Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mahesh Kandasamy and Version 2 by Camila Xu.
Huntington’s disease (HD) is a progressive neurodegenerative disorder characterized by abnormal progressive involuntary movements, cognitive deficits, sleep disturbances, and psychiatric symptoms. Notably, gamma-aminobutyric acid (GABA)-ergic neurons that express the vasoactive intestinal peptide (VIP) in the brain play a key role in the regulation of circadian rhythm and neuroplasticity. While an abnormal clock gene pathway has been associated with the inactivation of GABAergic VIP neurons, recent studies suggest the activation of this neuronal population in the brain positively contributes to neuroplasticity.
- Huntington’s disease
- circadian rhythm
- clock genes
1. Introduction
Huntington’s disease (HD) is an autosomal dominant hereditary neurodegenerative disorder that affects the structure and functions of the basal ganglia of the brain [1]. The progressive degeneration of gamma-aminobutyric acid (GABA)-ergic medium spiny (MSN) neurons in the brains of subjects with HD has been attributed to the expansion of polyglutamine (poly Q) segments in the huntingtin (HTT) protein resulting from more than 40 CAG repeats in the exon1 of the HTT gene [2][3][4][2,3,4]. Clinically, HD has been characterized by abnormal involuntary movements, neurocognitive impairments, and psychiatric disturbances [5]. In addition, abnormal sleep–wake cycles accounting for the abnormal circadian rhythm have been identified as non-motor clinical symptoms of HD [6]. Around 90% of HD subjects have been reported to suffer from sleep disturbances [7]. Chronic sleep disturbances appear to be detrimental to the neuroplasticity responsible for neurocognitive functions [8]. Ample research reports indicate that the occurrence of neurogenesis in the hippocampus in the brains of healthy subjects contributes to learning, memory, and mood [9]. Whereas neurogenic failure in the hippocampus has been considered an underlying cellular basis of neurocognitive decline in many neurodegenerative disorders, including HD [10][11][10,11]. While the expression of mutant HTT gene causes aberrant gliogenic events, the neurogenic potential of neural stem cells (NSCs) and survival of new-born neurons in different brain regions including the hippocampus have been reported to be drastically impaired in experimental models of HD and post-mortem human HD brains [12][13][14][15][16][17][12,13,14,15,16,17]. The underlying molecular mechanism for the impaired proliferative and differentiation potentials of NSCs in HD brains remains obscure. In a physiological state, circadian clock genes play important roles in the regulation of NSC-derived neurogenesis, whereas impairment in the neurogenic process has been linked to the irregular circadian clock pathway [18][19][18,19]. The expression of the mutant HTT gene interrupts the regulation and functions of the clock genes, thereby leading to the aberrant circadian rhythm in HD [7][20][21][7,20,21]. Therefore, the abnormal regulation of hippocampal neurogenesis and an irregular circadian rhythm may overlap and can collectively contribute to intertwining pathogenicity leading to psychiatric disturbances and cognitive deficits in HD. Considering the facts, it can be proposed that the reversal of an irregular circadian rhythm might contribute to repair mechanisms of the brain and regenerative plasticity in HD. Therefore, the identification of the prominent molecular pathway and cellular system involved in the regulation of circadian rhythm could serve as a potential therapeutic target in HD.
GABAergic vasoactive intestinal peptide (VIP)-expressing neurons in the suprachiasmatic nucleus (SCN) of the hypothalamus play a key role in the regulation of circadian rhythm. Neuropathogenic events mediated degeneration or functional defects in the VIP neurons of SCN and improper sensory inputs can trigger abnormal circadian rhythmicity in various brain diseases [22][23][24][22,23,24]. These VIP neurons play an important role in the control of GABAergic transmission responsible for the synaptic plasticity of the pyramidal neurons in the hippocampus [25][26][25,26]. Thus, the dysregulation of GABAergic transmission resulting from the mutant HTT protein might overlap with the altered expression and functions of VIP leading to neuroregenerative failure in HD. Therefore, the implementation of therapeutic strategies that aid in the restoration or activation of VIP neurons in the brain could contribute to rectifying sleep disorder in HD. Chemogenetics has been established as a potent molecular tool to specifically regulate the intracellular-signaling pathways in tissue and organs [27][28][27,28]. The chemogenetic-based approaches provide hope to mitigate the abnormal circadian clock pathways which may be coupled with improving neuroregeneration in the brain [29].
