Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriela Guillena | -- | 3502 | 2022-12-18 19:57:15 | | | |
| 2 | Rita Xu | Meta information modification | 3502 | 2022-12-19 04:38:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marset, X.; Guillena, G. Deep Eutectic Solvents as à-la-Carte Medium. Encyclopedia. Available online: https://encyclopedia.pub/entry/38933 (accessed on 06 February 2026).
Marset X, Guillena G. Deep Eutectic Solvents as à-la-Carte Medium. Encyclopedia. Available at: https://encyclopedia.pub/entry/38933. Accessed February 06, 2026.
Marset, Xavier, Gabriela Guillena. "Deep Eutectic Solvents as à-la-Carte Medium" Encyclopedia, https://encyclopedia.pub/entry/38933 (accessed February 06, 2026).
Marset, X., & Guillena, G. (2022, December 18). Deep Eutectic Solvents as à-la-Carte Medium. In Encyclopedia. https://encyclopedia.pub/entry/38933
Marset, Xavier and Gabriela Guillena. "Deep Eutectic Solvents as à-la-Carte Medium." Encyclopedia. Web. 18 December, 2022.
Copy Citation
Regarding the chemical industry, one of the most significant issues to be addressed is the use of volatile organic compounds (VOCs) as solvents because they are petrol-derived and most of them are toxic and flammable. Among the possible solutions, deep eutectic solvents (DESs) have emerged as sustainable alternatives to VOCs in organic catalyzed transformations and other fields. The advantages of these new reaction media are not only related to their more benign physical and chemical properties and, for most of them, their renewable sources but also due to the possibility of being recycled after their use, increasing the sustainability of the catalyzed process in which they are involved.
deep eutectic solvent
alternative solvent
sustainable chemistry
transition metal
1. Introduction
Over the last decades, sustainability has been a key factor in modern life. Rapidly evolving society and its needs cause an increase in industrial production, and thus more environmental concerns. This trend was reflected in 2015 by the United Nations when a development plan that was focused on sustainability was published under the title “Transforming Our World: The 2030 Agenda for Sustainable Development” [1]. This trend applies to all aspects of modern society, including chemistry.
The use of solvents in the chemical industry can easily be identified as one of the major concerns when applying sustainable principles. Traditionally, volatile organic compounds (VOCs) have been employed as solvents. Most of these compounds are fossil fuel derivatives, which are not renewable, highly toxic, non-degradable, and are accumulated in the atmosphere due to their low boiling points, leaving behind a very high carbon footprint. According to recent reports, solvents constitute 80 to 90% of the non-aqueous mass employed when synthesizing an active pharmaceutical ingredient (API) [1]. Owing to these facts, finding alternative solvents is crucial to improve the sustainability of these industries.
In this sense, deep eutectic solvents (DESs) have been proposed as alternative solvents in organic chemistry. DESs are defined as systems formed from a eutectic mixture of two or more components, usually Lewis or Brønsted acids and bases that can contain a variety of anionic and/or cationic species [2]. Eutectic formation occurs through intermolecular interactions between DES constituents (with hydrogen bonds being one of the most significant ones), but there are non-covalent or ionic bonds involved. These interactions decrease the lattice energy, explaining the drop in melting point, compared with their components.
-
Type I: formed by a quaternary ammonium salt and a metal chloride.
-
Type II: differs from type I in having hydrated metal halides instead of non-hydrated ones.
-
Type III: formed from quaternary ammonium salt and hydrogen bond donors (HBDs), e.g., alcohols, amino acids or amides.
-
Type IV: formed from transition-metal salts and HBDs.
-
Type V: formed exclusively by non-ionic components.
Eutectic mixtures can be synthesized by mixing their components in an appropriate ratio and heating them slightly until a melt is formed. Thus, the atom economy of the process is complete, and no by-products are formed. The easy preparation and atom economy are two of the main advantages offered by DESs, compared to the related ionic liquids. In addition to this, other advantages in their use are their negligible boiling points, the cost of their components, their renewable origin and low toxicity, and high biodegradability. These facts make them promising candidates to be used for the replacement of VOCs in organic reactions.
Not surprisingly, although early applications of DESs were related to metal extraction, electrodeposition or electropolishing were soon considered as a reaction media for organic transformations. Although, in the past 10 years, DESs proved to be useful reaction media in a myriad of organic transformations, this research will focus on transition-metal-catalyzed processes, tackling especially homogeneous catalysis. Attending to the previous classification, types I, II and IV can contain a transition metal in their composition and may act as both a solvent and catalyst, while the more environmentally friendly types III and V require the use of an external metal precursor as a reaction catalyst. In these catalytic transformations, it is essential to ensure the compatibility of the catalyst with the used DESs to provide the expected products selectively and efficiently. Due to the high polar nature of DESs, sometimes transition-metal salts can be used as a catalyst, but in most cases, the design of ligands to stabilize or modify the reactivity of the metals is required. In any case, DESs provide a unique advantage compared to VOCs, which is the possible recovery of the ligand–metal–solvent system. This allows their recyclability and enhances the sustainability of a given process. Additionally, DESs can solubilize some gases to a greater extent, compared to VOCs and, therefore, they are excellent solvent systems to perform hydrogenations, oxidations and carbonylation reactions. In fact, through the careful choice of the appropriate DESs and catalysts, sulfonylation reactions can be performed in DESs by generating in situ SO2 from simple inorganic sulfur salts.
2. RedOx Processes
As commented in the introduction, types I, II and IV DESs contain metallic salts as part of their structure. Therefore, some of these eutectic mixtures can be used as solvents and catalysts at the same time. One catalytic DES system used for oxidation reactions is the mixture FeCl3.6H2O:(CH2OH)2 (2:1), which has been applied to the oxidation of cellulose to gluconic acid. The product, which could be obtained in up to 52% yield, precipitated from the reaction medium, facilitating product isolation. In addition, the DES could be reused up to five times without losing its activity with an O2 treatment after each cycle (Figure 1, Table 1, entry 1) [4]. Similarly, 5-hydroxymethylfurfural (HMF) has been obtained in high yields by treatment of glucose at 130 °C with the eutectic ChCl:CrCl3.6H2O in a biphasic mixture with solvents, such as EtOAc, through different dehydration processes [5].
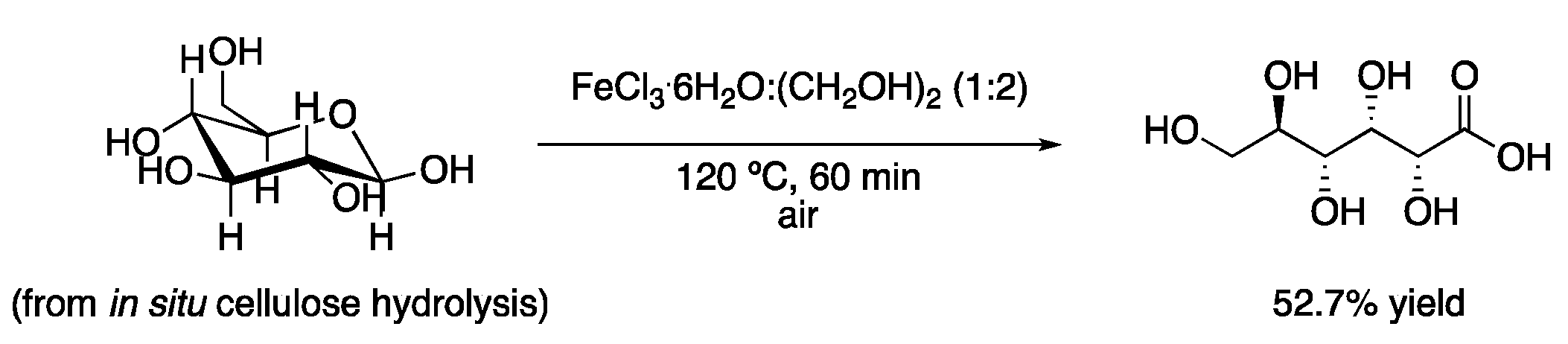
Figure 1. Hydrolysis and oxidation of cellulose to gluconic acid.
Another example of a catalytic DES system was employed for the oxidation of sulfides to sulfoxides by H2O2 using catalytic amounts of the mixture ZrOCl2.H2O:urea (1:5), although MeOH was needed as a solvent. However, in the case of oxidizing aryl boronic acids to phenols, no methanol as a co-solvent was required, performing the reaction with only DESs and 30% aqueous H2O2 as the solvent. The DES dissolved in water could be concentrated and reused up to three times, observing a slight decrease in the reaction yield [6]. Much more recently, the catalytic system based on CuCl2, TMEDA and TEMPO was employed in water and in the mixture d-fructose:urea (3:2) to perform the selective aerobic oxidation of alcohols. In addition, telescoped one-pot-hybrid reactions are feasible, in such a way that either an addition of Grignard reagents or a Henry reaction can be performed on the in situ generated aldehyde (Figure 2, Table 1, entry 2) [7].
