
E. coli,
Mycobacterium tuberculosum,
Yersinia pestis, etc. [
26,
27]. Streptomyces laccases work on pigmentation, antibiotics, and morphogenesis, and they are helpful in lignin degradation [
28]. Many species have also been reported for the detoxication of post-methanated distillery effluents and pulp–paper waste, which contains chlorolignin [
29,
30]. According to a recent study,
Bacillus atrophaeus laccases’ genes were coded, which corresponds to a protein with 278 amino acids [
31]. Many species of laccase-producing bacteria were reported in the last decade, including
Bacillus [
32],
Pseudomonas species [
33], the
Geobacillus species [
34],
Marinomonas mediterrânea, and
Pseudomonas putida [
35]. The latest research isolated different strains of laccase-producing bacteria in waste released from the soap industry [
36]. The researchers emphasized that the bacterial species may be of significant importance commercially in producing laccase during the scaling-up process at the bioreactor level.
1.2. Production Conditions, Properties, Substrates, and Mediators
Some species of bacteria, such as the
Streptomyces sp., are known to produce extracellular laccases that are useful in micropollutant degradations [
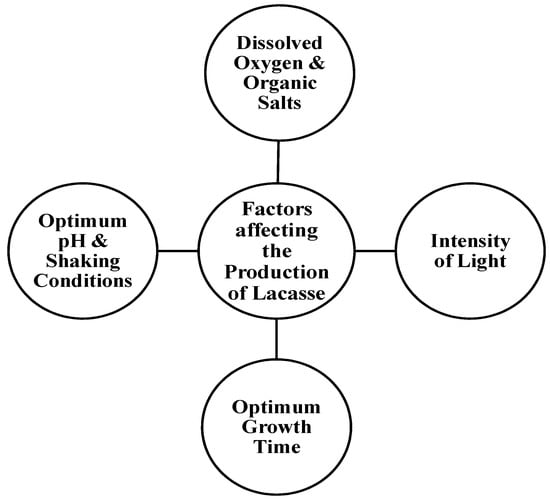
37]. One of the cheaper and more easily available substrates for laccase production is agricultural waste, such as rice bran, banana peel, and sawdust. The production of laccase is significantly affected by the optimum growth time, the intensity of light, the optimum pH and shaking conditions, and the amount of dissolved oxygen and organic salts, though different microorganisms require different amounts of time for an optimum yield of laccase [
38] (
Figure 2).
Figure 2. Factors affecting the production of Laccase.
A wide range of molecules can be oxidized by laccases, and more than a hundred compounds have been identified as substrates for laccases; however, it is difficult to oxidize all types of substrates directly by laccases due to their large sizes, which obstruct their penetration into the active site of the enzyme, and their high redox potentials. To remove this difficulty, many chemical mediators that are suitably oxidized by laccase are used, and, eventually, the oxidized forms are able to interact with the substrate with high redox potential. Bacterial laccases comprise enzymes with low redox potential, from 0.4 to 0.5 V, which can withstand more difficult conditions than fungal laccases [
30].
Laccase is a type of enzyme that is substrate-specific, which oxidizes a wide range of substrates, acts as a biocatalyst in the synthesis of organic compounds, and stops reactions of many aromatic organic contaminants. The degradation of highly toxic contaminants leads to a green and eco-friendly environment, and the organic synthesis via the production of nonhazardous by-products leads to bioremediation [
38,
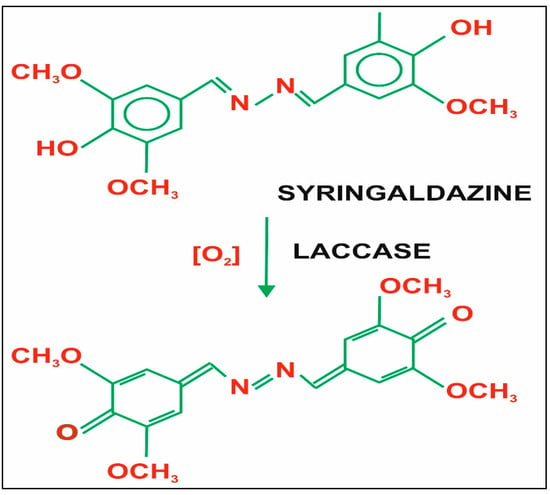
39]. Substrates such as 2,2 ′-azino-bis (3-ethylbenzothiazoline6-sulfonic acid), 2,6-dimethylphenol, syringaldazine, guaiacol, etc. are extensively used and are the most commonly used substrates for enzyme assays. The binding of the substrate with protein by using syringaldazine is shown in
Figure 3.
Figure 3. Structure of the substrate syringaldazine [
55].
Substrates with a large redox potential, such as azo dyes, anthraquinolic dyes, etc., are not degraded or oxidized by laccase, directly. These kinds of substrates require an electron shuttle mediator between the laccase and themselves [
40]. Basically, the mediators are those low molecular weight laccase substrates whose enzymatic oxidation produces stable intermediates with high oxidation potential. The first kind of such a synthetic mediator was 2,2′-azino-bis (3-ethylbenzothiazoline6-sulfonic acid), which is used to function as a laccase substrate mediator with enhanced enzyme action [
41].
