You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luigi Rosati | -- | 1555 | 2022-12-19 11:43:47 | | | |
| 2 | Rita Xu | Meta information modification | 1555 | 2022-12-20 03:48:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rosati, L.; Chianese, T.; Simoniello, P.; Motta, C.M.; Scudiero, R. Podarcis siculus Spermatogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/38956 (accessed on 29 December 2025).
Rosati L, Chianese T, Simoniello P, Motta CM, Scudiero R. Podarcis siculus Spermatogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/38956. Accessed December 29, 2025.
Rosati, Luigi, Teresa Chianese, Palma Simoniello, Chiara Maria Motta, Rosaria Scudiero. "Podarcis siculus Spermatogenesis" Encyclopedia, https://encyclopedia.pub/entry/38956 (accessed December 29, 2025).
Rosati, L., Chianese, T., Simoniello, P., Motta, C.M., & Scudiero, R. (2022, December 19). Podarcis siculus Spermatogenesis. In Encyclopedia. https://encyclopedia.pub/entry/38956
Rosati, Luigi, et al. "Podarcis siculus Spermatogenesis." Encyclopedia. Web. 19 December, 2022.
Copy Citation
Spermatogenesis is a genetically driven differentiation process that occurs in the testis and leads to the formation of spermatozoa. This process is extensively studied in several experimental models, particularly in vertebrates that share the morphological structure and functionality of the mammalian testis. Reptiles are not generally considered biological models, the lizard Podarcis siculus has represented a suitable organism for the study of spermatogenesis. In this lizard, the process of spermatogenesis is regulated by the interaction between systemic factors such as gonadotropins and local factors, i.e., molecules produced by the somatic and germinal cells of the testis.
reptiles
spermatogenesis
steroidogenesis
reproduction
1. Introduction
Among the reptiles inhabiting the Italian peninsula, the specimens of the field lizard Podarcis siculus (Rafinesque-Schmaltz, 1810) (Sauria, Lacertidae) are the most abundant (Figure 1). The Italian wall lizard, formerly known as Lacerta sicula, then Podarcis sicula, and currently P. siculus, is an endemic species of the Mediterranean regions, from the Iberian Peninsula to Tunisia [1]. It prefers warm, arid climates and for this it tends to disappear over 1000 m altitude. Their habitat varies from rural areas to cultivated fields and city gardens. It is often considered an invasive alien species dangerous to the native species [2]; a population had even been introduced in 1967 to New York [3], and, more recently, an abundant population has been found in a Natural Ornithological Park in Russia [4]. Adult specimens reach a length of 25 cm and a weight of 15 g; males are larger, have larger heads, and longer hind limbs. Sexual dimorphism becomes particularly evident during the reproductive season, when males develop femoral pores and aggressive behavior. They feed on larvae and adult insects, worms, and, occasionally, fruits and vegetables; several cases of cannibalism have been reported [5].

Figure 1. Male specimen of Podarcis siculus in an antrophic environment.
Its presence close to urban centers and the ability to live in altered anthropogenic habitats, without a significant loss of biodiversity over time, allows it to be considered a non-threatened IUCN species [6]. In addition, the possibility of keeping animals even for a long time in terrariums has made this lizard an interesting experimental model to study the biology of Reptilia in general, and reproduction in particular, in a typical oviparous species. In captivity, P. siculus specimens in fact continue to exhibit the essential features shown in the wild provided that natural photothermal conditions are maintained. Reptiles are pivotal in vertebrate evolution and were the first to develop the amniote condition. Although they are an often-neglected biological model, they can provide interesting information on comparative endocrinology and, above all, on developmental biology.
Studies on P. siculus gametogenesis and embryonic development have accumulated since the 1960s. The advancement of morphological and molecular techniques has provided increasing details on mechanisms of neuroendocrine control, on hormones, and on hormone receptors; many studies have also concerned the processes of vitellogenesis and oocyte growth.
P. siculus has been used also in toxicity studies providing evidence on responses to soil contamination; in fact, this lizard lives in strict contact with the soil, and contaminants can be absorbed via skin, inhalation, or diet [7]. Eggs are laid in soil, and since they have a water-semipermeable shell, they can absorb contaminants such as pesticides or pollutants dispersed by contaminated superficial water used for irrigation [8][9][10]. For this reason, toxicological studies have increased considerably in recent years in the attempt to determine the impact of environmental pollution on reproductive fitness.