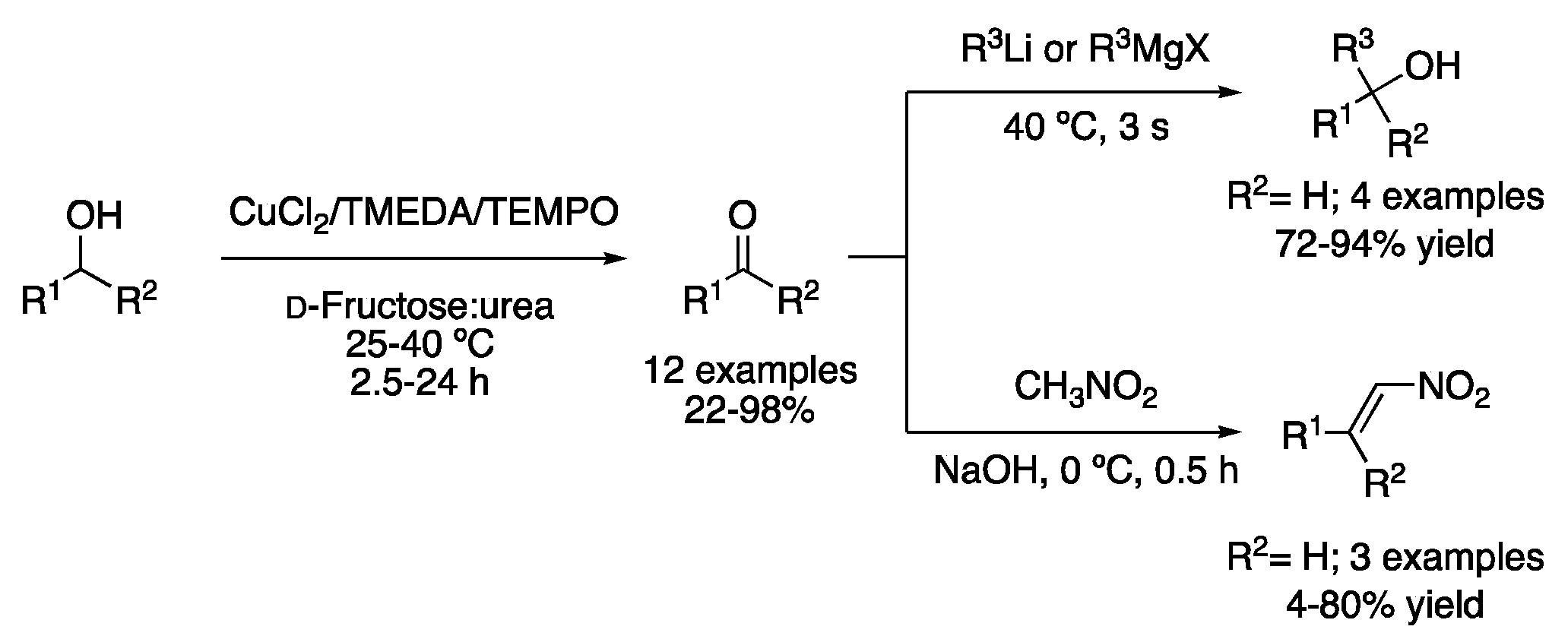
Figure 2. Oxidation of alcohols in DES and telescoped one-pot reactions.
Other oxidations are based on heterogeneous catalysts, such as metal-organic frameworks. These catalysts have been used in combination with DESs to perform reactions, such as the synthesis of HMF from carbohydrates [8] or the epoxidation of styrene [9].
Hydrogenation and hydroformylation reactions have been more extensively studied. One of the earliest examples was performed in a mixture of DMU and a ß-cyclodextrin derivative as a solvent, using Rh(acac)(CO)2 in combination with the ligand triphenylphosphine-3,3′,3′′-trisulfonic acid trisodium salt. This ionic phosphine derivative enhanced the compatibility of the catalysts with the polar solvent. Therefore, 1-decene was converted at 90 °C, 1 h into the corresponding aldehyde using 50 bar of a mixture of CO/H2, affording a ratio of the linear to the branched product of 2.3:1 [10]. Further studies by the same group on this topic using different solvent mixtures determined that the conversion was highly dependent on the solubility of the substrate and inversely dependent on the solvent viscosity [11]. The hydroformylation of 1-decene was also studied in different choline chloride-based eutectic mixtures. After screening different conditions, including several eutectic mixtures and ligands, the best results were obtained with Rh(acac)(CO)2 using BiPhePhos as a ligand in ChCl:urea (1:2) as reaction media. In this way, the yield of aldehydes was 41%, showing high selectivity to the linear aldehyde over the branched one (97:3, Figure 3, Table 1, entry 3) [12].
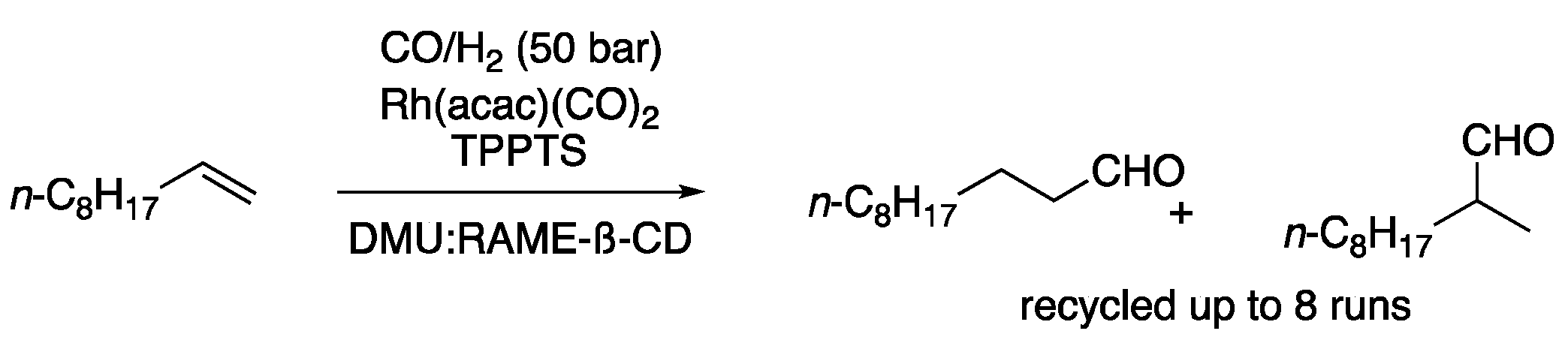
Figure 3. Hydroformylation of 1-decene.
Palladium-catalyzed hydrogenations have also been investigated in DESs media, although given the nature of these reactions, most of the systems are based on heterogeneous catalysts. One of the earliest examples is the Pd/C-catalyzed hydrogenation of phenacyl azides in ChCl:glycerol (1:2), providing intermediates, which yielded symmetrical 2,5-diarylpyrazines. In addition, the nucleophilic substitution of α-halo ketones with sodium azide can be performed in a one-pot manner using the eutectic mixture as a solvent (Figure 4, Table 1, entry 4) [13].
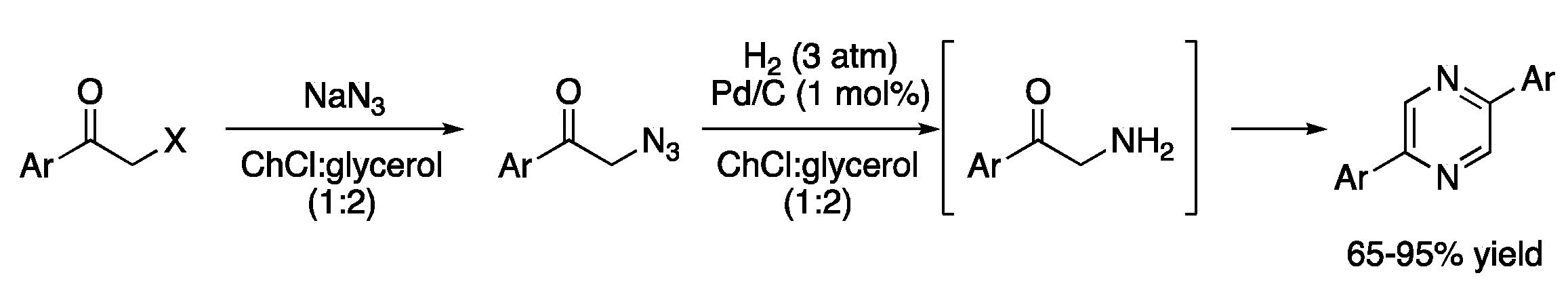
Figure 4. Hydrogenation of phenacyl azides.
The use of a mixture of choline tosylalaninate with glycerol was reported for performing Pd-NPs-catalyzed hydrogenations of 4-phenylbut-3-en-2-one and 4-phenylbutan-2-one. An interesting effect must be pointed out. This is the fact that the use of high pressures of mixtures of CO2 and H2 improved the results over the use of pure H2 atmospheres. It was rationalized that high pressures of CO2 decreased the viscosity of the DESs while improving the diffusion coefficient of H2 and reactants to almost two times their initial values, thus enhancing the catalytic activity [14]. The depolymerization of lignin is another pertinent topic in current sustainable chemistry. A linear homopolymer can be found in castor seed coats and a model benzodioxane compound present in this polymer was subjected to hydrogenolysis using Pd/C or Ru/C at 180 °C for 3 h under 3 MPa H2 atmosphere and ChCl:(CH2OH)2 (1:2) as a solvent (Figure 5, Table 1, entry 5) [15].
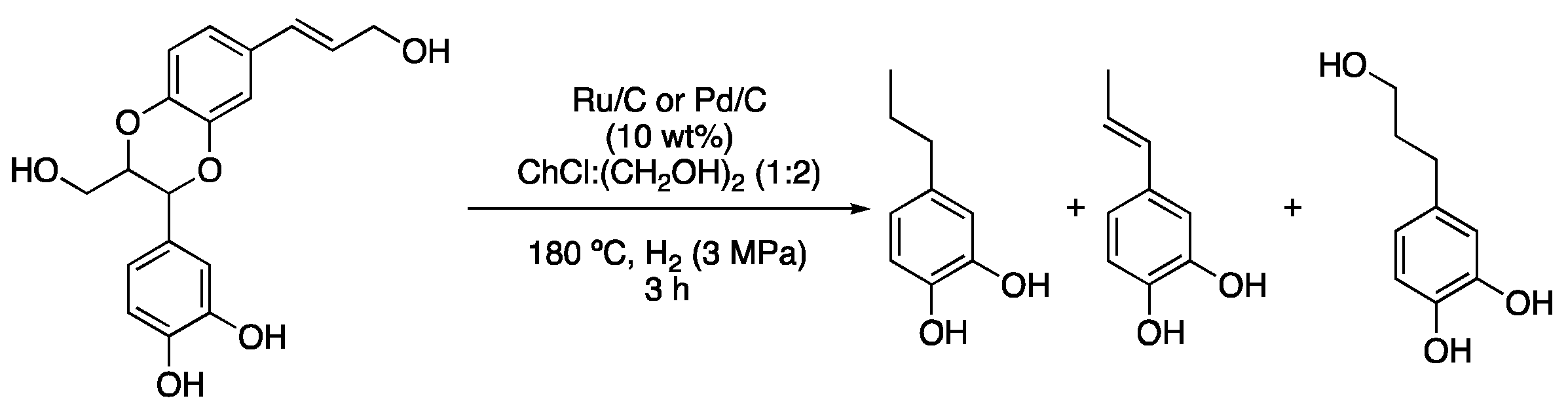
Figure 5. Hydrogenolysis of benzodioxane derivative.
