Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahesh Kandasamy | -- | 2319 | 2022-12-21 18:04:10 | | | |
| 2 | Camila Xu | Meta information modification | 2319 | 2022-12-22 05:10:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ravichandran, S.; Suhasini, R.; Deepa, S.M.; Selvaraj, D.B.; Andrews, J.F.V.; Thiagarajan, V.; Kandasamy, M. Aberrant Circadian Rhythm in Huntington’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/39054 (accessed on 06 March 2026).
Ravichandran S, Suhasini R, Deepa SM, Selvaraj DB, Andrews JFV, Thiagarajan V, et al. Aberrant Circadian Rhythm in Huntington’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/39054. Accessed March 06, 2026.
Ravichandran, Sowbarnika, Ramalingam Suhasini, Sudhiksha Madheswaran Deepa, Divya Bharathi Selvaraj, Jemi Feiona Vergil Andrews, Viruthachalam Thiagarajan, Mahesh Kandasamy. "Aberrant Circadian Rhythm in Huntington’s Disease" Encyclopedia, https://encyclopedia.pub/entry/39054 (accessed March 06, 2026).
Ravichandran, S., Suhasini, R., Deepa, S.M., Selvaraj, D.B., Andrews, J.F.V., Thiagarajan, V., & Kandasamy, M. (2022, December 21). Aberrant Circadian Rhythm in Huntington’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/39054
Ravichandran, Sowbarnika, et al. "Aberrant Circadian Rhythm in Huntington’s Disease." Encyclopedia. Web. 21 December, 2022.
Copy Citation
Huntington’s disease (HD) is a progressive neurodegenerative disorder characterized by abnormal progressive involuntary movements, cognitive deficits, sleep disturbances, and psychiatric symptoms. Notably, gamma-aminobutyric acid (GABA)-ergic neurons that express the vasoactive intestinal peptide (VIP) in the brain play a key role in the regulation of circadian rhythm and neuroplasticity. While an abnormal clock gene pathway has been associated with the inactivation of GABAergic VIP neurons, recent studies suggest the activation of this neuronal population in the brain positively contributes to neuroplasticity.
Huntington’s disease
circadian rhythm
clock genes
1. Introduction
Huntington’s disease (HD) is an autosomal dominant hereditary neurodegenerative disorder that affects the structure and functions of the basal ganglia of the brain [1]. The progressive degeneration of gamma-aminobutyric acid (GABA)-ergic medium spiny (MSN) neurons in the brains of subjects with HD has been attributed to the expansion of polyglutamine (poly Q) segments in the huntingtin (HTT) protein resulting from more than 40 CAG repeats in the exon1 of the HTT gene [2][3][4]. Clinically, HD has been characterized by abnormal involuntary movements, neurocognitive impairments, and psychiatric disturbances [5]. In addition, abnormal sleep–wake cycles accounting for the abnormal circadian rhythm have been identified as non-motor clinical symptoms of HD [6]. Around 90% of HD subjects have been reported to suffer from sleep disturbances [7]. Chronic sleep disturbances appear to be detrimental to the neuroplasticity responsible for neurocognitive functions [8]. Ample research reports indicate that the occurrence of neurogenesis in the hippocampus in the brains of healthy subjects contributes to learning, memory, and mood [9]. Whereas neurogenic failure in the hippocampus has been considered an underlying cellular basis of neurocognitive decline in many neurodegenerative disorders, including HD [10][11]. While the expression of mutant HTT gene causes aberrant gliogenic events, the neurogenic potential of neural stem cells (NSCs) and survival of new-born neurons in different brain regions including the hippocampus have been reported to be drastically impaired in experimental models of HD and post-mortem human HD brains [12][13][14][15][16][17]. The underlying molecular mechanism for the impaired proliferative and differentiation potentials of NSCs in HD brains remains obscure. In a physiological state, circadian clock genes play important roles in the regulation of NSC-derived neurogenesis, whereas impairment in the neurogenic process has been linked to the irregular circadian clock pathway [18][19]. The expression of the mutant HTT gene interrupts the regulation and functions of the clock genes, thereby leading to the aberrant circadian rhythm in HD [7][20][21]. Therefore, the abnormal regulation of hippocampal neurogenesis and an irregular circadian rhythm may overlap and can collectively contribute to intertwining pathogenicity leading to psychiatric disturbances and cognitive deficits in HD. Considering the facts, it can be proposed that the reversal of an irregular circadian rhythm might contribute to repair mechanisms of the brain and regenerative plasticity in HD. Therefore, the identification of the prominent molecular pathway and cellular system involved in the regulation of circadian rhythm could serve as a potential therapeutic target in HD.
