
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucinda J. Bessa | -- | 2637 | 2022-12-20 15:31:29 | | | |
| 2 | Lindsay Dong | Meta information modification | 2637 | 2022-12-23 03:46:54 | | |
Video Upload Options
The oral microbiome plays a major role in shaping oral health/disease state; thus, a main challenge for dental practitioners is to preserve or restore a balanced oral microbiome. Nonetheless, when pathogenic microorganisms install in the oral cavity and are incorporated into the oral biofilm, oral infections, such as gingivitis, dental caries, periodontitis, and peri-implantitis, can arise. Several prophylactic and treatment approaches are available nowadays, but most of them have been antibiotic-based. Given the actual context of antimicrobial resistance (AMR), antibiotic stewardship in dentistry would be a beneficial approach to optimize and avoid inappropriate or even unnecessary antibiotic use, representing a step towards precision medicine.
1. Oral Microbiome
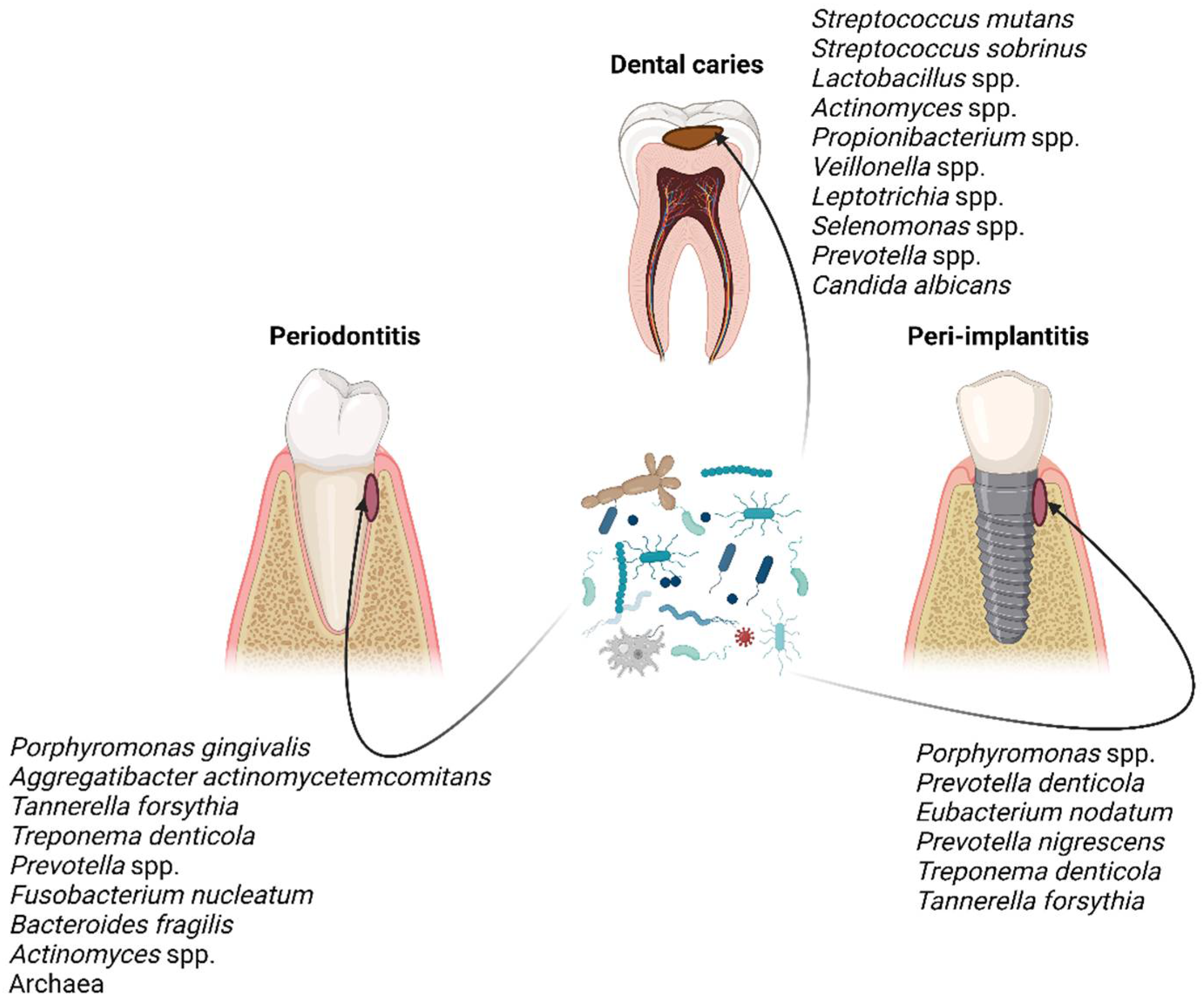
2. Oral Biofilms and Biofilm-Related Oral Diseases
3. Antimicrobial Resistance (AMR) in Dental Practice
Dentists prescribe antibiotics for two purposes: (1) prophylaxis, to improve the outcome success of surgical interventions and reduce complications and symptoms, and (2) therapeutics, for treating oral infections [35]. However, in dental practice, antibiotic indication has been long based on personal experience or judgment and on old evidence, rather than on effective diagnosis [36]; and it has been mostly an empirical drug prescription, with a predominant choice for broad-spectrum antibiotics [35][37]. Furthermore, guidelines for the prudent usage of antibiotics were scarce or not generally shared among dental practitioners before [38].
It is evident that the implementation of antibiotic stewardship programs in the dental setting is of great need [39]. Antibiotic stewardship entails a set of coordinated interventions to promote the correct use of antibiotics (optimal selection, dosing, route, and duration of administration), to improve clinical outcomes and minimize side effects to patients, and to reduce the development and spread of multidrug-resistant bacteria [40].