Pd NPs have also been synthetized directly on DES based on bio-ammonium salts and glycerol methyl ethers. The corresponding solutions were applied to the hydrogenation of alkenes, alkynes and carbonyl compounds (Figure 6) [16].
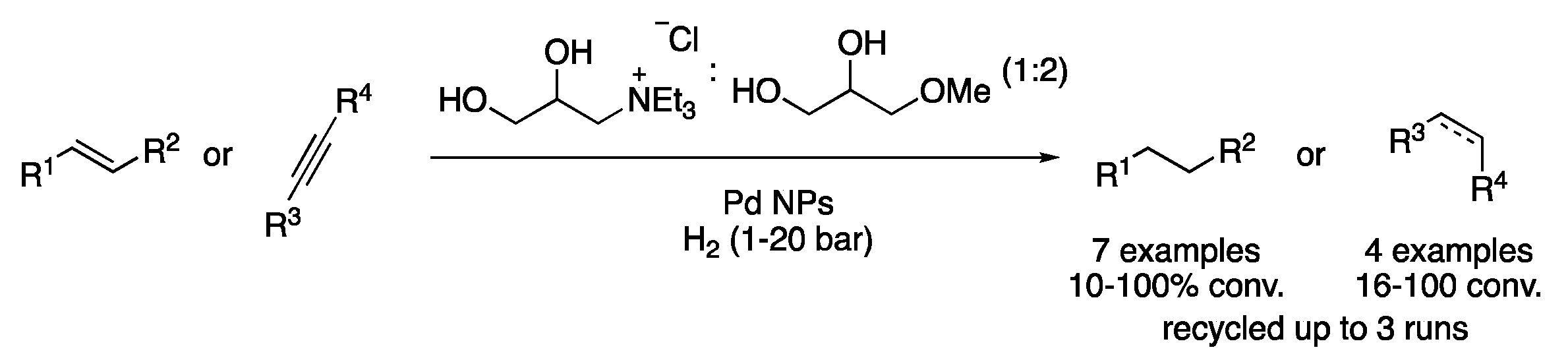
Figure 6. Pd-NPs-catalyzed hydrogenation of unsaturated compounds.
Other hydrogenation processes involve the use of DESs to prepare supported metallic catalysts, although the hydrogenation reactions are performed in other solvents, as is the case of Pd supported on pyrolyzed DES [17], Pd-DES@SiO2 [18], Pd immobilized on a composite of halloysite and DES [19] or gold nanoparticles dispersed in ChCl:urea (1:2) [20].
All these DES-mediated procedures try to reduce the environmental impact of chemical transformations by using sustainable solvents. Hydrogenations, however, fail to follow another principle of green chemistry, namely, safety, since hydrogenations require high hydrogen pressures. To avoid that, a safer method was developed by the in situ generation of hydrogen from aluminum powder and water in ChCl:glycerol (1:2) as a solvent. Thus, the generated hydrogen was employed to reduce a wide variety of functional groups, such as aldehydes, epoxides, nitro-compounds, imines, nitriles, alkenes or alkynes in good yields at only 40 °C in 8 h reaction time (Figure 7, Table 1, entry 6) [21].
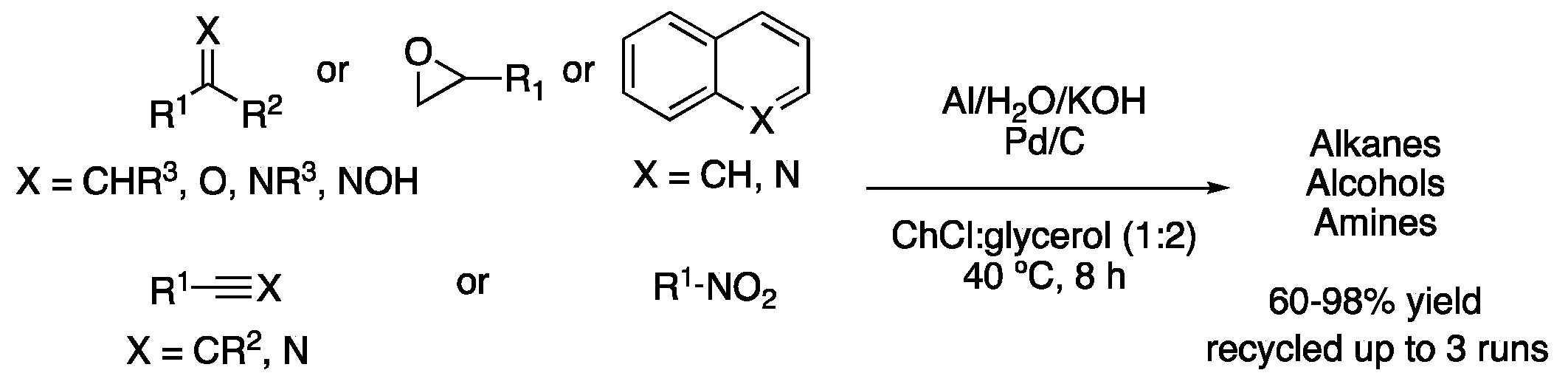
Figure 7. Hydrogenation using in situ generated H2 from Al and H2O.
To conclude, although most hydrogenations are performed using a palladium catalyst, a Ru(II)-transfer hydrogenation of carbonyl compounds was described in the biphasic mixture of TBABr:HCOOH (1:1) and cyclopentyl methyl ether (CPME). In this case, the eutectic mixture acts as a reaction medium, as well as a hydrogen source, with the complex [RuCl(p-cymene)Cl2]2 in combination with the ligand 1,1′-Ferrocenediyl-bis(diphenylphosphine) (dppf) being the catalyst. Reactions were performed between 40 and 80 °C (depending on the substrate reactivity) using triethylamine as a base (Figure 8, Table 1, entry 7) [22].
Table 1. Selected examples of RedOx processes catalyzed by transition metals in DESs.
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | Hydrolysis and oxidation | FeCl3·6H2O:(CH2OH)2 (1:2) | 120 °C | Gluconic acid | [4] |
| 2 | Alcohol Oxidation | d-fructose:urea (3:2) | 25–40 °C | Ketones/Aldehydes | [7] |
| 3 | Hydroformylation | DMU: RAME-ß-CD | CO/H2 (50 bar) | Aldehydes | [12] |
| 4 | Hydrogenations | ChCl:glycerol (1:2) | Pd/C, H2 | Diarylpyrazines | [13] |
| 5 | Hydrogenolysis | ChCl:(CH2OH)2 (1:2) | Ru/C or Pd/C, H2 | Resorcinol derivatives | [15] |
| 6 | Hydrogenation | ChCl:glycerol (1:2) | Al, KOH, H2O | Alkanes/alcohols/amines | [21] |
| 7 | Transfer hydrogenation | TBAB:HCOOH (1:1) | [Ru], NEt3, CPME | Secondary alcohols | [22] |
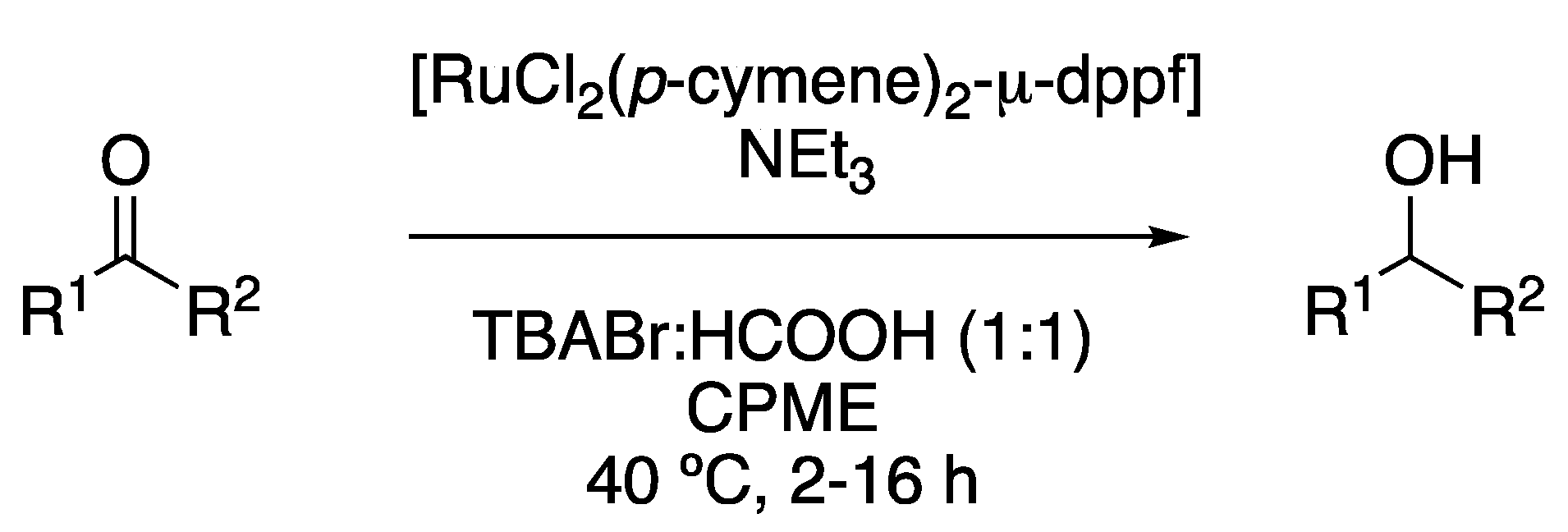
Figure 8. Ru-catalyzed transfer hydrogenation.