GABAergic vasoactive intestinal peptide (VIP)-expressing neurons in the suprachiasmatic nucleus (SCN) of the hypothalamus play a key role in the regulation of circadian rhythm. Neuropathogenic events mediated degeneration or functional defects in the VIP neurons of SCN and improper sensory inputs can trigger abnormal circadian rhythmicity in various brain diseases [22][23][24]. These VIP neurons play an important role in the control of GABAergic transmission responsible for the synaptic plasticity of the pyramidal neurons in the hippocampus [25][26]. Thus, the dysregulation of GABAergic transmission resulting from the mutant HTT protein might overlap with the altered expression and functions of VIP leading to neuroregenerative failure in HD. Therefore, the implementation of therapeutic strategies that aid in the restoration or activation of VIP neurons in the brain could contribute to rectifying sleep disorder in HD. Chemogenetics has been established as a potent molecular tool to specifically regulate the intracellular-signaling pathways in tissue and organs [27][28]. The chemogenetic-based approaches provide hope to mitigate the abnormal circadian clock pathways which may be coupled with improving neuroregeneration in the brain [29].
2. Regulation of Circadian Rhythm in Physiological State
Circadian rhythm represents a biological chronometer of the living system that regulates, intertwines, overlaps, and synchronizes various physiological, biochemical, cellular, and genetic events, in response to the gut–brain axis, atmospheric temperature, and different sensory inputs from light and dark conditions [30][31][32]. In mammals, the periodic modulation of circadian rhythm has been tightly linked to both photic and non-photic stimuli [33]. In the eyes, retinal ganglion cells (RGCs) express the photopigment known as melanopsin, a key photoreceptor that mediates the non-image-forming functions of the light and pupillary light reflexes [34]. Similarly, to rod and cone cells, RGCs are also intrinsically photosensitive units that play a key role in transmitting photic signals from the eyes to the SCN through optic chiasma [35] (Figure 1). In mammals. the master clock for circadian rhythm is positioned in the SCN that synchronizes the regulation of neuroplasticity with the daily variation of the photic signals and nonphotic inputs. The SCN is compartmentalized into the dorsal shell and ventral core subdivisions and receives inputs from three afferent pathways, namely the retinohypothalamic tract (RHT), the genicular-hypothalamic tract (GHT), and a compact serotonergic plexus of the raphe nucleus (RN) [36] (Figure 1).
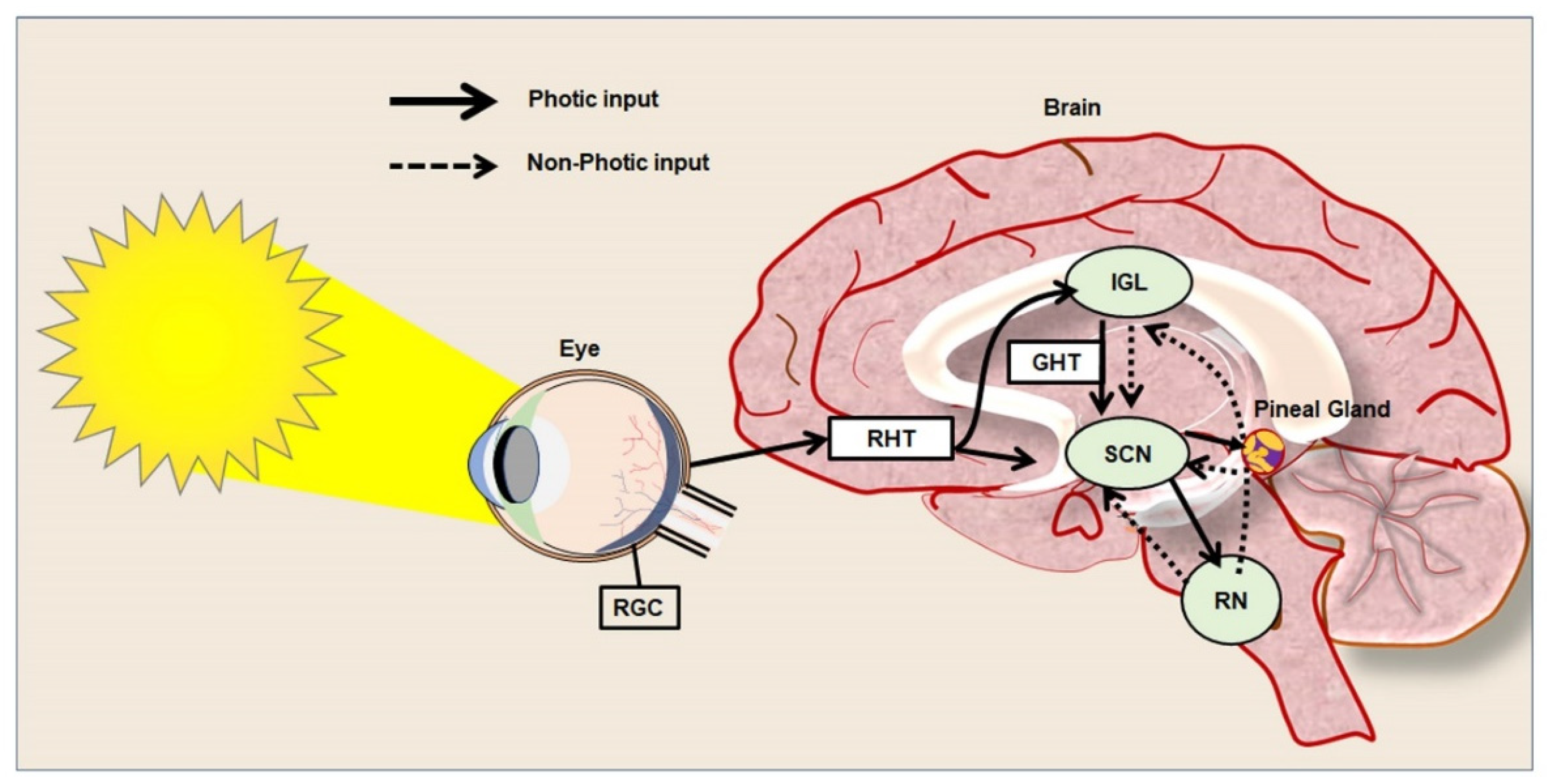
Figure 1. Photic and non-photic input of circadian rhythm in the healthy brain.