A multidisciplinary antibiotic stewardship team, involving the dental team in close collaboration with pharmacists, microbiologists, or other health care professionals, is fundamental to assure the execution of an antibiotic stewardship program in a dental setting [41]. A set of antibiotic stewardship interventions recommended for dental practice are compiled in Table 1.
| • Ponder patient conditions and look for a clear diagnosis before prescribing antibiotics; discuss with peers and other specialists if needed |
| • Follow updated and standardized guidelines |
| • Receive feedback on previous acts of antibiotic prescribing |
| • Warrant ongoing education and appropriate training |
| • Educate the dental patient and establish good communication to ensure the patient will follow the correct instructions when taking antibiotics |
| • Audit how appropriately antimicrobials are prescribed |
Among these interventions, the education of both dentists and patients on the adequate use of antibiotics and the current problem of AMR should be encouraged. Several studies have confirmed the critical need for dental students’ education and specific training in the prescription of antibiotics [43][44][45][46]. Educational interventions can include lectures, didactic meetings, workshops, and practice campaigns. A recent study showed that final-year undergraduates from the Glasgow Dental School were enthusiastic about attending a supplemental, yet mandatory, online course on the essential role of dental teams in antimicrobial stewardship and in reducing AMR; the students’ feedback was positive, and they recognized they could play an important role in stewardship [47].
4. Antibiotic Prophylaxis (AP) and Treatment of Oral Infections
4.1. Recent Changes for AP
4.2. Antibiotic Treatment of Dental Caries, Periodontal Diseases, and Peri-Implantitis
5. Oral Resistome
6. Current Alternatives to Antibiotics to Prevent and Treat Oral Infections
6.1. Antimicrobial Photodynamic Therapy (APDT)
6.2. Cold Atmospheric Plasma (CAP)
6.3. Natural Products
6.4. Antimicrobial Peptides (AMPs)
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103.
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666.
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128.
- Xu, X.; He, J.; Xue, J.; Wang, Y.; Li, K.; Zhang, K.; Guo, Q.; Liu, X.; Zhou, Y.; Cheng, L.; et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ. Microbiol. 2015, 17, 699–710.