3. Cyclization Reactions
Heterocyclic compounds are broadly present in nature, as well as in biologically active compounds used in the pharmaceutical industry. Therefore, loads of synthetic methods have been developed, with several strategies being carried out in DESs as reaction media. Two main strategies to generate new rings will be discussed, i.e., cycloadditions and condensation reactions.
3.1. Cycloadditions
One of the first examples of cycloadditions in DESs is based on the cycloisomerization of γ-alkynoic acids in ChCl:urea (1:2) using a gold-based catalyst. The reaction was performed under air atmosphere at room temperature and completed in 0.25–3.5 h, with the catalyst being reusable in up to four cycles without losing its catalytic activity (Table 2, entry 1) [23]. Shortly after, the same group reported the cycloisomerization of (Z)-enynols into furans. In that case, a bis(iminophosphorane)-Au(I) complex was used in the mixture ChCl:glycerol (1:2) as a reaction medium in the absence of any other co-catalyst. In addition, the in situ generated furan derivative could be further transformed via a Diels–Alder reaction in a one-pot manner (Table 2, entry 2) [24]. Another iminophosphorane-Au complex was also described to catalyze the cycloisomerization of various alkynyl amides into alkylidene lactams using water or ChCl:urea (1:2) as solvents in good-to-excellent yields [25]. Moreover, the cycloisomerization of γ-alkynoic acids was described using a recyclable palladium supported on a magnetite catalyst. Although water was found to be an excellent solvent for this transformation, the reaction also proceeded with good yields using the mixture ChCl:urea (1:2) at 90 °C in 10 min reaction time (Figure 9) [26].
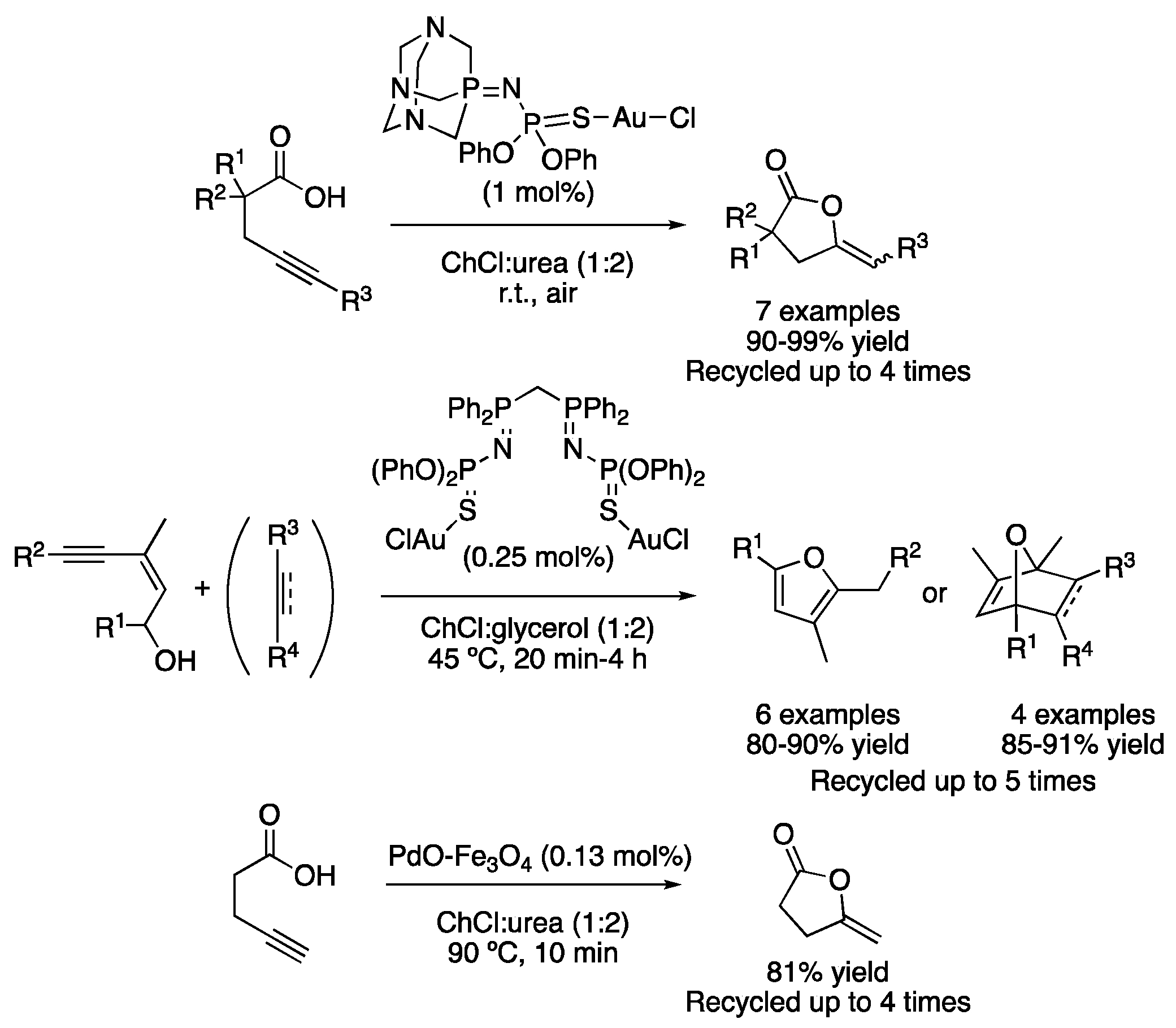
Figure 9. Cycloisomerization reactions catalyzed by Au and Pd.
Other cycloadditions take advantage of the properties of type I DESs, using them as solvents and catalysts. In this sense, the mixture ChCl:ZnCl2 (1:2) was employed for promoting the [2 + 3] cycloaddition of nitriles and sodium azide. The reaction, performed at 140 °C for 0.5–12 h, afforded excellent yields of 5-substituted 1H-tetrazoles using aromatic or benzylic nitriles as starting materials [27]. However, the more sustainable mixture ChCl:urea (1:2) can also be used to carry out this transformation using Cu(OAc)2 as catalyst. Thus, the one-pot reaction of aromatic aldehydes, hydroxylamine hydrochloride and sodium azide was performed at 100 °C for 12 h (Figure 10, Table 2, entry 3) [28].
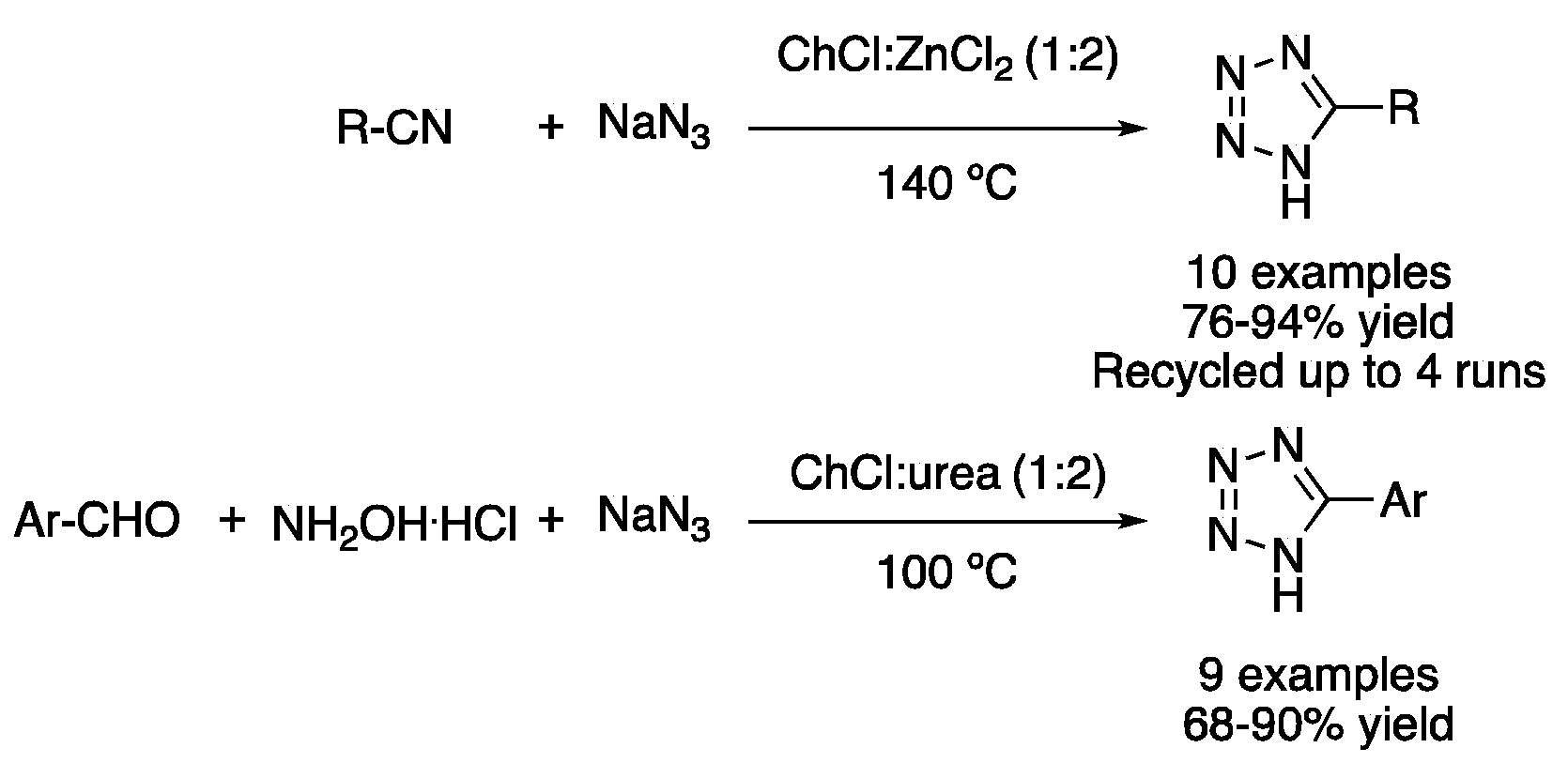
Figure 10. Tetrazole syntheses through [3 + 2] cycloadditions.