The digital diagram represents the photic and non-photic inputs from the retina to the brain and neural pathways among the hypothalamus, pineal gland, and raphe nucleus (RN) that regulate circadian rhythm in a healthy brain. The straight line represents the photic inputs and the dotted line represents the non-photic inputs. The photic information is generally transmitted to the suprachiasmatic nucleus (SCN) both directly, as well as indirectly from the retina. While the retinohypothalamic tract (RHT) is directly involved in the transfer of light-based input from the retina to the SCN, the retinorecipient intergeniculate leaflet (IGL) and geniculohypothalamic tract (GHT) play an indirect role in the regulation of circadian rhythm.
Eventually, the efferent projections of the SCN target the pineal gland through the VIP [37]. VIP is a major neuropeptide that is widely expressed in the gut, pancreas, and brain [38]. In particular, VIP neurons are highly present in various regions of the brain, including the cerebral cortex, amygdala, septum, hippocampus, thalamus, and hypothalamus [39][40]. VIP acts through VPAC1 and 2 receptors to stimulate the secondary messengers Cyclic adenosine 3′,5′-monophosphate (cAMP), and protein kinase A (PKA) signaling cascade, and presynaptically enhance gamma-aminobutyric acid (GABA) release in the neuronal population brain [40]. VIP receptors are widely present in the GABAergic interneurons of the hippocampus and VIP-mediated enhancement of synaptic transmission to cornu Ammonis (CA) 1 pyramidal cells involves the inhibition of GABAergic interneurons that controls the synaptic plasticity of the pyramidal neurons [40]. In the SCN, VIP neurons present contribute an important role in synchronizing the circadian cycle [41]. VIP-secreting neurons are mainly located in the ventrolateral area of the SCN, which receives the environmental input from the optic chiasm through the retinohypothalamic tract and plays a vital role in regulating the circadian cycle [23][42][43]. The release of VIP from the neurons of the SCN regulates the biosynthesis of melatonin in the pineal gland [44]. The synergistic coactivation of VIP and GABAergic pathways in the brain has been identified as a key step in priming the molecular oscillation responsible for the circadian rhythm [22]. Thus, inactivation or defects in the VIP neuronal pathway appears to be a key determinant of the circadian rhythm dysfunction seen in many pathogenic conditions resulting from GABAergic dysfunction, including HD.
3. Neuropathogenic Input of Abnormal Regulation of Clock Genes in HD
HD patients have been found to display decreased activity during the day time as they show increased activity during the night-time [6]. Polysomnographic and actigraphic findings in HD patients indicate frequent eye and leg movements during sleep [45]. Several neuroimaging studies of the hypothalamus have revealed prominent neuropathological alterations in the SCN in corroboration with abnormal sleep–wake cycles in HD [6]. Aziz NA et al. reported that there is a delay in the release of melatonin from the pineal gland in HD patients due to abnormal neurotransmission in the SCN [46]. The drosophila model of HD has been seen to exhibit sedentary behaviors as a reflection of impaired circadian rhythm [47]. Experimental data gathered from the sheep model of HD reveals that the sleep disorder resulting from abnormal circadian rhythm is an early sign of the onset of the disease [7]. Kuljis DA et al. indicated that the expression of the mutant HTT gene in the brain is responsible for sleep disorder in a bacterial artificial chromosome-based transgenic mouse model of HD [48][49]. In addition, the R6/2 mouse model of HD exhibits progressive disruption in the circadian rhythm leading to reduced physical activity and sluggish behavior [50]. Loh DH et al. observed the progressive deterioration of motor function in association with altered sleep patterns due to defects in the circadian rhythm in the Q175 mouse model of HD [19]. Furthermore, experimental subjects with HD have been reported to display depression and progressive forms of memory deficit resulting from an abnormal circadian rhythm [51]. Considering the aforementioned facts, insights into the mutant HTT protein-mediated dysregulation of circadian clock genes pathway in HD has become an important scientific quest.