2. Podarcis siculus Spermatogenesis
2.1. Testis Morphology
In this lizard, testes have a typical tubular organization that changes during the annual cycle determining six different conditions [11][12][13][14]. During the breeding season (May–June), the seminiferous epithelium is thick, germ cells are in all stages of differentiation, and it is rich in spermatozoa ready to be ejaculated. In the summer stasis (July–August), tubules regress and the epithelium becomes thin and is composed only of spermatogonia and Sertoli cells. In early (September) and mid-autumn (October–November), a recovery occurs: spermatogenesis is resumed, spermatocytes I (early autumn) are produced, and then all the germ cells, including few spermatozoa (mid-autumn). The latter, however, are not used for reproduction; this spermatogenic recovery is considered the reminiscence of two reproductive events once present in the ancestor of this lizard, living in a milder environment [14]. This morphological organization of the seminiferous tubules is maintained during the winter stasis, while during spring recovery there is the complete activation of spermatogenesis, and the tubules are rich in spermatozoa ready to be ejaculated (Figure 2). Photoperiod and temperature are the most important players in the regulation of spermatogenesis [13][15][16][17].
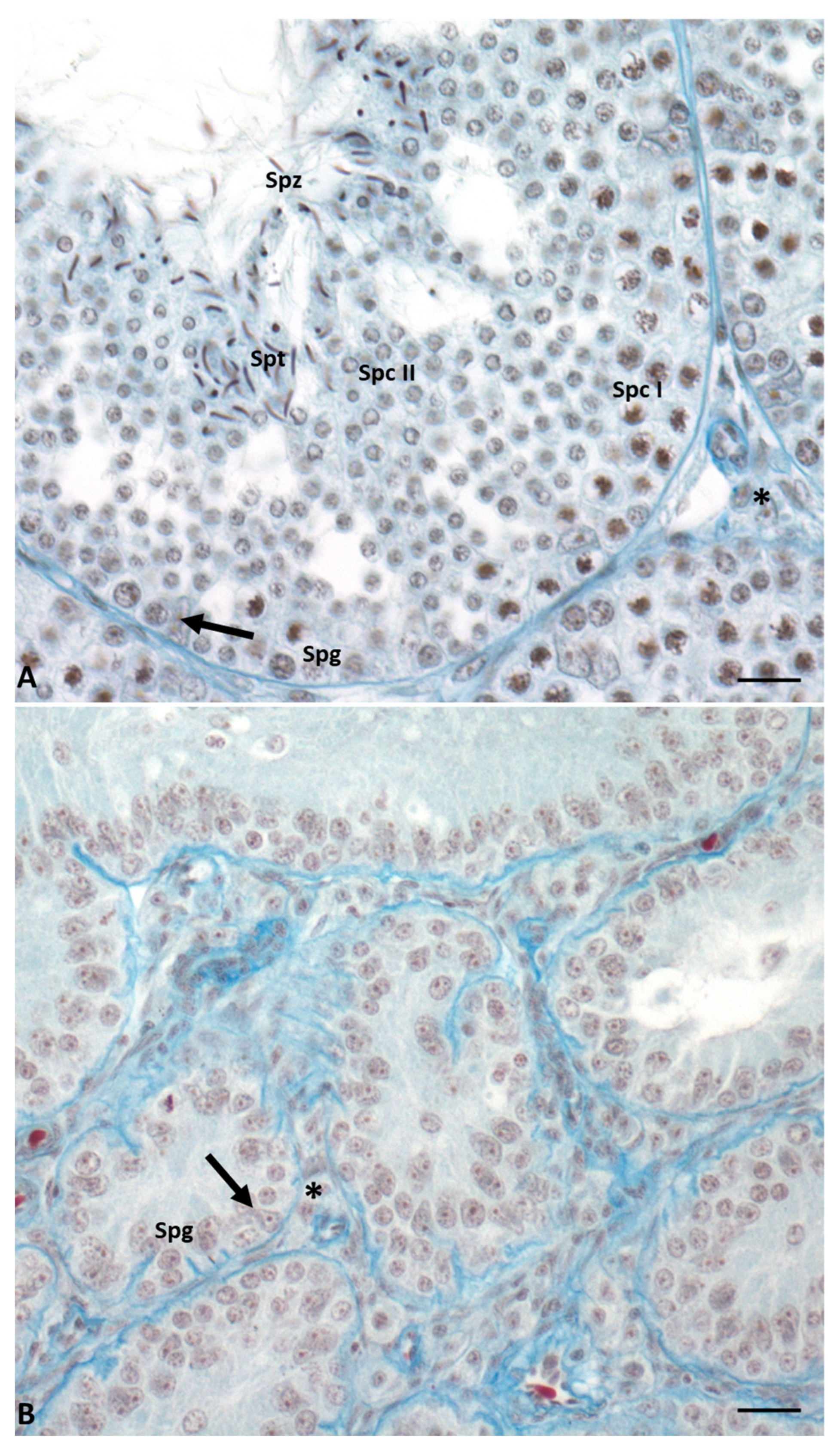
Figure 2. Cross-section of control lizard testis, stained with Mallory’s trichrome, during the two main phases of the reproductive cycle: reproductive period (A) and summer stasis (B). (A) During this period, the seminiferous tubules are characterized by a large lumen and a thick wall with germ cells in all stages of differentiation, i.e., spermatogonia (Spg), spermatocytes I (Spc I) and II (Spc II), spermatids (Spt), and spermatozoa (Spz). Triangular-shaped Sertoli cells (arrow) and Leydig cells in the interstitial space (asterisk) are clearly visible. (B) In this period, seminiferous tubules are smaller and characterized by the absence of lumen and the presence of only spermatogonia (Spg) in the thin wall. Sertoli cells (arrow) and Leydig cells in the interstitial space (asterisk) are always present. Scale bars at 10 µm.
2.2. Control of Spermatogenesis
