Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luigi Rosati and Version 2 by Rita Xu.
Spermatogenesis is a genetically driven differentiation process that occurs in the testis and leads to the formation of spermatozoa. This process is extensively studied in several experimental models, particularly in vertebrates that share the morphological structure and functionality of the mammalian testis. Reptiles are not generally considered biological models, the lizard
Podarcis siculus
has represented a suitable organism for the study of spermatogenesis. In this lizard, the process of spermatogenesis is regulated by the interaction between systemic factors such as gonadotropins and local factors, i.e., molecules produced by the somatic and germinal cells of the testis.
- reptiles
- spermatogenesis
- steroidogenesis
- reproduction
1. Introduction
Among the reptiles inhabiting the Italian peninsula, the specimens of the field lizard Podarcis siculus (Rafinesque-Schmaltz, 1810) (Sauria, Lacertidae) are the most abundant (Figure 1). The Italian wall lizard, formerly known as Lacerta sicula, then Podarcis sicula, and currently P. siculus, is an endemic species of the Mediterranean regions, from the Iberian Peninsula to Tunisia [1]. It prefers warm, arid climates and for this it tends to disappear over 1000 m altitude. Their habitat varies from rural areas to cultivated fields and city gardens. It is often considered an invasive alien species dangerous to the native species [2]; a population had even been introduced in 1967 to New York [3], and, more recently, an abundant population has been found in a Natural Ornithological Park in Russia [4]. Adult specimens reach a length of 25 cm and a weight of 15 g; males are larger, have larger heads, and longer hind limbs. Sexual dimorphism becomes particularly evident during the reproductive season, when males develop femoral pores and aggressive behavior. They feed on larvae and adult insects, worms, and, occasionally, fruits and vegetables; several cases of cannibalism have been reported [5].

Figure 1. Male specimen of Podarcis siculus in an antrophic environment.
Its presence close to urban centers and the ability to live in altered anthropogenic habitats, without a significant loss of biodiversity over time, allows it to be considered a non-threatened IUCN species [6]. In addition, the possibility of keeping animals even for a long time in terrariums has made this lizard an interesting experimental model to study the biology of Reptilia in general, and reproduction in particular, in a typical oviparous species. In captivity, P. siculus specimens in fact continue to exhibit the essential features shown in the wild provided that natural photothermal conditions are maintained. Reptiles are pivotal in vertebrate evolution and were the first to develop the amniote condition. Although they are an often-neglected biological model, they can provide interesting information on comparative endocrinology and, above all, on developmental biology.
Studies on P. siculus gametogenesis and embryonic development have accumulated since the 1960s. The advancement of morphological and molecular techniques has provided increasing details on mechanisms of neuroendocrine control, on hormones, and on hormone receptors; many studies have also concerned the processes of vitellogenesis and oocyte growth.
P. siculus has been used also in toxicity studies providing evidence on responses to soil contamination; in fact, this lizard lives in strict contact with the soil, and contaminants can be absorbed via skin, inhalation, or diet [7]. Eggs are laid in soil, and since they have a water-semipermeable shell, they can absorb contaminants such as pesticides or pollutants dispersed by contaminated superficial water used for irrigation [8][9][10][8,9,10]. For this reason, toxicological studies have increased considerably in recent years in the attempt to determine the impact of environmental pollution on reproductive fitness.
2. Podarcis siculus Spermatogenesis
2.1. Testis Morphology
In this lizard, testes have a typical tubular organization that changes during the annual cycle determining six different conditions [11][12][13][14][17,18,19,20]. During the breeding season (May–June), the seminiferous epithelium is thick, germ cells are in all stages of differentiation, and it is rich in spermatozoa ready to be ejaculated. In the summer stasis (July–August), tubules regress and the epithelium becomes thin and is composed only of spermatogonia and Sertoli cells. In early (September) and mid-autumn (October–November), a recovery occurs: spermatogenesis is resumed, spermatocytes I (early autumn) are produced, and then all the germ cells, including few spermatozoa (mid-autumn). The latter, however, are not used for reproduction; this spermatogenic recovery is considered the reminiscence of two reproductive events once present in the ancestor of this lizard, living in a milder environment [14][20]. This morphological organization of the seminiferous tubules is maintained during the winter stasis, while during spring recovery there is the complete activation of spermatogenesis, and the tubules are rich in spermatozoa ready to be ejaculated (Figure 2). Photoperiod and temperature are the most important players in the regulation of spermatogenesis [13][15][16][17][15,19,21,22].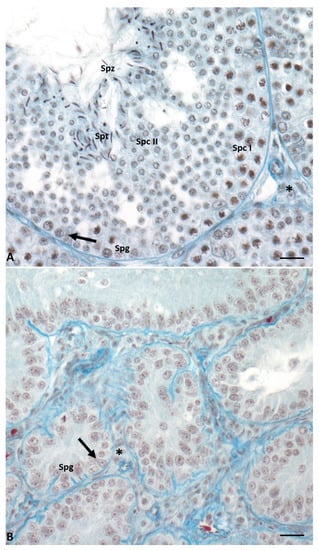
Figure 2. Cross-section of control lizard testis, stained with Mallory’s trichrome, during the two main phases of the reproductive cycle: reproductive period (A) and summer stasis (B). (A) During this period, the seminiferous tubules are characterized by a large lumen and a thick wall with germ cells in all stages of differentiation, i.e., spermatogonia (Spg), spermatocytes I (Spc I) and II (Spc II), spermatids (Spt), and spermatozoa (Spz). Triangular-shaped Sertoli cells (arrow) and Leydig cells in the interstitial space (asterisk) are clearly visible. (B) In this period, seminiferous tubules are smaller and characterized by the absence of lumen and the presence of only spermatogonia (Spg) in the thin wall. Sertoli cells (arrow) and Leydig cells in the interstitial space (asterisk) are always present. Scale bars at 10 µm.