- Seidel, C.L.; Gerlach, R.G.; Wiedemann, P.; Weider, M.; Rodrian, G.; Hader, M.; Frey, B.; Gaipl, U.S.; Bozec, A.; Cieplik, F.; et al. Defining metaniches in the oral cavity according to their microbial composition and cytokine profile. Int. J. Mol. Sci. 2020, 21, 8218.
- NIH Human Microbiome Project—Core Microbiome Sampling Protocol A (HMP-A). Available online: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/document.cgi?study_id=phs000228.v3.p1&phd=3190 (accessed on 10 June 2022).
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66.
- Willis, J.R.; Saus, E.; Iraola-Guzmán, S.; Ksiezopolska, E.; Cozzuto, L.; Bejarano, L.A.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Blanco, A.; Puig-Sola, A.; et al. Citizen-science reveals changes in the oral microbiome in Spain through age and lifestyle factors. NPJ Biofilms Microbiomes 2022, 8, 38.
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131.
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810.
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259.
- Neu, A.T.; Allen, E.E.; Roy, K. Defining and quantifying the core microbiome: Challenges and prospects. Proc. Natl. Acad. Sci. USA 2021, 118, e2104429118.
- Risely, A. Applying the core microbiome to understand host–microbe systems. J. Anim. Ecol. 2020, 89, 1549–1558.
- Jovel, J.; Nimaga, A.; Jordan, T.; O’Keefe, S.; Patterson, J.; Thiesen, A.; Hotte, N.; Bording-Jorgensen, M.; Subedi, S.; Hamilton, J.; et al. Metagenomics versus metatranscriptomics of the murine gut microbiome for assessing microbial metabolism during inflammation. Front. Microbiol. 2022, 13, 829378.
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the oral microbiome in personalized dentistry. Arch. Oral Biol. 2019, 104, 7–12.
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120.
- Ribeiro, M.; Simões, M. Oral biofilms. In Recent Trends in Biofilm Science and Technology; Simões, M., Borges, A., Simões, L.C., Eds.; Academic Press: Salt Lake City, UT, USA, 2020; pp. 89–99.
- Al-Shahrani, M.A. Microbiology of dental caries: A literature review. Ann. Med. Health Sci. Res. 2019, 9, 655–659.
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569.
- Komatsu, K.; Shiba, T.; Takeuchi, Y.; Watanabe, T.; Koyanagi, T.; Nemoto, T.; Shimogishi, M.; Shibasaki, M.; Katagiri, S.; Kasugai, S.; et al. Discriminating microbial community structure between peri-implantitis and periodontitis with integrated metagenomic, metatranscriptomic, and network analysis. Front. Cell. Infect. Microbiol. 2020, 10, 596490.
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The oral microbiome in health and its Implication in oral and systemic diseases. Adv. Appl. Microbiol. 2016, 97, 171–210.
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427.
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886.
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms—A review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601.
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.; Mei, M.L.; Chu, C.H. Acquired salivary pellicle and oral diseases: A literature review. J. Dent. Sci. 2021, 16, 523–529.
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6 (Suppl. S1), S14.
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759.
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of dental caries and dental caries management: Consensus report of a workshop organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14.
- Boisen, G.; Davies, J.R.; Neilands, J. Acid tolerance in early colonizers of oral biofilms. BMC Microbiol. 2021, 21, 45.
- Moussa, D.G.; Ahmad, P.; Mansour, T.A.; Siqueira, W.L. Current state and challenges of the global outcomes of dental caries research in the meta-omics era. Front. Cell. Infect. Microbiol. 2022, 12, 887907.
- Simón-Soro, A.; Mira, A. Solving the etiology of dental caries. Trends Microbiol. 2015, 23, 76–82.
- Peterson, S.N.; Meissner, T.; Su, A.I.; Snesrud, E.; Ong, A.C.; Schork, N.J.; Bretz, W.A. Functional expression of dental plaque microbiota. Front. Cell. Infect. Microbiol. 2014, 4, 108.