Similarly, the synthesis of triazoles via copper-catalyzed azide–alkyne cycloaddition (CuAAc) has been described in DESs media. Namely, ChCl:glycerol (1:2) was employed as a solvent to perform the copper-catalyzed azidation of aryl bromides, followed by the cycloaddition to an alkyne to yield the corresponding 1,4-disubstituted triazole derivatives. In addition, the catalytic system could be reused several times, although results were improved when fresh ligand (N,N′-dimethylethylendiamine) was added after the third cycle [29]. NADES have also been shown to promote this transformation, acting both as solvents and in stabilizing catalytic copper intermediates, as well as by interacting with the alkyne through the H bond, as demonstrated by DFT calculations. Thus, the mixtures ascorbic acid:choline chloride (1:2) and glycolic acid:trimethylglycine (2:1) were effectively used as solvents and avoided the need of using external bases or reducing agents, with the solvent being recyclable up to three times without loss of activity (Table 2, entry 4) [30]. In addition, a heterogeneous catalyst based on copper iodide supported on 3-thionicotinyl-urea-modified magnetic nanoparticles was also employed to perform this transformation using ChCl:PEG (1:4) as a solvent (Figure 11) [31].
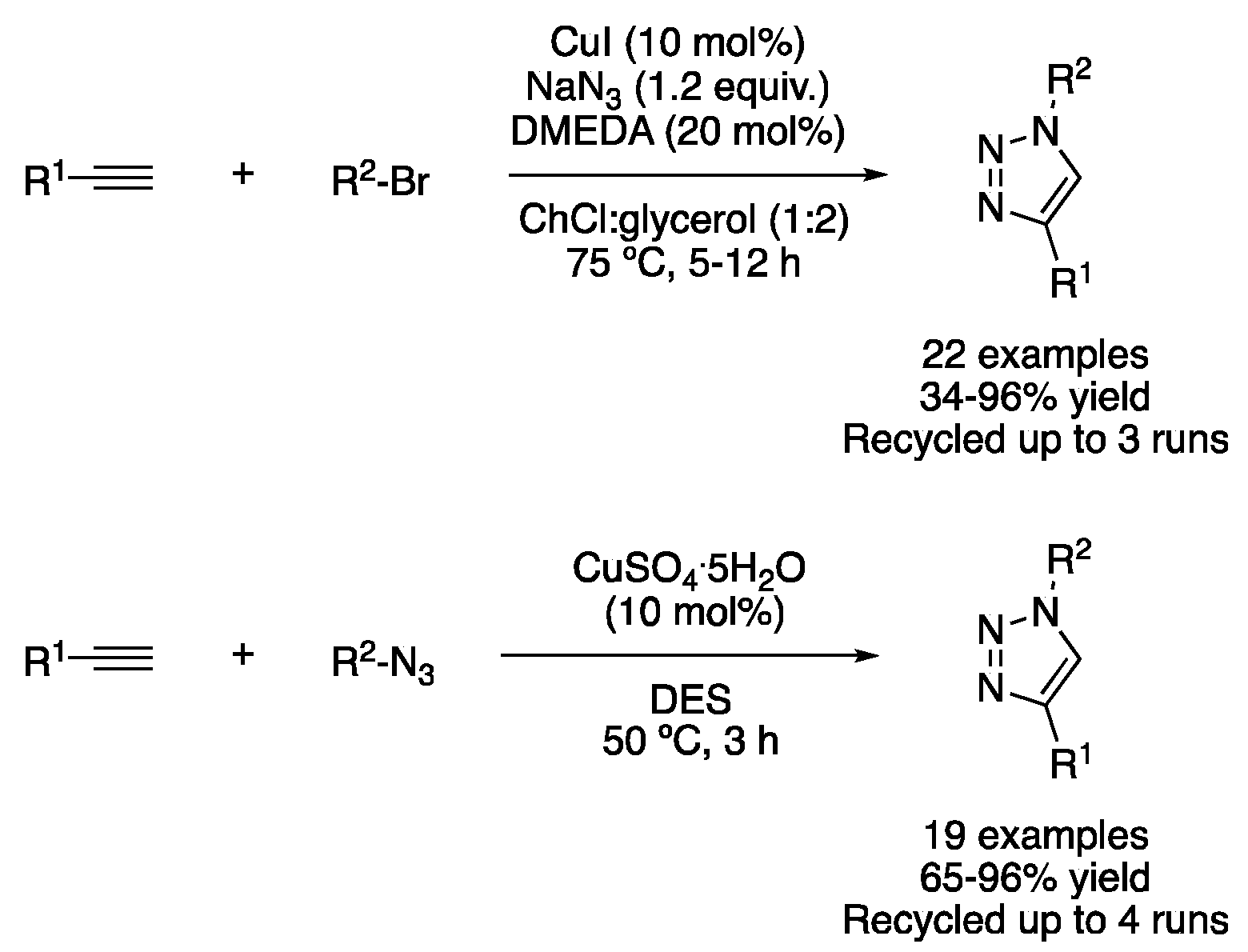
Figure 11. Syntheses of triazole derivatives.
3.2. Cyclizations through Condensation
Condensation reactions are a second approach to perform cyclization reactions. Early reports of condensation-based cyclizations in DES media were reported using heterogeneous catalysts. For instance, the mixture citric acid:DMU (7:2) was used as a solvent for performing the synthesis of [1,2-a]pyridines using 5 mol% of recyclable CuFeO2 to catalyze the reaction between 2-aminopyridine derivatives, aldehydes and alkynes. The reaction did not proceed in traditional solvents under these conditions. However, the use of an acid as a component of the eutectic mixture was crucial to obtaining good yields, proving that DES played a double role, both as a solvent and also as an acidic catalyst (Table 2, entry 5) [32]. These compounds have also been prepared in TBAB:glycerol (1:2) using copper iodide supported on magnetic nanoparticles [33]. Another example of a heterogeneous catalyst for performing cyclization reactions in DESs is based on molybdenum supported on graphene oxide/Fe3O4, which was employed for the synthesis of spiro-oxindole dihydropyridines in ChCl:urea (1:2). These reactions were carried out by the combination of isatins, malononitrile and anilinolactones at 90 °C under microwave irradiation. The catalytic system could be reused up to eight times, showing almost negligible leaching for Fe and Mo in the ChCl:urea (1:2) phase (Figure 12, Table 2, entry 6) [34]. Similarly, 2,3-dihydroquinazolin-4-(1H)-ones and spiroquinazolin-4(3H)-ones derivatives were prepared with Fe3O4@SiO2@TiO2-OSO3H magnetic nanoparticles as a catalyst in the mixture ChCl:urea (1:2), by reaction of isatonic anhydride, anilines (or ammonium chloride) and cyclic aliphatic ketones or benzaldehyde derivatives [35]. The same eutectic mixture was also employed for the preparation of 7-aryl-benzo[h]tetrazolo[5,1-b]quinazoline-5,6-dione, employing a magnetic polymeric nanostructure, with DES-catalyst being recyclable up to six times [36]. Finally, a DES-coated metal-organic framework was employed for the multicomponent synthesis of 2-amino-4H-chromenes, although, in this case, reactions were carried out in a solvent-free fashion [37].
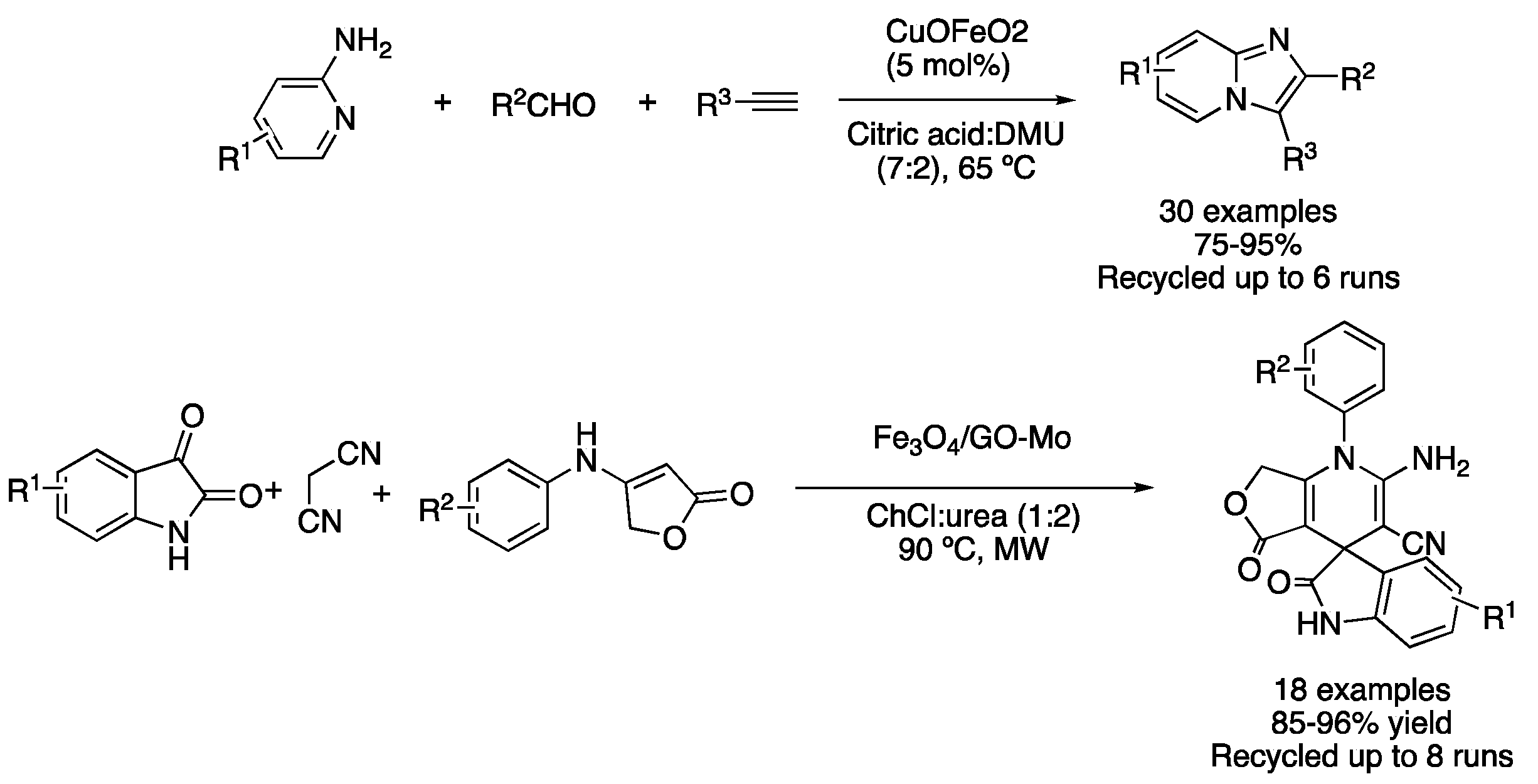
Figure 12. Heterocycle synthesis using heterogeneous catalysts in DESs.