P. siculus represents one of the main animal models used to study the intricate process of spermatogenesis since the 1950s, as evidenced by the studies of Galgano and D’Amore [11]. Over time, an abundance of studies has documented spermatogenesis control mechanisms in P. siculus, many of which helped to elucidate the spermatogenic process in other vertebrates that share a similar testicular organization, including mammals. It has been shown that the process is regulated by a network of interaction between several factors of both systemic and testicular origin [14]; among these, the pituitary adenylate cyclase activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) [18][19][20][21][22][23], D-aspartic amino acid (D-Asp) [24][25], beta-endorphin [26][27], retinoic acid [28], and steroidogenic enzymes [22][29][30] deserve to be mentioned. Numerous studies have demonstrated the presence in P. siculus testis of these molecules with their corresponding receptors both in the germline and in somatic cells, suggesting their involvement in the control of testicular activities [19][20][21]. In detail, qPCR experiments revealed a cyclical pattern of PACAP and VIP mRNA levels during the different phases of the reproductive cycle, with the lowest mRNA expression levels recorded during the summer and winter stasis, when spermatogenesis is blocked; the highest transcript levels were detected during the reproductive period, characterized by maximum testicular activity; finally, intermediate expression levels were found in the recovery phases, when spermatogenesis is reactivated [19][20][21]. These data, therefore, highlight that the seasonal changes in PACAP and VIP mRNA levels are directly linked to changes in P. siculus testis activity. Furthermore, in situ hybridization and immunocytochemical investigations have shown that PACAP, VIP, and their receptors are widely distributed in the Podarcis testis throughout the reproductive cycle and localized in both germ cells and somatic cells [19][20][21][22][23][29]. Taken together, all these data suggest that PACAP and VIP could act as local control factors of spermatogenesis and spermiogenesis in P. siculus.
In addition to neuropeptides, the presence in the testis of P. siculus of beta-endorphin (β-EP), an endogenous opioid peptide involved in the control of reproductive processes in vertebrates, has also been demonstrated [26][27][31][32]. Immunohistochemistry demonstrates a seasonal variation in β-EP positivity, with a strong signal, especially in the interstitial cells of sexually quiescent lizards (December) [26].