2. Regulation of Circadian Rhythm in Physiological State
Circadian rhythm represents a biological chronometer of the living system that regulates, intertwines, overlaps, and synchronizes various physiological, biochemical, cellular, and genetic events, in response to the gut–brain axis, atmospheric temperature, and different sensory inputs from light and dark conditions [30][31][32][30,31,32]. In mammals, the periodic modulation of circadian rhythm has been tightly linked to both photic and non-photic stimuli [33]. In the eyes, retinal ganglion cells (RGCs) express the photopigment known as melanopsin, a key photoreceptor that mediates the non-image-forming functions of the light and pupillary light reflexes [34]. Similarly, to rod and cone cells, RGCs are also intrinsically photosensitive units that play a key role in transmitting photic signals from the eyes to the SCN through optic chiasma [35] (Figure 1). In mammals. the master clock for circadian rhythm is positioned in the SCN that synchronizes the regulation of neuroplasticity with the daily variation of the photic signals and nonphotic inputs. The SCN is compartmentalized into the dorsal shell and ventral core subdivisions and receives inputs from three afferent pathways, namely the retinohypothalamic tract (RHT), the genicular-hypothalamic tract (GHT), and a compact serotonergic plexus of the raphe nucleus (RN) [36] (Figure 1).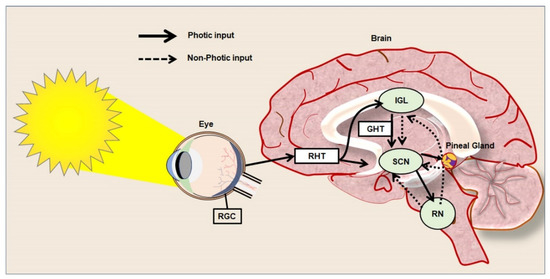
Figure 1.
Photic and non-photic input of circadian rhythm in the healthy brain.
3. Neuropathogenic Input of Abnormal Regulation of Clock Genes in HD
HD patients have been found to display decreased activity during the day time as they show increased activity during the night-time [6]. Polysomnographic and actigraphic findings in HD patients indicate frequent eye and leg movements during sleep [45]. Several neuroimaging studies of the hypothalamus have revealed prominent neuropathological alterations in the SCN in corroboration with abnormal sleep–wake cycles in HD [6]. Aziz NA et al. reported that there is a delay in the release of melatonin from the pineal gland in HD patients due to abnormal neurotransmission in the SCN [46]. The drosophila model of HD has been seen to exhibit sedentary behaviors as a reflection of impaired circadian rhythm [47]. Experimental data gathered from the sheep model of HD reveals that the sleep disorder resulting from abnormal circadian rhythm is an early sign of the onset of the disease [7]. Kuljis DA et al. indicated that the expression of the mutant HTT gene in the brain is responsible for sleep disorder in a bacterial artificial chromosome-based transgenic mouse model of HD [48][49][48,49]. In addition, the R6/2 mouse model of HD exhibits progressive disruption in the circadian rhythm leading to reduced physical activity and sluggish behavior [50]. Loh DH et al. observed the progressive deterioration of motor function in association with altered sleep patterns due to defects in the circadian rhythm in the Q175 mouse model of HD [19]. Furthermore, experimental subjects with HD have been reported to display depression and progressive forms of memory deficit resulting from an abnormal circadian rhythm [51]. Considering the aforementioned facts, insights into the mutant HTT protein-mediated dysregulation of circadian clock genes pathway in HD has become an important scientific quest. Circadian rhythms have been known to be regulated by key clock genes such as Period1 and 2 (Per1/2), Cryptochrome1/2 (Cry1/2), Brain and muscle Arnt-like protein 1 (Bmal1), and Circadian Locomotor Output Cycle Kaput (CLOCK) [52][53][54][52,53,54]. Bmal1 functions as a transcriptional activator in heterodimeric form in the cytoplasm and it enters the nucleus and binds with the promoter region of Per and Cry, called the enhancer box (E-box), to regulate the expression of various genes [55][56][55,56]. Recently, a gene knockout study in embryonic stem cells (ESCs) indicates that the Bmal1/CLOCK gene regulates the transcription of REV-ERBα/β, which plays an important role in neuronal growth, lipid metabolism, and inflammatory processes [57]. Cry1 and Bmal1/CLOCK also modulate the feedback loop of D-box binding protein and interleukin-3-regulated protein which is also important for the regulation of neuroplasticity [58][59][58,59]. Clock genes have also been involved in non-circadian phenotypes such as the regulation of immune cells, metabolic