Circadian rhythms have been known to be regulated by key clock genes such as Period1 and 2 (Per1/2), Cryptochrome1/2 (Cry1/2), Brain and muscle Arnt-like protein 1 (Bmal1), and Circadian Locomotor Output Cycle Kaput (CLOCK) [52][53][54]. Bmal1 functions as a transcriptional activator in heterodimeric form in the cytoplasm and it enters the nucleus and binds with the promoter region of Per and Cry, called the enhancer box (E-box), to regulate the expression of various genes [55][56]. Recently, a gene knockout study in embryonic stem cells (ESCs) indicates that the Bmal1/CLOCK gene regulates the transcription of REV-ERBα/β, which plays an important role in neuronal growth, lipid metabolism, and inflammatory processes [57]. Cry1 and Bmal1/CLOCK also modulate the feedback loop of D-box binding protein and interleukin-3-regulated protein which is also important for the regulation of neuroplasticity [58][59]. Clock genes have also been involved in non-circadian phenotypes such as the regulation of immune cells, metabolic pathways, and their loss of function which leads to abnormal aging and the progression of malignant disorders [52]. Notably, the genetic ablation of the Per gene in the drosophila model has been reported to induce mitochondrial dysfunction and oxidative stress, leading to prominent neurodegeneration in the brain [60]. In addition, the Per mutant mouse model has been reported to display abnormal mitotic events due to defects in tumor suppressor genes, thereby indicating the roles of circadian clock genes in cell-cycle control [61]. In addition, an experimental mouse model with the conditional deletion of the Bmal1 gene in the excitatory forebrain neurons has been reported to exhibit cognitive impairments [62]. Furthermore, several experimental studies reported that the aberrant expression or dysfunction of clock genes leads to cognitive impairment, movement, and mood-related disorders in many neurodegenerative conditions, including HD [63]. Circadian rhythm abnormalities following sleep disruption appear to be the prominent clinical manifestation of human subjects, as well as many experimental models of Alzheimer’s disease (AD) and Parkinson’s disease (PD) [64]. In addition, the abnormal regulation of clock genes has been identified to be associated with various neuropsychiatric manifestations observed in autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), major depressive disorder (MDD), bipolar disorder (BP) and schizophrenia (SCZ) [53].
Ample reports indicate that expression of mutant HTT gene alters the circadian rhythms often before the appearance of involuntary movements in HD [6]. Sleep during night time appears to be progressively reduced and fragmented as neurological symptoms of HD progress [7]. Moreover, abnormal circadian rhythms have been reported to aggravate the progression of the clinical symptoms of HD [65]. HD has been characterized by dysfunctions in the transcriptional regulation of clock genes, which in turn are considered to be an initial trigger for various neuropathogenic changes and mental illnesses [6][51][66]. During the early stage of pathogenesis, HD displays various neuropsychiatric symptoms such as depression, anxiety, stress aggression, psychosis, apathy, obsessive-compulsive behaviors, and psychosis [67][68]. These neuropsychiatric symptoms are multifactorial in origin and are known to be associated with sleep disruption resulting from abnormal circadian rhythmicity [67]. Thus, it can be speculated that the dysregulation of clock genes might be an early pathogenic molecular event prior to the obvious motor and behavioral manifestation of HD. However, the initiation of the abnormalities in the regulation of clock genes upon the pathogenic onset in HD, and the molecular mechanisms by which mutant HTT proteins impair their functions, remain largely unknown.
The abnormal sleep patterns noticed in the fly model of HD have been reported to be linked with an alteration in the transcription of clock genes [18]. Abnormal sleep–wake disorders noticed in the R6/2 mouse model of HD have been reported to be associated with aberrant expressions of Per2 and Bmal1 in the striatum and SCN [50][51]. Moreover, R6/2 mouse models have also been characterized by low levels of VIP expression and its receptor VIPR2 in the brain [69]. Alteration in the metabolic events in the liver of R6/2 mice has been reported to be associated with the abnormal expression of Cry1, D site of albumin promoter binding protein (DBP), and Per2 [50]. Further, the Bmal1 knockout mouse has been characterized by gliosis, neuronal loss, the degeneration of presynaptic terminals, and decreased neural connectivity upon the 3-nitropropionic acid-induced acute HD condition [70][71]. Notably, the supplement of sleeping pills in the R6/2 mouse has been reported to revert the function of Per2 resulting in a significant improvement in cognitive performance [72][73]. While the involvement of clock genes in neuroplasticity has been increasingly noticed, prolonged sleep disruption and the expression of the mutant HTT gene have been known to interfere with the regulation of neuroregenerative plasticity [74] (Figure 2). Therefore, regulation of neurogenesis in stem cell niches of the brain can be expected to be linked with the expression of the clock genes.