- Zhang, Y.; Li, Y.; Yang, Y.; Wang, Y.; Cao, X.; Jin, Y.; Xu, Y.; Li, S.C.; Zhou, Q. Periodontal and peri-implant microbiome dysbiosis is associated with alterations in the microbial community structure and local stability. Front. Microbiol. 2022, 12, 785191.
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol. 2000 2021, 86, 231–240.
- Buonavoglia, A.; Leone, P.; Solimando, A.G.; Fasano, R.; Malerba, E.; Prete, M.; Corrente, M.; Prati, C.; Vacca, A.; Racanelli, V. Antibiotics or no antibiotics, that is the question: An update on efficient and effective use of antibiotics in dental practice. Antibiotics 2021, 10, 550.
- Palmer, N.O.A. Antimicrobial resistance and antibiotic prescribing in dental practice. Dent. Update 2016, 43, 954–960.
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884.e5.
- Tong, D.C.; Rothwell, B.R. Antibiotic prophylaxis in dentistry: A review and practice recommendations. J. Am. Dent. Assoc. 2000, 131, 366–374.
- Löffler, C.; Böhmer, F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care—A systematic review. PLoS ONE 2017, 12, e0188061.
- Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries, A WHO Practical Toolkit; World Health Organization. Available online: https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf (accessed on 28 June 2022).
- Teoh, L.; Thompson, W.; Suda, K. Antimicrobial stewardship in dental practice. J. Am. Dent. Assoc. 2020, 151, 589–595.
- Montero, M. Antimicrobial Resistance: What should Dentists be doing? Odovtos-Int. J. Dent. Sci. 2016, 18, 10–14.
- Kyles, B.J.; Spivakovsky, S. Toward the development of an antibiotic stewardship competency in dental education. J. Dent. Educ. 2022, 86, 883–886.
- McMaster, D.; Courtenay, M.; Santucci, C.; Davies, A.P.; Kirby, A.; Seddon, O.; Price, D.A.; Barlow, G.; Lim, F.H.; Davies, B.S.; et al. Consensus-based antimicrobial resistance and stewardship competencies for UK undergraduate medical students. JAC Antimicrob. Resist. 2020, 2, dlaa096.
- Holz, M.; Naavaal, S.; Stilianoudakis, S.; Carrico, C.; Byrne, B.E.; Myers, G.L. Antibiotics and antimicrobial resistance: Evaluation of the knowledge, attitude, and perception among students and faculty within US dental schools. J. Dent. Educ. 2021, 85, 383–391.
- Veses, V.; Del Mar Jovani-Sancho, M.; González-Martínez, R.; Cortell-Ballester, I.; Sheth, C.C. Raising awareness about microbial antibiotic resistance in undergraduate dental students: A research-based strategy for teaching non-laboratory elements of a microbiology curriculum. BMC Med. Educ. 2020, 20, 47.
- Cooper, L.; Sneddon, J.; Thompson, W.; Guise, T.; Robertson, D.; Smith, A. Tackling antimicrobial resistance in practice: Dental students’ evaluation of university teaching supplemented by an online course. JAC Antimicrob. Resist. 2022, 4, dlac039.
- Palmer, N. (Ed.) Antimicrobial Prescribing in Dentistry: Good Practice Guidelines, 3rd ed.; Faculty of General Dental Practice and Faculty of Dental Surgery: London, UK, 2020; pp. 1–128.
- Kirnbauer, B.; Jakse, N.; Truschnegg, A.; Dzidic, I.; Mukaddam, K.; Payer, M. Is perioperative antibiotic prophylaxis in the case of routine surgical removal of the third molar still justified? A randomized, double-blind, placebo-controlled clinical trial with a split-mouth design. Clin. Oral Investig. 2022, 26, 6409–6421.
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; Aminoshariae, A.; Durkin, M.J.; Fouad, A.F.; Gopal, P.; Hatten, B.W.; Kennedy, E.; Lang, M.S.; et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J. Am. Dent. Assoc. 2019, 150, 906–921.e12.
