The oral microbiome plays a major role in shaping oral health/disease state; thus, a main challenge for dental practitioners is to preserve or restore a balanced oral microbiome. Nonetheless, when pathogenic microorganisms install in the oral cavity and are incorporated into the oral biofilm, oral infections, such as gingivitis, dental caries, periodontitis, and peri-implantitis, can arise. Several prophylactic and treatment approaches are available nowadays, but most of them have been antibiotic-based. Given the actual context of antimicrobial resistance (AMR), antibiotic stewardship in dentistry would be a beneficial approach to optimize and avoid inappropriate or even unnecessary antibiotic use, representing a step towards precision medicine.
1. Oral Microbiome
The term microbiome has been connected to various definitions, but it has been recognized that there is a need to converge to a clear and commonly agreed upon definition of the microbiome
[1][2]. Considering the recent definition proposed for microbiome by Berg et al.
[1][2], it may define the oral microbiome as a dynamic ecosystem composed of the oral microbiota, viruses, their structural elements, and metabolites, as well as molecules produced by the coexisting host and controlled by the surrounding environment. The oral microbiome, like every microbiome, is continuously and functionally evolving due to the microbe–host and inter-species interactions.
In the oral cavity, there are distinctive niches, each with its associated microbiome, including the gingival sulcus, tongue, cheek, hard and soft palate, floor of the mouth, throat, saliva, teeth, and, if present, dental implants
[2][3][4][5][3,14,15,16]. The Human Microbiome Project defined nine anatomical locations in the human mouth in a state of health: the tongue dorsum, the hard palate, the tonsils, sub- and supra-gingival plaque on teeth, the keratinized gingiva, the buccal mucosa, the throat, and saliva
[6][17].
The microbial oral community shifts constantly throughout an individual’s life due to extrinsic and intrinsic factors
[2][7][8][9][10][3,18,19,20,21]. The oral cavity is exposed to exogenous microorganisms mainly through diet, drinking, air, kissing, and lifestyle
[7][18]. Changes in oral pH, depressed immune system, presence of chronic diseases, and intake of antibiotics also affect the composition of the oral microbiome
[8][10][19,21]. Nonetheless, despite all these factors accounting for inter-individual variability, an oral core microbiome has been pinpointed
[3][11][14,22], and it refers to the microbial taxa or the genomic and functional attributes associated with those taxa that are characteristic of the oral cavity under healthy conditions
[12][13][23,24].
Quantitative and qualitative variations in the composition of the core microbiome cause dysbiosis, which correlates with the disease state
[14][15][29,30]. The taxonomic composition of microbial communities implicated in dental caries, periodontitis, and peri-implantitis has been pinpointed by several studies throughout the years
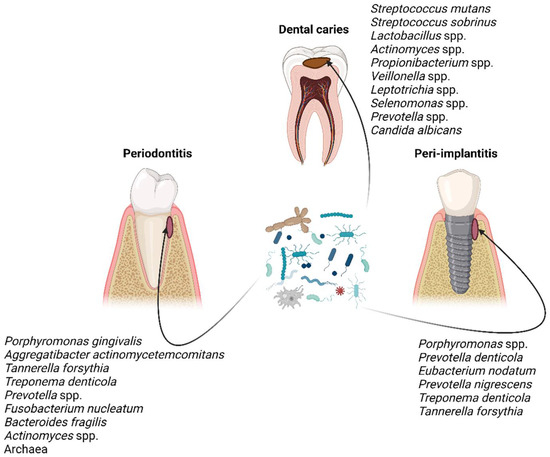
[15][16][17][18][19][20][30,31,32,33,34,35] (
Figure 1), which have disclosed that the microbial communities are distinct in those three oral diseases in terms of composition and/or abundance. Nonetheless, the taxonomic composition is unsurprisingly not definite; it is being constantly updated as knowledge and technologies are advancing.
Figure 1. Characteristic taxa associated with dental caries, periodontitis, and peri-implantitis
[15][16][17][18][19][20][30,31,32,33,34,35]. Figure created with BioRender.com.
2. Oral Biofilms and Biofilm-Related Oral Diseases
Bacteria play a major role in biofilm formation as the initial colonizers and in terms of abundance and function in a normal oral microbiome. However, fungi, viruses, archaea, and protozoa are also constituents of the oral community and must be taken into consideration when studying the complete oral microbiome
[21][5].
Entamoeba gingivalis and
Trichomonas tenax are the most commonly found protozoa in the oral cavity, while
Candida species are the most prevalent fungi
[7][18].
Bacteria within a biofilm communicate via quorum sensing, through which signaling molecules are produced and detected by neighboring bacteria. This communication system enables bacteria to regulate several bacterial mechanisms, such as the production of virulence factors and biofilm formation
[22][39]. In addition, biofilm seems to boost bacterial protection against the host immune system, environmental factors (such as shear stress), and antimicrobial agents
[23][24][10,11]. Thus, any biofilm-associated infection, including those of the oral cavity, represents a therapeutic conundrum.