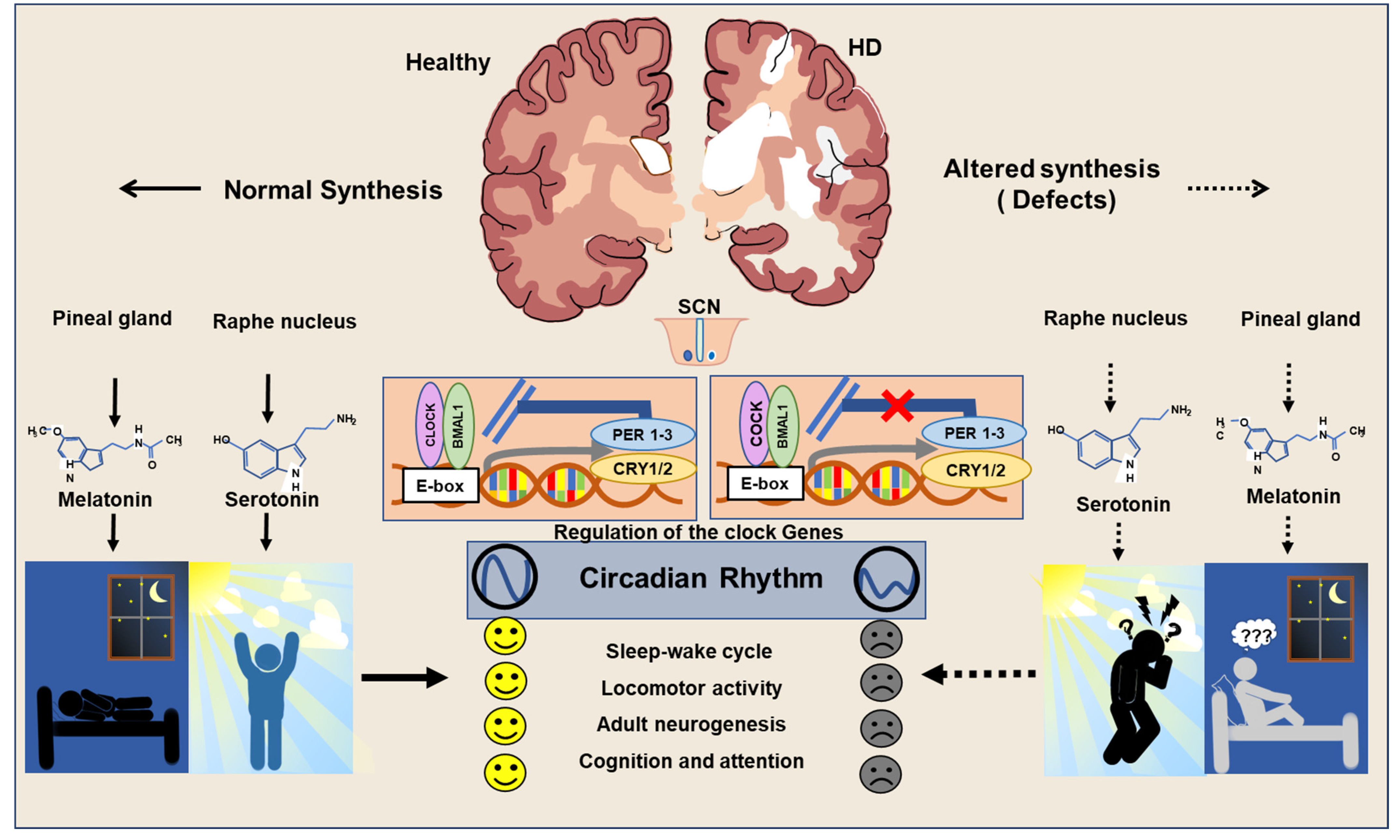
Figure 2. Regulation of circadian clock in Healthy and HD brains.
In a healthy brain, the normal secretion of melatonin and serotonin takes place to ensure the proper sleep–wake cycle and neuroplasticity. In the HD brain, an imbalance in secretions of melatonin and serotonin is responsible for abnormal circadian rhythms and leads to depression, cognitive deficits, and impaired neurogenesis.
References
- Gusella, J.F.; Wexler, N.S.; Conneally, P.M.; Naylor, S.L.; Anderson, M.A.; Tanzi, R.E.; Watkins, P.C.; Ottina, K.; Wallace, M.R.; Sakaguchi, A.Y.; et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 1983, 306, 234–238.
- Moily, N.S.; Kota, L.N.; Anjanappa, R.M.; Venugopal, S.; Vaidyanathan, R.; Pal, P.; Purushottam, M.; Jain, S.; Kandasamy, M. Trinucleotide repeats and haplotypes at the huntingtin locus in an Indian sample overlaps with European haplogroup A. PLoS Curr. 2014, 6.
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240.
- Harper, P.S. The epidemiology of Huntington’s disease. Hum. Genet. 1992, 89, 365–376.
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228.
- Herzog–Krzywoszanska, R.; Krzywoszanski, L. Sleep Disorders in Huntington’s Disease. Front. Psychiatry 2019, 10, 221.