The S-heterocyclodehydration of 1-mercapto-3-yn-2-ols catalyzed by PdI2 was reported in ChCl:glycerol (1:2) at 50 °C, affording seven examples of thiophene derivatives with good yields. The catalytic system was recycled up to six times without any loss of activity (Figure 13; Table 2, entry 7). Moreover, the starting material of this reaction could also be prepared in eutectic solvent as reaction media [38].
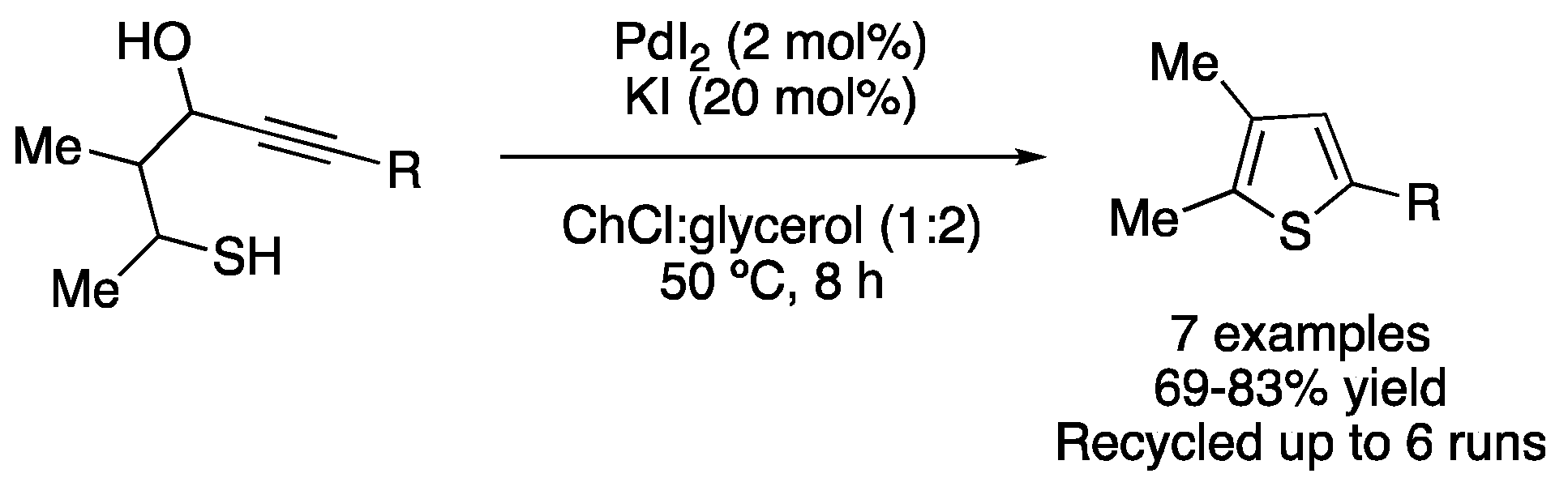
Figure 13. Synthesis of substituted thiophenes by PdI2/KI-catalyzed heterocyclization.
Due to the nature of most condensation reactions, the presence of a Lewis acid catalyst is beneficial for the reaction to take place in good yields. Thus, type II DESs based on hydrated metallic salts and quaternary ammonium salts can be applied not only as solvents but also as catalysts to perform these transformations. In this sense, the mixture ChCl:ZnCl2 (3:2) was used for synthesizing tetrahydroquinolines from anilines, aromatic aldehydes and trans-anethole, a naturally occurring essential oil. Firstly, condensation takes place to form an imine derivative, followed by an aza-Diels–Alder reaction to afford the desired heterocycle. Moderate-to-excellent yields were obtained with high diastereoselectivity and wide substrate scope (Table 2, entry 8) [39]. The same eutectic mixture was also reported as a catalyst for the synthesis of 4-aryldeneisoxazol-5(4H)-ones, although the reaction was performed in water as a solvent [40]. In addition, the type-IV mixture ZnCl2:urea (2:7) has also been employed as a catalytic solvent for performing reactions, such as the synthesis of 2,4,5-triaryl-1H-imidazoles and 2-aryl-1H-phenantro[9,10-d]imidazoles. Those compounds were prepared by the reaction between dicarbonyl compounds, ammonium acetate and aromatic aldehydes. Up to 25 examples were reported with excellent yields, with the DES being recovered by dissolution in water, filtration and lyophilization. This allowed the DES to be reused up to four times with only a slight decrease in the catalytic activity (Table 2, entry 9) [41]. Another heterocyclic synthesis in ZnCl2:urea (2:7) is the synthesis of 2-benzimidazolones and 2-imidazolones, which relies on a triple role for the DES, i.e., the eutectic mixture acts as a solvent, as a Lewis acid catalyst and as a reagent (urea). Therefore, arene-1,2-diamine derivatives were heated at 100 °C for 4 h in the aforementioned DES, affording seven examples of the corresponding 2-benzimidazolone derivatives in moderate-to-excellent yields. If benzoin derivates were employed instead of the diamine compounds, the corresponding 2-imidazolone products could be obtained in good yields, employing a method that avoids the generation of gas ammonia evolution (Figure 14, Table 2, entry 10) [42].
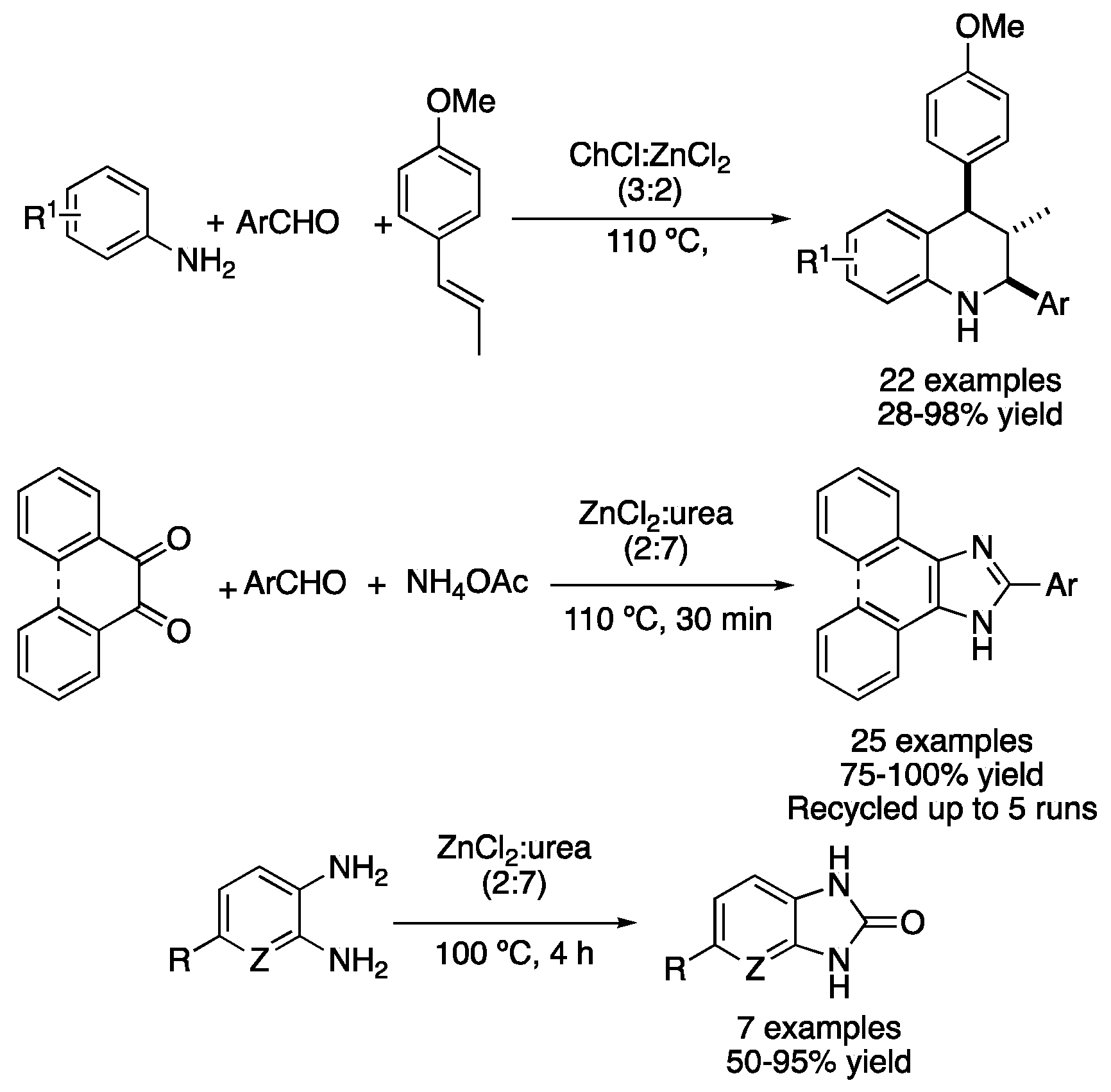
Figure 14. Condensation-based cyclization reactions using Zn-based type I and IV DESs.