In another study, it was shown that an additional molecule such as vitamin A and its main biologically active derivative, retinoic acid (RA), is involved in the control of lizard spermatogenesis [28]. Indeed, quantitative PCR analysis showed a different expression of its receptors (RAR α and RAR β) during the different phases of the reproductive cycle. In the reproductive period (May), only RAR β is expressed, while in the post-reproductive phase (August) only the transcript for RAR α is present [33].
More recently, through biochemical and molecular investigations in the testis of P. siculus, the expression of the Vasa gene, which encodes a protein involved in different aspects of germ cell development in many animals [34], has been demonstrated in three significant moments of the reproductive cycle: reproductive period, summer stasis, and autumn recovery [35][36]. Using immunohistochemical investigations, the authors also demonstrated the presence of the Vasa protein in germ cells, from spermatogonia to spermatids, but not in spermatozoa [36].
A major role in the control of P. siculus spermatogenesis is also played by steroidogenic enzymes, which represent the key to managing the local action of sex hormones on spermatogenesis, whose localization and levels have been studied (StAR, 3β-HSD, 17β-HSD, P450 aromatase, and 5α-Red) [23][30]. The presence and localization of these enzymes in the P. siculus testis throughout the reproductive cycle have been established by immunocytochemistry; this analysis showed that, in lizard testis, as occurs in mammals [37][38][39], sex hormones are produced by both germ cells and somatic cells [23][30].
The balance between cytochrome P450 aromatase expression and sex hormone levels is really interesting: the highest levels of P450 aromatase, the enzyme responsible for estrogen biosynthesis, and 17β-estradiol were recorded during the summer and winter stasis when the hormone is involved in blocking the spermatogenesis [15][23][40]; conversely, the highest level of testosterone and the concurrently low level of P450 aromatase were detected during the recovery period and, in particular, the reproductive period, when testosterone is responsible for the massive production of spermatozoa and the development and maintenance of secondary sexual characteristics [13][23][40].
References
- Corti, C.; Biaggini, M.; Capula, M. Podarcis siculus (Rafinesque–Schmaltz, 1810). In Fauna d’ Italia: Reptilia; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Edizioni Calderini: Bologna, Italy, 2011; Volume 4, pp. 407–417.
- Adamopoulou, C.; Paburkefilis, P. Eaten or beaten? Severe population decline of the invasive lizard Podarcis siculus (Rafinesque-Schmaltz, 1810) after an eradication project in Athens, Greece. Herpetozoa 2019, 32, 165–169.
- Burke, R.L.; Hussain, A.A.; Storey, J.M.; Kenneth, B.; Storey, K.B. Freeze Tolerance and Supercooling Ability in the Italian Wall Lizard, Podarcis sicula, Introduced to Long Island, New York. Copeia 2002, 3, 836–842.
- Tuniyev, B.S.; Shagarov, L.M.; Arribas, O.J. Podarcis siculus (Reptilia: Sauria: Lacertidae), a new alien species for Russian fauna. Proc. Zool. Inst. RAS 2020, 324, 364–370.
- Grano, M.; Cattaneo, C.; Cattaneo, A. A case of cannibalism in Podarcis siculus campestris De Betta, 1857 (Reptilia, Lacertidae). Biodivers. J. 2011, 2, 151–152.
- Rondinini, C.; Battistoni, A.; Peronace, V.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2013.
- Sciarrillo, R.; Falzarano, A.; Gallicchio, V.; Mileo, A.; De Falco, M. Toxic effects on the thyroid gland of adult male lizards (Podarcis siculus) in contact with soil contaminated with polychlorinated biphenyls (PCBs). Int. J. Mol. Sci. 2022, 23, 4790.
- Trinchella, F.; Cannetiello, M.; Simoniello, P.; Filosa, S.; Scudiero, R. Differential gene expression profiles in embryos of the lizard Podarcis sicula under in ovo exposure to cadmium. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 33–39.
- Simoniello, P.; Trinchella, F.; Filosa, S.; Scudiero, R.; Magnani, D.; Theil, T.; Motta, C.M. Cadmium contaminated soil affects retinogenesis in lizard embryos. J. Exp. Zool. 2014, 321A, 207–219.