- Voysey, Z.; Fazal, S.V.; Lazar, A.S.; Barker, R.A. The sleep and circadian problems of Huntington’s disease: When, why and their importance. J. Neurol. 2021, 268, 2275–2283.
- Banks, S.; Dinges, D.F. Behavioral and Physiological Consequences of Sleep Restriction. J. Clin. Sleep Med. 2007, 3, 519–528.
- Toda, T.; Parylak, S.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87.
- Kandasamy, M.; Anusuyadevi, M.; Aigner, K.M.; Unger, M.S.; Kniewallner, K.M.; de Sousa, D.M.B.; Altendorfer, B.; Mrowetz, H.; Bogdahn, U.; Aigner, L. TGF-β Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia? Aging Dis. 2020, 11, 828–850.
- Kandasamy, M.; Reilmann, R.; Winkler, J.; Bogdahn, U.; Aigner, L. Transforming Growth Factor-Beta Signaling in the Neural Stem Cell Niche: A Therapeutic Target for Huntington’s Disease. Neurol. Res. Int. 2011, 2011, 124256.
- Kandasamy, M.; Couillard-Despres, S.; Raber, K.A.; Stephan, M.; Lehner, B.; Winner, B.; Kohl, Z.; Rivera, F.J.; Nguyen, H.P.; Riess, O.; et al. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J. Neuropathol. Exp. Neurol. 2010, 69, 717–728.
- Kohl, Z.; Kandasamy, M.; Winner, B.; Aigner, R.; Gross, C.; Couillard-Despres, S.; Bogdahn, U.; Aigner, L.; Winkler, J. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington’s disease. Brain Res. 2007, 1155, 24–33.
- Kandasamy, M.; Aigner, L. Reactive Neuroblastosis in Huntington’s Disease: A Putative Therapeutic Target for Striatal Regeneration in the Adult Brain. Front. Cell. Neurosci. 2018, 12, 37.
- Kandasamy, M.; Rosskopf, M.; Wagner, K.; Klein, B.; Couillard-Despres, S.; Reitsamer, H.A.; Stephan, M.; Nguyen, H.P.; Riess, O.; Bogdahn, U.; et al. Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington’s disease is accompanied by striatal invasion of neuroblasts. PLoS ONE 2015, 10, e0116069.
- Lazic, S.E.; Grote, H.; Armstrong, R.J.E.; Blakemore, C.; Hannan, A.J.; van Dellen, A.; Barker, R.A. Decreased hippocampal cell proliferation in R6/1 Huntington’s mice. Neuroreport 2004, 15, 811–813.
- Curtis, M.A.; Penney, E.B.; Pearson, A.G.; van Roon-Mom, W.M.C.; Butterworth, N.J.; Dragunow, M.; Connor, B.; Faull, R.L.M. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc. Natl. Acad. Sci. USA 2003, 100, 9023–9027.
- Ali, A.A.H.; von Gall, C. Adult Neurogenesis under Control of the Circadian System. Cells 2022, 11, 764.
- Malik, A.; Kondratov, R.V.; Jamasbi, R.J.; Geusz, M.E. Circadian Clock Genes Are Essential for Normal Adult Neurogenesis, Differentiation, and Fate Determination. PLoS ONE 2015, 10, e0139655.
- Faragó, A.; Zsindely, N.; Bodai, L. Mutant huntingtin disturbs circadian clock gene expression and sleep patterns in Drosophila. Sci. Rep. 2019, 9, 7174.
- Loh, D.H.; Kudo, T.; Truong, D.; Wu, Y.; Colwell, C.S. The Q175 Mouse Model of Huntington’s Disease Shows Gene Dosage- and Age-Related Decline in Circadian Rhythms of Activity and Sleep. PLoS ONE 2013, 8, e69993.
- Fan, J.; Zeng, H.; Olson, D.P.; Huber, K.M.; Gibson, J.R.; Takahashi, J.S. Vasoactive Intestinal Polypeptide (VIP)-Expressing Neurons in the Suprachiasmatic Nucleus Provide Sparse GABAergic Outputs to Local Neurons with Circadian Regulation Occurring Distal to the Opening of Postsynaptic GABAA Ionotropic Receptors. J. Neurosci. 2015, 35, 1905–1920.
- Ono, D.; Honma, K.; Honma, S. Roles of Neuropeptides, VIP and AVP, in the Mammalian Central Circadian Clock. Front. Neurosci. 2021, 15, 650154. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2021.650154 (accessed on 26 September 2022).
- Ono, D.; Honma, K.-I.; Yanagawa, Y.; Yamanaka, A.; Honma, S. Role of GABA in the regulation of the central circadian clock of the suprachiasmatic nucleus. J. Physiol. Sci. 2018, 68, 333–343.