In liquid media, the growth of bacteria is usually faster than that of fungi, which favors scaling-up processes for the production of laccase [
56]. In different bacteria, different inorganic metals and detergents affect the yield of laccase distinctly. In many cases, inorganic metals (Mg
+2, Hg
+2, and Zn
+2) highly inhibit the activity by changing the protein conformation, indicating that the enzymatic yield of laccase was not dependent on positively charged metals [
57]. According to one hypothesis, Hg
+2 decreases the yield of laccase, which shows the important role of a thiol-possessing amino acid in laccase activity [
58,
59].
2. Applications of Laccases
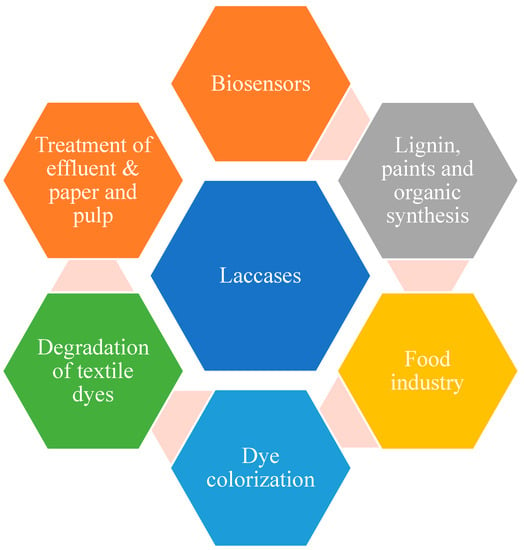
Laccases as biocatalysts are gaining popularity in different fields from the application point of view. Laccases are potential green, biological tools that work efficiently in the air and release water as the only by-product. Hence, laccases, especially bacterial and fungal laccases, have wide applications when applied in different areas (Figure 5).
Figure 5. Applications of laccases.
2.1. Detoxification and Bioremediation of Industrial Effluents
Bacterial laccases have the capability of oxidizing all kinds of substrates, whether toxic or non-toxic. Various effluents from industries such as the pulp–paper, textile, pharmaceutical, distillery, and food industries are also treated by bacterial laccases. In the pulp–paper industry, laccase has become known for the removal of black liquor and the wastewater produced from pulp–paper mills [
65]. Laccases work on phenolic lignin fragments in which the substrate reacts with the lignin polymer, resulting in the degradation of the lignin. Though decolorization by chemical bleaching is very effective, these methods have serious drawbacks due to the release of toxic byproducts. Delignification by laccase systems is a better method for reducing toxicity, and it has been adapted to current pulp production lines as a green and sustainable alternative.
2.2. Textile Dye Degradation and Decolorization
Chemicals of diverse compositions, ranging from organic to polymer products, are used in the textile industry. The chemical structure of dyes makes them fade-resistant on exposure to chemicals, heat, light, and water, and synthetic dyes hardly decolorize due to their synthetic nature. Many dyes are prepared from aromatic organic compounds, such as benzidine, which are highly carcinogenic [
65]. Consequently, the textile industry’s effluents, when disposed of in water, reduce light penetration into the water and strongly affect the photosynthetic process of green aquatic plants [
66].
The contaminated water may be carcinogenic and pose threats to the environment and marine life due to the presence of degraded dye products, metals, halogens, etc. [
67,
68,
69]. The laccases are considered promising solutions for chemically diversified dyes, including synthetic dyes [
70,
71]. The released reactive dyestuff can be bleached quickly by laccase as a part of the washing solution, which results in less processing time, cost, energy, and volume of water required for the desired quality of textile [
72]. Laccases have been widely studied for the degradation of azo dyes [
73,
74]. The decolorization of some synthetic dyes, such as methyl orange, Congo red, methylene, and toluidine blue, etc., and the industrial effluents were achieved by the bacterial species
S. maltophilia AAP56 [
75].
2.3. Bioremediation of Food Industry Wastewater Effluents
The wastewater effluents released from the food industry contain a remarkable number of aromatic compounds, especially phenols, which have toxic effects on health [
76]. According to a study, approximately 40–90% of phenolic compounds are removed in a co-immobilized form by 95% of the laccase units in a bioreactor [
77]. Organic gel-trapped laccase removes organic aromatic compounds from aqueous suspensions, and the enzyme is reused without any efficiency loss [
78]. Dark brown wastewater released from beer factories has a high concentration of polyphenols in bioremediation via
C. gallica [
79]. Sugarcane factories release vinasse as a by-product in the production of ethanol, which contains toxic organic matter and is also treated by the laccase from
T. versicolor [
74,
80].
2.4. Other Applications
Many reports have shown that xenobiotics can also be degraded by laccases. According to a recent report, the bacterial laccase CueO’s mutations of chemical plant sludge displayed that the mutants G276R, G276N, G276Y, and G276K can oxidize the carcinogen benzo[
ɑ]pyrene very efficiently [
81]. The degradation of Tyramine (a toxic compound in food) by laccases can resolve the problems generated in food. In addition to the applications discussed above, laccases are also used in the production of polymers [
82], indo-dye synthesis [
83], biosensors, and bioremediation [
84,
85]. Laccases are green catalytic enzymes with great potential for the biodegradation of environmental xenobiotics. They have great potential for biotechnological applications, such as biosensors, biopulping, biobleaching, organic synthesis, biofuels, antimicrobial applications, etc. Laccases are currently being represented as the latest topic of research for the biodegradation of xenobiotic compounds, pharmaceutical products, and different dyes in an eco-friendly manner [
86,
87,
88,
89,
90,
91,
92].
This entry is adapted from the peer-reviewed paper 10.3390/w14244068