pathways, and their loss of function which leads to abnormal aging and the progression of malignant disorders [52]. Notably, the genetic ablation of the Per gene in the drosophila model has been reported to induce mitochondrial dysfunction and oxidative stress, leading to prominent neurodegeneration in the brain [60]. In addition, the Per mutant mouse model has been reported to display abnormal mitotic events due to defects in tumor suppressor genes, thereby indicating the roles of circadian clock genes in cell-cycle control [61]. In addition, an experimental mouse model with the conditional deletion of the Bmal1 gene in the excitatory forebrain neurons has been reported to exhibit cognitive impairments [62]. Furthermore, several experimental studies reported that the aberrant expression or dysfunction of clock genes leads to cognitive impairment, movement, and mood-related disorders in many neurodegenerative conditions, including HD [63]. Circadian rhythm abnormalities following sleep disruption appear to be the prominent clinical manifestation of human subjects, as well as many experimental models of Alzheimer’s disease (AD) and Parkinson’s disease (PD) [64]. In addition, the abnormal regulation of clock genes has been identified to be associated with various neuropsychiatric manifestations observed in autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), major depressive disorder (MDD), bipolar disorder (BP) and schizophrenia (SCZ) [53]. Ample reports indicate that expression of mutant HTT gene alters the circadian rhythms often before the appearance of involuntary movements in HD [6]. Sleep during night time appears to be progressively reduced and fragmented as neurological symptoms of HD progress [7]. Moreover, abnormal circadian rhythms have been reported to aggravate the progression of the clinical symptoms of HD [65]. HD has been characterized by dysfunctions in the transcriptional regulation of clock genes, which in turn are considered to be an initial trigger for various neuropathogenic changes and mental illnesses [6][51][66][6,51,66]. During the early stage of pathogenesis, HD displays various neuropsychiatric symptoms such as depression, anxiety, stress aggression, psychosis, apathy, obsessive-compulsive behaviors, and psychosis [67][68][67,68]. These neuropsychiatric symptoms are multifactorial in origin and are known to be associated with sleep disruption resulting from abnormal circadian rhythmicity [67]. Thus, it can be speculated that the dysregulation of clock genes might be an early pathogenic molecular event prior to the obvious motor and behavioral manifestation of HD. However, the initiation of the abnormalities in the regulation of clock genes upon the pathogenic onset in HD, and the molecular mechanisms by which mutant HTT proteins impair their functions, remain largely unknown. The abnormal sleep patterns noticed in the fly model of HD have been reported to be linked with an alteration in the transcription of clock genes [18]. Abnormal sleep–wake disorders noticed in the R6/2 mouse model of HD have been reported to be associated with aberrant expressions of Per2 and Bmal1 in the striatum and SCN [50][51][50,51]. Moreover, R6/2 mouse models have also been characterized by low levels of VIP expression and its receptor VIPR2 in the brain [69]. Alteration in the metabolic events in the liver of R6/2 mice has been reported to be associated with the abnormal expression of Cry1, D site of albumin promoter binding protein (DBP), and Per2 [50]. Further, the Bmal1 knockout mouse has been characterized by gliosis, neuronal loss, the degeneration of presynaptic terminals, and decreased neural connectivity upon the 3-nitropropionic acid-induced acute HD condition [70][71][70,71]. Notably, the supplement of sleeping pills in the R6/2 mouse has been reported to revert the function of Per2 resulting in a significant improvement in cognitive performance [72][73][72,73]. While the involvement of clock genes in neuroplasticity has been increasingly noticed, prolonged sleep disruption and the expression of the mutant HTT gene have been known to interfere with the regulation of neuroregenerative plasticity [74] (Figure 2). Therefore, regulation of neurogenesis in stem cell niches of the brain can be expected to be linked with the expression of the clock genes.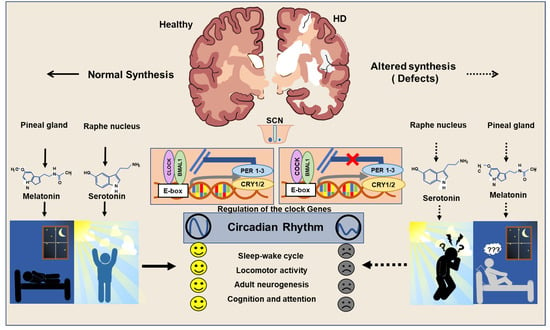
Figure 2.
Regulation of circadian clock in Healthy and HD brains.