- Daly, C.G. Antibiotic prophylaxis for dental procedures. Aust. Prescr. 2017, 40, 184–188.
- Vähäsarja, N.; Lund, B.; Ternhag, A.; Götrick, B.; Olaison, L.; Hultin, M.; Warnqvist, A.; Krüger Weiner, C.; Naimi-Akbar, A. Infective Endocarditis Among High-risk Individuals Before and After the Cessation of Antibiotic Prophylaxis in Dentistry: A National Cohort Study. Clin. Infect. Dis. 2022, 75, 1171–1178.
- Kopra, E.; Lahdentausta, L.; Pietiäinen, M.; Buhlin, K.; Mäntylä, P.; Hörkkö, S.; Persson, R.; Paju, S.; Sinisalo, J.; Salminen, A.; et al. Systemic Antibiotics Influence Periodontal Parameters and Oral Microbiota, but Not Serological Markers. Front. Cell. Infect. Microbiol. 2021, 11, 774665.
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 257–281.
- Khattri, S.; Nagraj, S.K.; Arora, A.; Eachempati, P.; Kusum, C.K.; Bhat, K.G.; Johnson, T.M.; Lodi, G. Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis. Cochrane Database Syst. Rev. 2020, 11, CD012568.
- Cope, A.L.; Francis, N.; Wood, F.; Chestnutt, I.G. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst. Rev. 2018, 9, CD010136.
- Robertson, K.; Shahbazian, T.; MacLeod, S. Treatment of peri-implantitis and the failing implant. Dent. Clin. N. Am. 2015, 59, 329–343.
- Roccuzzo, A.; Stähli, A.; Monje, A.; Sculean, A.; Salvi, G.E. Peri-Implantitis: A clinical update on prevalence and surgical treatment outcomes. J. Clin. Med. 2021, 10, 1107.
- Passarelli, P.C.; Netti, A.; Lopez, M.A.; Giaquinto, E.F.; De Rosa, G.; Aureli, G.; Bodnarenko, A.; Papi, P.; Starzyńska, A.; Pompa, G.; et al. Local/topical antibiotics for peri-implantitis treatment: A systematic review. Antibiotics 2021, 10, 1298.
- Almeida, V.S.M.; Azevedo, J.; Leal, H.F.; Queiroz, A.T.L.; da Silva Filho, H.P.; Reis, J.N. Bacterial diversity and prevalence of antibiotic resistance genes in the oral microbiome. PLoS ONE 2020, 15, e0239664.
- Carr, V.R.; Witherden, E.A.; Lee, S.; Shoaie, S.; Mullany, P.; Proctor, G.B.; Gomez-Cabrero, D.; Moyes, D.L. Abundance and diversity of resistomes differ between healthy human oral cavities and gut. Nat. Commun. 2020, 11, 693.
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the antibiotic resistome. Science 2006, 311, 374–377.
- Kim, D.W.; Cha, C.J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309.
- Aguiar-Pulido, V.; Huang, W.; Suarez-Ulloa, V.; Cickovski, T.; Mathee, K.; Narasimhan, G. Metagenomics, metatranscriptomics, and metabolomics approaches for microbiome analysis. Evol. Bioinform. Online 2016, 12, 5–16.
- Sukumar, S.; Roberts, A.P.; Martin, F.E.; Adler, C.J. Metagenomic insights into transferable antibiotic resistance in oral bacteria. J. Dent. Res. 2016, 95, 969–976.
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28.
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of photodynamic therapy in dentistry: Current concepts. Dent. J. 2020, 8, 43.
- Gu, M.; Jiang, S.; Xu, X.; Wu, M.Y.; Chen, C.; Yuan, Y.; Chen, Q.; Sun, Y.; Chen, L.; Shen, C.; et al. Simultaneous photodynamic eradication of tooth biofilm and tooth whitening with an aggregation-induced emission luminogen. Adv. Sci. 2022, 9, e2106071.