Streptococci and some
Actinomyces species are known to be the early colonizers of the salivary pellicle, an organic film composed mostly of proteins on the tooth surface, epithelium, and restorations
[17][25][32,40]. The colonization of the salivary pellicle is the starting point for the co-aggregation of new species to previously adhered bacteria and the subsequent formation of a polymicrobial biofilm known as dental plaque, which is a natural phenomenon involved in the physiology and defenses of the host
[26][41], as long as a certain degree of stability, known as microbial homeostasis, is maintained despite regular environmental perturbations, such as dietary intake and oral hygiene. Consequently, dysbiotic dental plaque is implicated in the development of common oral diseases, such as erosion, dental caries, periodontal disease, and peri-implantitis
[25][40], and is characterized by an imbalance in the biofilm composition favoring oral pathogens to take the lead. It is noteworthy that the appearance and persistence of dysbiosis rely on both microbial changes and host factors, namely the development of inflammation and the intake of dietary sugars
[27][42].
Dental caries is a multifactorial oral disease, which is biofilm-mediated and modulated by dietary carbohydrates
[28][43]. High and frequent exposure to fermentable carbohydrates is the driver for the development of a supragingival dysbiotic biofilm, where aciduric bacteria prevail and lead to a pH decline that can no longer be buffered by saliva. Therefore, there is a selection for more acid-tolerant microorganisms, which in turn favors the persistence of this acidic environment that enables enamel demineralization
[27][29][30][42,44,45]. In early studies,
Streptococcus mutans has been recognized as ‘the cariogenic keystone pathogen’; however, next-generation sequencing (NGS) and omics studies have disclosed that dental caries is more a polymicrobial disease based on a variable and diverse pathogenic community that relies on sugar consumption, rather than a classic Koch’s postulate (a single agent related disease)
[30][31][45,46]. Despite the substantial inter-individual variability composition of this dysbiotic biofilm in dental caries, different microbial combinations have been identified to have a similar functional profile
[32][47]. This suggests that the pathogenesis of dental caries is controlled by complex and intricate host, microbial, and environmental factors and interactions, and for that reason, it remains an entangled research issue
[30][45].
Periodontitis and peri-implantitis are also biofilm-mediated oral diseases with marked microbial dysbiosis and inflammation. In both, alveolar bone is lost, in addition to the loss of tooth-supporting tissue in periodontitis and peri-implant tissue in peri-implantitis, respectively
[33][48]. If untreated, these oral non-communicable diseases can lead to tooth or dental implant loss. On the one hand, both diseases share many etiological and clinical features. On the other hand, they differ in the microbial community present. This is mostly due to the material (dentin or titanium) that serves as the substratum for biofilm formation and affects bacterial adhesion, determining differences in the type of initial bacterial colonizers and dictating the formation of distinct subgingival biofilms
[34][49].
3. Antimicrobial Resistance (AMR) in Dental Practice
Dentists prescribe antibiotics for two purposes: (1) prophylaxis, to improve the outcome success of surgical interventions and reduce complications and symptoms, and (2) therapeutics, for treating oral infections [35][57]. However, in dental practice, antibiotic indication has been long based on personal experience or judgment and on old evidence, rather than on effective diagnosis [36][58]; and it has been mostly an empirical drug prescription, with a predominant choice for broad-spectrum antibiotics [35][37][57,59]. Furthermore, guidelines for the prudent usage of antibiotics were scarce or not generally shared among dental practitioners before [38][60].
It is evident that the implementation of antibiotic stewardship programs in the dental setting is of great need [39][68]. Antibiotic stewardship entails a set of coordinated interventions to promote the correct use of antibiotics (optimal selection, dosing, route, and duration of administration), to improve clinical outcomes and minimize side effects to patients, and to reduce the development and spread of multidrug-resistant bacteria [40][69].
A multidisciplinary antibiotic stewardship team, involving the dental team in close collaboration with pharmacists, microbiologists, or other health care professionals, is fundamental to assure the execution of an antibiotic stewardship program in a dental setting [41][70]. A set of antibiotic stewardship interventions recommended for dental practice are compiled in Table 1.
Table 1.
Antibiotic stewardship interventions in dental practice.
| • Ponder patient conditions and look for a clear diagnosis before prescribing antibiotics; discuss with peers and other specialists if needed |
| • Follow updated and standardized guidelines |
| • Receive feedback on previous acts of antibiotic prescribing |
| • Warrant ongoing education and appropriate training |
| • Educate the dental patient and establish good communication to ensure the patient will follow the correct instructions when taking antibiotics |
| • Audit how appropriately antimicrobials are prescribed |