Apart from ZnCl2-based DES, the use of ZrOCl2.8H2O in combination with different hydrogen bond donors (HBD) has also been explored. One example is the synthesis of 1,8-dioxooctahydroxanthenes from dimedone and aromatic aldehydes. This reaction was explored using urea, ethylene glycol and glycerol as HBD. The best results were obtained with the mixture ZrOCl2.8H2O:(CH2OH)2 (1:2), which was explained by the ability of ethylene glycol to activate the aldehyde through hydrogen bonding and the lower viscosity of the mixture, compared to the glycerol one, which favors the free motion of reactants. Furthermore, DES could be recovered and reused up to five times without a noticeable loss in its catalytic activity (Figure 15, Table 2, entry 11) [43]. However, the synthesis of benzimidazole derivatives was far more efficient in the mixture ZrOCl2.8H2O:urea (1:5), which was achieved by the reaction of arene-1,2-diamine with aromatic aldehydes. The main reasons behind the good results obtained with this DES are the higher acidity and lower viscosity of this mixture compared with their ethylene glycol or glycerol HBD analogues (Table 2, entry 12) [44].
Table 2. Selected examples of cyclization processes catalyzed by transition metals in DESs.
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | Cycloisomerization of alkynoic acids | ChCl:urea (1:2) | [Au] | Enol-lactones | [23][26] |
| 2 | Cycloisomerization of Z-enynols | ChCl:glycerol (1:2) | [Au] | Furans | [24] |
| 3 | 3+2 Cycloaddition | ChCl:ZnCl2 (1:2) | 100–140 °C | 1H-Tetrazoles | [27][28] |
| 4 | 3+2 Cycloaddition | ChCl:glycerol (1:2) | [Cu], 50–75 °C | Triazoles | [29][30] |
| 5 | A3 coupling | Citric acid:DMU (7:2) | CuFeO2 | imidazo[1,2a]pyridines | [32][33] |
| 6 | Condensation | ChCl:urea (1:2) | [Fe/Mo], MW | spirooxyndoles | [34] |
| 7 | S-heterocyclization | ChCl:glycerol (1:2) | PdI2/KI | Thiophene derivatives | [38] |
| 8 | Condensation + D.A. | ChCl:ZnCl2 (3:2) | 110 °C | Tetrahydroisoquinolines | [39] |
| 9 | Condensation | ZnCl2:urea (2:7) | 110 °C | phenantro[9,10a]imidazoles | [41] |
| 10 | Condensation | ZnCl2:urea (2:7) | 100 °C | 2-benzimidazolones | [42] |
| 11 | Condensation | ZrOCl2:(CH2OH)2 (1:2) | r.t. | dioxooctahydroxanthenes | [43] |
| 12 | Condensation | ZrOCl2:urea (1:5) | r.t. | benzimidazoles | [44] |
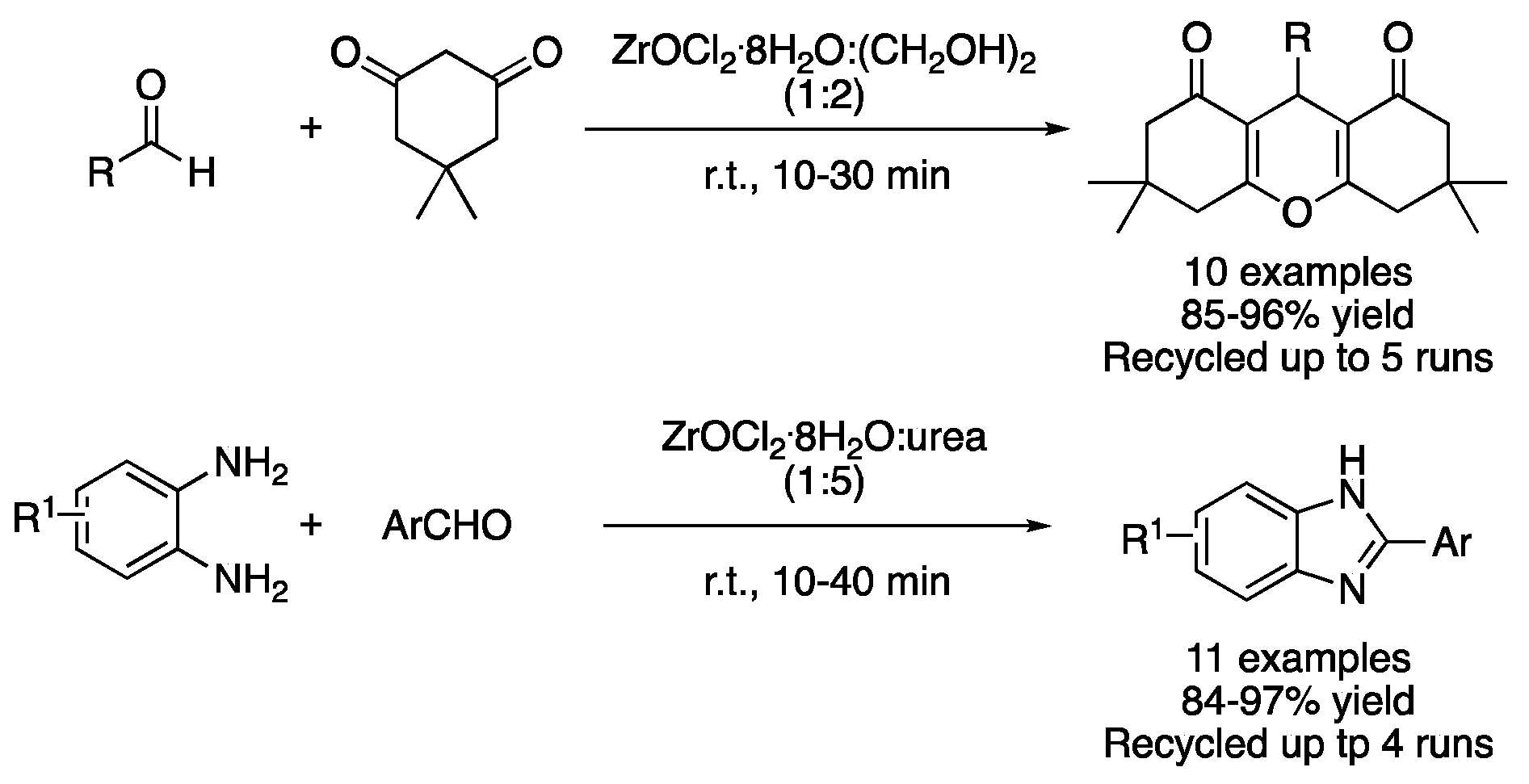
Figure 15. Cyclizations using Zr-based type IV DESs.
References
- Constable, D.J.C.; Jiménez-González, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Proc. Res. Dev. 2007, 11, 133–137.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Abranches, D.O.; Coutinho, J.A.P. Type V deep eutectic solvents: Design and applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100612.
- Liu, F.; Xue, Z.; Zhao, X.; Mou, H.; He, J.; Mu, T. Catalytic deep eutectic solvents for highly efficient conversion of cellulose to gluconic acid with gluconic acid self-precipitation separation. Chem. Commun. 2018, 54, 6140–6143.
- Guo, X.; Zhu, H.; Si, Y.; Lyu, X.; Cheng, Y.; Zheng, L.; Wang, L.; Li, X. Conversion of Glucose to 5-Hydroxymethylfurfural in Deep Eutectic Solvent of Choline Chloride–Chromium Chloride. Ind. Eng. Chem. Res. 2022, 61, 7216–7224.
- Dutta, A.; Garg, A.; Borah, J.; Borah, R.P.; Sarma, D. Deep eutectic solvent mediated controlled and selective oxidation of organic sulfides and hydroxylation of arylboronic acids. Curr. Res. Green Sustain. Chem. 2021, 4, 100107.
- Cicco, L.; Roggio, M.; López-Aguilar, M.; Ramos-Martín, M.; Perna, F.M.; García-Álvarez, J.; Vitale, P.; Capriati, V. Selective Aerobic Oxidation of Alcohols in Low Melting Mixtures and Water and Use for Telescoped One-Pot Hybrid Reactions. ChemistryOpen 2022, 11, e202200160.
- Feng, Y.; Yan, G.; Wang, T.; Jia, W.; Zeng, X.; Sperry, J.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Synthesis of MCM-41-Supported Metal Catalysts in Deep Eutectic Solvent for the Conversion of Carbohydrates into 5-Hydroxymethylfurfural. ChemSusChem 2019, 12, 978–982.
- Zhao, M.-Y.; Zhu, J.-N.; Li, P.; Li, W.; Cai, T.; Cheng, F.-F.; Xiong, W.-W. Structural variation of transition metal–organic frameworks using deep eutectic solvents with different hydrogen bond donors. Dalton Trans. 2019, 48, 10199–10209.
- Jérôme, F.; Ferreira, M.; Bricout, H.; Menuel, S.; Monflier, E.; Tilloy, S. Low melting mixtures based on β-cyclodextrin derivatives and N,N′-dimethylurea as solvents for sustainable catalytic processes. Green Chem. 2014, 16, 3876–3880.
- Ferreira, M.; Jérôme, F.; Bricout, H.; Menuel, S.; Landy, D.; Fourmentin, S.; Tilloy, S.; Monflier, E. Rhodium catalyzed hydroformylation of 1-decene in low melting mixtures based on various cyclodextrins and N,N′-dimethylurea. Catal. Commun. 2015, 63, 62–65.