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Aminian, M.; Bahador, A. Anti-biofilm and anti-metabolic effects of antimicrobial photodynamic therapy using chlorophyllin-phycocyanin mixture against Streptococcus mutans in experimental biofilm caries model on enamel slabs. Photodiagnosis Photodyn. Ther. 2020, 29, 101620.
- Tennert, C.; Zinovieva, Y.; Shishkov, K.; Karygianni, L.; Altenburger, M.J.; Wierichs, R.J.; Al-Ahmad, A. Improving the efficiency of photodynamic chemotherapy in root canals against Enterococcus faecalis in vitro. Antibiotics 2020, 9, 543.
- Lopes, C.B.; Motta, P.B.; Campos, T.M.; Leite, D.P.V.; Araki Yamamoto, Â.T.; Mota, M.S.A.; Navarro, R.S.; Santos, E.M.; Horliana, A.C.R.T.; Bussadori, S.K.; et al. Protocol for the clinical practice of photodynamic therapy in endodontics: Assessment of guideline quality using the AGREE II instrument. Photodiagnosis Photodyn. Ther. 2022, 38, 102835.
- Yamashita, Y.; Mae, M.; Oohira, M.; Ozaki, Y.; Ohba, S.; Asahina, I.; Yoshimura, A. Clinical efficacy and safety of antimicrobial photodynamic therapy in residual periodontal pockets during the maintenance phase. Pharmaceuticals 2022, 15, 924.
- Lafzi, A.; Mojahedi, S.M.; Mirakhori, M.; Torshabi, M.; Kadkhodazadeh, M.; Amid, R.; Karamshahi, M.; Arbabi, M.; Torabi, H. Effect of one and two sessions of antimicrobial photodynamic therapy on clinical and microbial outcomes of non-surgical management of chronic periodontitis: A clinical study. J. Adv. Periodontol. Implant. Dent. 2019, 11, 85–93.
- Sivaramakrishnan, G.; Sridharan, K. Photodynamic therapy for the treatment of peri-implant diseases: A network meta-analysis of randomized controlled trials. Photodiagnosis Photodyn. Ther. 2018, 21, 1–9.
- Wang, H.; Liu, Y.; Li, W.; Li, W.; Xu, H.; Niu, G.; Wang, Z. Microbiota in gingival crevicular fluid before and after mechanical debridement with antimicrobial photodynamic therapy in peri-implantitis. Front. Cell. Infect. Microbiol. 2022, 11, 777627.
- Afrasiabi, S.; Partoazar, A.; Chiniforush, N.; Goudarzi, R. The potential application of natural photosensitizers used in antimicrobial photodynamic therapy against oral infections. Pharmaceuticals 2022, 15, 767.
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Bahador, A. Propolis nanoparticle enhances the potency of antimicrobial photodynamic therapy against Streptococcus mutans in a synergistic manner. Sci. Rep. 2020, 10, 15560.
- Pourhajibagher, M.; Alaeddini, M.; Etemad-Moghadam, S.; Rahimi Esboei, B.; Bahrami, R.; Miri Mousavi, R.S.; Bahador, A. Quorum quenching of Streptococcus mutans via the nano-quercetin-based antimicrobial photodynamic therapy as a potential target for cariogenic biofilm. BMC Microbiol. 2022, 22, 125.
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold plasmas for biofilm control: Opportunities and challenges. Trends Biotechnol. 2018, 36, 627–638.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial peptides: A promising therapeutic strategy in tackling antimicrobial resistance. Curr. Med. Chem. 2017, 24, 4303–4314.
- Griffith, A.; Mateen, A.; Markowitz, K.; Singer, S.R.; Cugini, C.; Shimizu, E.; Wiedman, G.R.; Kumar, V. Alternative antibiotics in dentistry: Antimicrobial peptides. Pharmaceutics 2022, 14, 1679.
- Dostert, M.; Trimble, M.J.; Hancock, R.E.W. Antibiofilm peptides: Overcoming biofilm-related treatment failure. RSC Adv. 2021, 11, 2718–2728.