- Simoniello, P.; Motta, C.M.; Scudiero, R.; Trinchella, F.; Filosa, S. Cadmium-induced teratogenicity in lizard embryos: Correlation with metallothionein gene expression. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 119–127.
- Galgano, M.; D’Amore, C. Il ciclo sessuale annuo nel maschio di Lacerta s. sicula Raf. Monit. Zool. Ital. 1954, 62, 320–325.
- Angelini, F.; Picariello, O. The course of spermatogenesis in reptilia. Accad. Delle Sci. Fis. E Mat. 1975, 6, 62–107.
- Angelini, F.; Botte, V. Spermatogenesis in Reptiles, Dynamic and Regulatory Aspect. In Sex Origin and Evolution; Dallai, R., Ed.; Mucchi Selected Symposia and Monographs UZI: Modena, Italy, 1992; Volume 6, pp. 211–223.
- Rosati, L.; Agnese, M.; Di Lorenzo, M.; Barra, T.; Valiante, S.; Prisco, M. Spermatogenesis and regulatory factors in the wall lizard Podarcis sicula. Gen. Comp. Endocrinol. 2020, 298, 113579.
- Angelini, F.; Botte, V.; D’Alterio, E. Autumn photothermal regimes and reproductive functions in the female lizard Podarcis S. sicula Raf. Monit. Zool. Ital. 1982, 16, 133–148.
- Angelini, F.; Picariello, O.; Botte, V. Influence of photoperiod and temperature on the testicular activity of the lizard, Lacerta sicula sicula Raf. Boll. Zool. 1976, 43, 111–123.
- Angelini, F.; Ciarcia, G.; Botte, V. Ambient Crues and Sexual Cycle in the Lizard, Podarcis s. sicula Raf. In Endocrine Regulations as Adaptative Mechanisms to Environments; Assenmacher, I., Boissin, J., Eds.; Editions CNRS: Paris, France, 1986; pp. 227–232.
- Agnese, M.; Rosati, L.; Muriano, F.; Valiante, S.; Laforgia, V.; Andreuccetti, P.; Prisco, M. Expression of VIP and its receptors in the testis of the spotted ray Torpedo marmorata (Risso 1880). J. Mol. Neurosci. 2012, 48, 638–646.
- Agnese, M.; Rosati, L.; Coraggio, F.; Valiante, S.; Prisco, M. Molecular cloning of VIP and distribution of VIP/VPACR system in the testis of Podarcis sicula. J. Etuniyevxp. Zool. A Ecol. Genet. Physiol. 2014, 321, 334–347.
- Agnese, M.; Rosati, L.; Prisco, M.; Coraggio, F.; Valiante, S.; Scudiero, R.; Laforgia, V.; Andreuccetti, P. The VIP/VPACR system in the reproductive cycle of male lizard Podarcis sicula. Gen. Comp. Endocrinol. 2014, 205, 94–101.
- Rosati, L.; Prisco, M.; Coraggio, F.; Valiante, S.; Scudiero, R.; Laforgia, V.; Andreuccetti, P.; Agnese, M. PACAP and PAC₁ receptor in the reproductive cycle of male lizard Podarcis sicula. Gen. Comp. Endocrinol. 2014, 205, 102–108.
- Rosati, L.; Prisco, M.; Di Fiore, M.M.; Santillo, A.; Sciarrillo, R.; Valiante, S.; Laforgia, V.; Coraggio, F.; Andreuccetti, P.; Agnese, M. Sex steroid hormone secretion in the wall lizard Podarcis sicula testis: The involvement of VIP. J. Exp. Zool. A Ecol. Genet. Physiol. 2015, 323, 714–721.
- Rosati, L.; Prisco, M.; Di Fiore, M.M.; Santillo, A.; Valiante, S.; Andreuccetti, P.; Agnese, M. Role of PACAP on testosterone and 17β-estradiol production in the testis of wall lizard Podarcis sicula. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 180–186.
- Raucci, F.; D’Aniello, S.; Di Fiore, M.M. Endocrine roles of D-aspartic acid in the testis of lizard Podarcis s. sicula. J. Endocrinol. 2005, 187, 347–359.