- Groen, M.R.; Paulsen, O.; Pérez-Garci, E.; Nevian, T.; Wortel, J.; Dekker, M.P.; Mansvelder, H.D.; van Ooyen, A.; Meredith, R.M. Development of dendritic tonic GABAergic inhibition regulates excitability and plasticity in CA1 pyramidal neurons. J. Neurophysiol. 2014, 112, 287–299.
- Turi, G.F.; Li, W.-K.; Chavlis, S.; Pandi, I.; O’Hare, J.; Priestley, J.B.; Grosmark, A.D.; Liao, Z.; Ladow, M.; Zhang, J.F.; et al. Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning. Neuron 2019, 101, 1150–1165.
- Campbell, E.J.; Marchant, N.J. The use of chemogenetics in behavioural neuroscience: Receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018, 175, 994–1003.
- Magnus, C.J.; Lee, P.H.; Bonaventura, J.; Zemla, R.; Gomez, J.L.; Ramirez, M.H.; Hu, X.; Galvan, A.; Basu, J.; Michaelides, M.; et al. Ultrapotent chemogenetics for research and potential clinical applications. Science 2019, 364, eaav5282.
- Rodriguez, G.A.; Barrett, G.M.; Duff, K.E.; Hussaini, S.A. Chemogenetic attenuation of neuronal activity in the entorhinal cortex reduces Aβ and tau pathology in the hippocampus. PLoS Biol. 2020, 18, e3000851.
- Reddy, S.; Reddy, V.; Sharma, S. Physiology, Circadian Rhythm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK519507/ (accessed on 26 September 2022).
- Czeisler, C.A.; Gooley, J.J. Sleep and circadian rhythms in humans. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 579–597.
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338.
- Challet, E.; Pévet, P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front. Biosci. 2003, 8, s246–s257.
- La Morgia, C.; Carelli, V.; Carbonelli, M. Melanopsin Retinal Ganglion Cells and Pupil: Clinical Implications for Neuro-Ophthalmology. Front. Neurol. 2018, 9, 1047.
- Do, M.T.H.; Yau, K.-W. Intrinsically Photosensitive Retinal Ganglion Cells. Physiol. Rev. 2010, 90, 1547–1581.
- Reghunandanan, V.; Reghunandanan, R. Neurotransmitters of the suprachiasmatic nuclei. J. Circadian Rhythms 2006, 4, 2.
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822.
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629.
- Gozes, I.; Brenneman, D.E. VIP: Molecular biology and neurobiological function. Mol. Neurobiol. 1989, 3, 201–236.
- Cunha-Reis, D.; Caulino-Rocha, A. VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy. Front. Cell. Neurosci. 2020, 14, 153. Available online: https://www.frontiersin.org/articles/10.3389/fncel.2020.00153 (accessed on 26 September 2022).
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577.
- Jones, J.R.; Simon, T.; Lones, L.; Herzog, E.D. SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of the Circadian System. J. Neurosci. 2018, 38, 7986–7995.
- Herzog, E.D.; Hermanstyne, T.; Smyllie, N.J.; Hastings, M.H. Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harb. Perspect. Biol. 2017, 9, a027706.
- Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK550972/ (accessed on 8 November 2020).
- Piano, C.; Losurdo, A.; Della Marca, G.; Solito, M.; Calandra-Buonaura, G.; Provini, F.; Bentivoglio, A.R.; Cortelli, P. Polysomnographic Findings and Clinical Correlates in Huntington Disease: A Cross-Sectional Cohort Study. Sleep 2015, 38, 1489–1495.
- Aziz, N.A.; Pijl, H.; Frölich, M.; Schröder-van der Elst, J.P.; van der Bent, C.; Roelfsema, F.; Roos, R.A.C. Delayed onset of the diurnal melatonin rise in patients with Huntington’s disease. J. Neurol. 2009, 256, 1961–1965.
- Gonzales, E.; Yin, J. Drosophila Models of Huntington’s Disease Exhibit Sleep Abnormalities. PLoS Curr. 2010, 2, RRN1185.
- Kuljis, D.; Schroeder, A.M.; Kudo, T.; Loh, D.H.; Willison, D.L.; Colwell, C.S. Sleep and circadian dysfunction in neurodegenerative disorders: Insights from a mouse model of Huntington’s disease. Minerva Pneumol. 2012, 51, 93–106.
- Kuljis, D.A.; Gad, L.; Loh, D.H.; MacDowell Kaswan, Z.; Hitchcock, O.N.; Ghiani, C.A.; Colwell, C.S. Sex Differences in Circadian Dysfunction in the BACHD Mouse Model of Huntington’s Disease. PLoS ONE 2016, 11, e0147583.
- Maywood, E.S.; Fraenkel, E.; McAllister, C.J.; Wood, N.; Reddy, A.B.; Hastings, M.H.; Morton, A.J. Disruption of Peripheral Circadian Timekeeping in a Mouse Model of Huntington’s Disease and Its Restoration by Temporally Scheduled Feeding. J. Neurosci. 2010, 30, 10199–10204.