- Esteban, J.; Warmeling, H.; Vorholt, A.J. Utilization of deep eutectic solvents based on choline chloride in the biphasic hydroformylation of 1-decene with rhodium complexes. Catal. Commun. 2019, 129, 105721.
- Vitale, P.; Cicco, L.; Messa, F.; Perna, F.M.; Salomone, A.; Capriati, V. Streamlined Routes to Phenacyl Azides and 2,5-Diarylpyrazines Enabled by Deep Eutectic Solvents. Eur. J. Org. Chem. 2019, 2019, 5557–5562.
- Garg, G.; Masdeu-Bultó, A.M.; Farfán, N.; Ordóñez-Hernández, J.; Gómez, M.; Medina-González, Y. Palladium Nanoparticles in Glycerol/Ionic Liquid/Carbon Dioxide Medium as Hydrogenation Catalysts. ACS Appl. Nano Mater. 2020, 3, 12240–12249.
- Liu, C.; Wang, S.; Wang, B.; Song, G. Catalytic hydrogenolysis of castor seeds C-lignin in deep eutectic solvents. Ind. Crops Prod. 2021, 169, 113666.
- Leal-Duaso, A.; Favier, I.; Pla, D.; Pires, E.; Gómez, M. Design of Glycerol-Based Solvents for the Immobilization of Palladium Nanocatalysts: A Hydrogenation Study. ACS Sustain. Chem. Eng. 2021, 9, 6875–6885.
- Iwanow, M.; Finkelmeyer, J.; Söldner, A.; Kaiser, M.; Gärtner, T.; Sieber, V.; König, B. Preparation of Supported Palladium Catalysts using Deep Eutectic Solvents. Chem. A Eur. J. 2017, 23, 12467–12470.
- Batarseh, C.; Levi-Zada, A.; Abu-Reziq, R. Preparation of catalytic deep eutectic solvent-based silica microreactors using a non-aqueous sol–gel route. J. Mater. Chem. A 2019, 7, 2242–2252.
- Sadjadi, S.; Akbari, M.; Kahangi, F.G.; Heravi, M.M. Pd immobilized on the composite of halloysite and low-cost bio-based eutectic solvent-derived carbon: An efficient catalyst for hydrogenation in the presence of cyclodextrin. Appl. Clay Sci. 2020, 192, 105640.
- O’Neill, M.; Raghuwanshi, V.S.; Wendt, R.; Wollgarten, M.; Hoell, A.; Rademann, K. Gold Nanoparticles in Novel Green Deep Eutectic Solvents: Self-Limited Growth, Self-Assembly & Catalytic Implications. Z. Für Phys. Chem. 2015, 229, 221–234.
- Messa, F.; Dilauro, G.; Paparella, A.N.; Silvestri, L.; Furlotti, G.; Iacoangeli, T.; Perrone, S.; Salomone, A. Deep eutectic solvents meet safe, scalable and sustainable hydrogenations enabled by aluminum powder and Pd/C. Green Chem. 2022, 24, 4388–4394.
- Cavallo, M.; Arnodo, D.; Mannu, A.; Blangetti, M.; Prandi, C.; Baratta, W.; Baldino, S. Deep eutectic solvents as H2-sources for Ru(II)-catalyzed transfer hydrogenation of carbonyl compounds under mild conditions. Tetrahedron 2021, 83, 131997.
- Rodríguez-Álvarez, M.J.; Vidal, C.; Díez, J.; García-Álvarez, J. Introducing deep eutectic solvents as biorenewable media for Au(i)-catalysed cycloisomerisation of γ-alkynoic acids: An unprecedented catalytic system. Chem. Commun. 2014, 50, 12927–12929.
- Vidal, C.; Merz, L.; García-Álvarez, J. Deep eutectic solvents: Biorenewable reaction media for Au(i)-catalysed cycloisomerisations and one-pot tandem cycloisomerisation/Diels–Alder reactions. Green Chem. 2015, 17, 3870–3878.
- Rodríguez-Álvarez, M.J.; Vidal, C.; Schumacher, S.; Borge, J.; García-Álvarez, J. AuI Iminophosphorane Complexes as Efficient Catalysts for the Cycloisomerization of Alkynyl Amides under Air, at Room Temperature, and in Aqueous or Eutectic-Mixture Solutions. Chem. A Eur. J. 2017, 23, 3425–3431.
- Saavedra, B.; Pérez, J.M.; Rodríguez-Álvarez, M.J.; García-Álvarez, J.; Ramón, D.J. Impregnated palladium on magnetite as a water compatible catalyst for the cycloisomerization of alkynoic acid derivatives. Green Chem. 2018, 20, 2151–2157.
- Padvi, S.A.; Dalal, D.S. Choline chloride–ZnCl2: Recyclable and efficient deep eutectic solvent for the cycloaddition reaction of organic nitriles with sodium azide. Synth. Commun. 2017, 47, 779–787.
- Xiong, X.; Yi, C.; Liao, X.; Lai, S. A practical multigram-scale method for the green synthesis of 5-substituted-1H-tetrazoles in deep eutectic solvent. Tetrahedron Lett. 2019, 60, 402–406.
- Kafle, A.; Handy, S.T. A one-pot, copper-catalyzed azidation/click reaction of aryl and heteroaryl bromides in an environmentally friendly deep eutectic solvent. Tetrahedron 2017, 73, 7024–7029.
- Giofrè, S.V.; Tiecco, M.; Ferlazzo, A.; Romeo, R.; Ciancaleoni, G.; Germani, R.; Iannazzo, D. Base-Free Copper-Catalyzed Azide-Alkyne Click Cycloadditions (CuAAc) in Natural Deep Eutectic Solvents as Green and Catalytic Reaction Media**. Eur. J. Org. Chem. 2021, 2021, 4777–4789.
- Mirshafiee, S.; Salamatmanesh, A.; Heydari, A. A sustainable approach for efficient one-pot synthesis of 1-aryl 1,2,3-triazoles using copper iodide supported on 3-thionicotinyl-urea-modified magnetic nanoparticles in DES. Appl. Organomet. Chem. 2021, 35, e6255.
- Lu, J.; Li, X.-T.; Ma, E.-Q.; Mo, L.-P.; Zhang, Z.-H. Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazopyridines. ChemCatChem 2014, 6, 2854–2859.
- Salamatmanesh, A.; Heydari, A. Magnetic nanostructure-anchored mixed-donor ligand system based on carboxamide and N-heterocyclic thiones: An efficient support of CuI catalyst for synthesis of imidazopyridines in eutectic medium. Appl. Catal. A: Gen. 2021, 624, 118306.
- Zhang, M.; Liu, Y.-H.; Shang, Z.-R.; Hu, H.-C.; Zhang, Z.-H. Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun. 2017, 88, 39–44.
- Maleki, A.; Kari, T.; Aghaei, M. @TiO2-OSO3H: An efficient hierarchical nanocatalyst for the organic quinazolines syntheses. J. Porous Mater. 2017, 24, 1481–1496.
- Maleki, A.; Aghaei, M.; Kari, T. Facile Synthesis of 7-Aryl-benzotetrazoloquinazoline-5,6-dione Fused Polycyclic Compounds by Using a Novel Magnetic Polyurethane Catalyst. Polycycl. Aromat. Compd. 2019, 39, 266–278.
- Akbarian, M.; Sanchooli, E.; Oveisi, A.R.; Daliran, S. Choline chloride-coated UiO-66-Urea MOF: A novel multifunctional heterogeneous catalyst for efficient one-pot three-component synthesis of 2-amino-4H-chromenes. J. Mol. Liq. 2021, 325, 115228.
- Mancuso, R.; Maner, A.; Cicco, L.; Perna, F.M.; Capriati, V.; Gabriele, B. Synthesis of thiophenes in a deep eutectic solvent: Heterocyclodehydration and iodocyclization of 1-mercapto-3-yn-2-ols in a choline chloride/glycerol medium. Tetrahedron 2016, 72, 4239–4244.
- Peñaranda Gómez, A.; Rodríguez Bejarano, O.; Kouznetsov, V.V.; Ochoa-Puentes, C. One-Pot Diastereoselective Synthesis of Tetrahydroquinolines from Star Anise Oil in a Choline Chloride/Zinc Chloride Eutectic Mixture. ACS Sustain. Chem. Eng. 2019, 7, 18630–18639.
- Damghani, K.F.; Kiyani, H.; Pourmousavi, A.S. Green Three-component Synthesis of Merocyanin Dyes Based on 4-Arylideneisoxazol-5(4H)-ones. Curr. Green Chem. 2020, 7, 217–225.
- Higuera, N.L.; Peña-Solórzano, D.; Ochoa-Puentes, C. Urea–Zinc Chloride Eutectic Mixture-Mediated One-Pot Synthesis of Imidazoles: Efficient and Ecofriendly Access to Trifenagrel. Synlett 2019, 30, 225–229.
- Aghapoor, K.; Mohsenzadeh, F.; Darabi, H.R.; Sayahi, H.; Jalali, M.R. ZnCl2/Urea Eutectic Solvent as Stable Carbonylation Source for Benign Synthesis of 2–Benzimidazolones and 2–Imidazolones: An Effective Strategy for Preventing NH3 Gas Evolution. ChemistrySelect 2019, 4, 11093–11097.
- Shaibuna, M.; Abbas, A.; Kariyottu Kuniyil, M.J.; Sreekumar, K. Sustainable synthesis of 1,8-dioxooctahydroxanthenes in deep eutectic solvents (DESs). New J. Chem. 2021, 45, 8335–8344.
- Shaibuna, M.; Hiba, K.; Shebitha, A.M.; Kariyottu Kuniyil, M.J.; Sherly mole, P.B.; Sreekumar, K. Sustainable and selective synthesis of benzimidazole scaffolds using deep eutectic solvents. Curr. Res. Green Sustain. Chem. 2022, 5, 100285.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
19 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