- Raucci, F.; Di Fiore, M.M. The reproductive activity in the testis of Podarcis s. sicula involves D-aspartic acid: A study on c-kit receptor protein, tyrosine kinase activity and PCNA protein during annual sexual cycle. Gen. Comp. Endocrinol. 2009, 161, 373–383.
- Ciarcia, G.; Facchinetti, F.; Vallarino, M.; Pestarino, M.; Paolucci, M.; Cardone, A.; Fasano, S.; Pierantoni, R.; Genazzani, A.R. Opioid peptides and testicular activity in the lizard Podarcis s. sicula Raf. J. Endocrinol. 1994, 143, 565–571.
- Ciarcia, G.; Cardone, A.; Paolucci, M.; Botte, V. In vitro effects of beta-endorphin on testicular release of androgens in the lizard Podarcis sicula Raf. Mol. Reprod. Dev. 1996, 45, 308–312.
- Comitato, R.; Esposito, T.; Cerbo, G.; Angelini, F.; Varriale, B.; Cardone, A. Impairment of spermatogenesis and enhancement of testicular germ cell apoptosis induced by exogenous all-trans-retinoic acid in adult lizard Podarcis sicula. J. Exp. Zool. A Comp. Exp. Biol. 2006, 305, 288–298.
- Rosati, L.; Agnese, M.; Di Fiore, M.M.; Andreuccetti, P.; Prisco, M. P450 aromatase: A key enzyme in the spermatogenesis of the Italian wall lizard, Podarcis sicula. J. Exp. Biol. 2016, 219, 2402–2408.
- Rosati, L.; Santillo, A.; Di Fiore, M.M.; Andreuccetti, P.; Prisco, M. Testicular steroidogenic enzymes in the lizard Podarcis sicula during the spermatogenic cycle. Comptes Rendus Biol. 2017, 340, 492–498.
- Knotts, L.K.; Glass, J.D. Effects of Photoperiod, Beta-Endorphin, and Naloxone on in Vitro Secretion of Testosterone in White-50 Footed Mouse (Peromyscus Leucopus) Testes. Biol. Reprod. 1988, 39, 205–212.
- Chandrashekar, V.; Bartke, A. The influence of beta-endorphin on testicular endocrine function in adult rats. Biol. Reprod. 1992, 47, 1–5.
- Esposito, T.; Caccavo, M.; Cianci, A.; Cardone, A.; Angelini, F.; Varriale, B. Sequence analysis of retinoic acid receptor alpha, beta and gamma isoforms in the lizard, Podarcis sicula. J. Steroid. Biochem. Mol. Biol. 2007, 104, 143–153.
- Saffman, E.; Lasko, P. Germline development in vertebrates and invertebrates. Cell. Mol. Life Sci. 1999, 55, 1141–1163.
- Milani, L.; Maurizii, M.G. First evidence of Vasa expression in differentiating male germ cells of a reptile. Mol. Reprod. Dev. 2014, 81, 678.
- Milani, L.; Maurizii, M.G. Vasa expression in spermatogenic cells during the reproductive-cycle phases of Podarcis sicula (Reptilia, Lacertidae). J. Exp. Zool. B Mol. Dev. Evol. 2015, 324, 424–434.
- Carreau, S.; Silandre, D.; Bourguiba, S.; Hamden, K.; Said, L.; Lambard, S.; Galeraud-Denis, I.; Delalande, C. Estrogens and male reproduction: A new concept. Braz. J. Med. Biol. Res. 2007, 40, 761–768.
- Carreau, S.; Bois, C.; Zanatta, L.; Silva, F.R.; Bouraima-Lelong, H.; Delalande, C. Estrogen signaling in testicular cells. Life Sci. 2011, 89, 584–587.
- O’Donnell, L.; Nicholls, P.K.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35.
- Andò, S.; Panno, M.L.; Ciarcia, G.; Imbrogno, E.; Buffone, M.; Beraldi, E.; Sisci, D.; Angelini, F.; Botte, V. Plasma sex hormone concentrations during the reproductive cycle in the male lizard, Podarcis s. sicula. J. Reprod. Fertil. 1990, 90, 353–360.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
20 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