- Aziz, N.A.; Anguelova, G.V.; Marinus, J.; Lammers, G.J.; Roos, R.A.C. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington’s disease. Parkinsonism Relat. Disord. 2010, 16, 345–350.
- Andreani, T.S.; Itoh, T.Q.; Yildirim, E.; Hwangbo, D.-S.; Allada, R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015, 10, 413–421.
- Charrier, A.; Olliac, B.; Roubertoux, P.; Tordjman, S. Clock Genes and Altered Sleep–Wake Rhythms: Their Role in the Development of Psychiatric Disorders. Int. J. Mol. Sci. 2017, 18, 938.
- Mazzoccoli, G.; Pazienza, V.; Vinciguerra, M. Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol. Int. 2012, 29, 227–251.
- Zheng, X.; Zhao, X.; Zhang, Y.; Tan, H.; Qiu, B.; Ma, T.; Zeng, J.; Tao, D.; Liu, Y.; Lu, Y.; et al. RAE1 promotes BMAL1 shuttling and regulates degradation and activity of CLOCK: BMAL1 heterodimer. Cell Death Dis. 2019, 10, 62.
- Ye, R.; Selby, C.P.; Chiou, Y.-Y.; Ozkan-Dagliyan, I.; Gaddameedhi, S.; Sancar, A. Dual modes of CLOCK:BMAL1 inhibition mediated by Cryptochrome and Period proteins in the mammalian circadian clock. Genes Dev. 2014, 28, 1989–1998.
- Ikeda, R.; Tsuchiya, Y.; Koike, N.; Umemura, Y.; Inokawa, H.; Ono, R.; Inoue, M.; Sasawaki, Y.; Grieten, T.; Okubo, N.; et al. REV-ERBα and REV-ERBβ function as key factors regulating Mammalian Circadian Output. Sci. Rep. 2019, 9, 10171.
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179.
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102.
- Krishnan, N.; Rakshit, K.; Chow, E.S.; Wentzell, J.S.; Kretzschmar, D.; Giebultowicz, J.M. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012, 45, 1129–1135.
- Lee, C.C. Tumor suppression by the mammalian Period genes. Cancer Causes Control 2006, 17, 525–530.
- Snider, K.H.; Dziema, H.; Aten, S.; Loeser, J.; Norona, F.E.; Hoyt, K.; Obrietan, K. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav. Brain Res. 2016, 308, 222–235.
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82.
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318.
- Videnovic, A.; Zee, P.C. Consequences of Circadian Disruption on Neurologic Health. Sleep Med. Clin. 2015, 10, 469–480.
- Moumné, L.; Betuing, S.; Caboche, J. Multiple Aspects of Gene Dysregulation in Huntington’s Disease. Front. Neurol. 2013, 4, 127.
- Anderson, K.E.; van Duijn, E.; Craufurd, D.; Drazinic, C.; Edmondson, M.; Goodman, N.; van Kammen, D.; Loy, C.; Priller, J.; Goodman, L.V. Clinical Management of Neuropsychiatric Symptoms of Huntington Disease: Expert-Based Consensus Guidelines on Agitation, Anxiety, Apathy, Psychosis and Sleep Disorders. J. Huntingt. Dis. 2018, 7, 355–366.
- Van Duijn, E.; Craufurd, D.; Hubers, A.A.M.; Giltay, E.J.; Bonelli, R.; Rickards, H.; Anderson, K.E.; van Walsem, M.R.; van der Mast, R.C.; Orth, M.; et al. Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J. Neurol. Neurosurg. Psychiatry 2014, 85, 1411–1418.
- Fahrenkrug, J.; Popovic, N.; Georg, B.; Brundin, P.; Hannibal, J. Decreased VIP and VPAC2 receptor expression in the biological clock of the R6/2 Huntington’s disease mouse. J. Mol. Neurosci. 2007, 31, 139–148.
- Musiek, E.S. Circadian clock disruption in neurodegenerative diseases: Cause and effect? Front. Pharmacol. 2015, 6, 29.
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400.
- Pallier, P.N.; Maywood, E.S.; Zheng, Z.; Chesham, J.E.; Inyushkin, A.N.; Dyball, R.; Hastings, M.H.; Morton, A.J. Pharmacological Imposition of Sleep Slows Cognitive Decline and Reverses Dysregulation of Circadian Gene Expression in a Transgenic Mouse Model of Huntington’s Disease. J. Neurosci. 2007, 27, 7869–7878.
- Pallier, P.N.; Morton, A.J. Management of sleep/wake cycles improves cognitive function in a transgenic mouse model of Huntington’s disease. Brain Res. 2009, 1279, 90–98.
- Fernandes, C.; Rocha, N.B.F.; Rocha, S.; Herrera-Solís, A.; Salas-Pacheco, J.; García-García, F.; Murillo-Rodríguez, E.; Yuan, T.-F.; Machado, S.; Arias-Carrión, O. Detrimental role of prolonged sleep deprivation on adult neurogenesis. Front. Cell. Neurosci. 2015, 9, 140.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
22 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





