Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Makoto Hosoya | -- | 2541 | 2022-12-18 01:16:20 | | | |
| 2 | Peter Tang | Meta information modification | 2541 | 2022-12-19 02:37:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hosoya, M.; Wakabayashi, T.; Wasano, K.; Nishiyama, T.; Tsuzuki, N.; Oishi, N. Vestibular Schwannoma for Hearing Preservation Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/38928 (accessed on 07 February 2026).
Hosoya M, Wakabayashi T, Wasano K, Nishiyama T, Tsuzuki N, Oishi N. Vestibular Schwannoma for Hearing Preservation Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/38928. Accessed February 07, 2026.
Hosoya, Makoto, Takeshi Wakabayashi, Koichiro Wasano, Takanori Nishiyama, Nobuyoshi Tsuzuki, Naoki Oishi. "Vestibular Schwannoma for Hearing Preservation Surgery" Encyclopedia, https://encyclopedia.pub/entry/38928 (accessed February 07, 2026).
Hosoya, M., Wakabayashi, T., Wasano, K., Nishiyama, T., Tsuzuki, N., & Oishi, N. (2022, December 18). Vestibular Schwannoma for Hearing Preservation Surgery. In Encyclopedia. https://encyclopedia.pub/entry/38928
Hosoya, Makoto, et al. "Vestibular Schwannoma for Hearing Preservation Surgery." Encyclopedia. Web. 18 December, 2022.
Copy Citation
Vestibular schwannoma is a clinically benign schwannoma that arises from the vestibulocochlear nerve that causes sensorineural hearing loss. This tumor is clinically and oncologically regarded as a benign tumor as it does not metastasize or invade surrounding tissues. Despite being a benign tumor, its management is difficult and controversial due to the potential serious complications, such as irreversible sensorineural hearing loss, of the interventions. Therefore, preventing hearing loss due to the natural course of the disease and complications of surgery is a challenging issue for an otologist.
vestibular schwannoma
hearing loss
hearing preservation surgery
FREMAP
DNAP
1. Introduction
Vestibular schwannoma is a clinically benign schwannoma that arises from the vestibulocochlear nerve (VIIIth cranial nerve) that runs through the internal auditory canal. The vestibulocochlear nerve comprises of the cochlear nerve, the superior vestibular nerve, and the inferior vestibular nerve. Most vestibular schwannomas originate from the inferior vestibular nerves between the other two nerves [1][2]. Vestibular schwannoma can arise anywhere along the course of the vestibular nerve, running from the cistern to inside the vestibular organ. However, the vast majority of vestibular schwannomas are observed from the cistern portion to the porus of the inner ear canal. Therefore, typical vestibular schwannomas present as cerebellopontine angle tumors. Vestibular schwannoma accounts for about 8–10% of intracranial tumors and almost 80% of cerebellopontine angle tumors [3].
The incidence of vestibular schwannoma is estimated to be approximately 20 new cases per million people each year [4] with a lifetime prevalence of approximately one case in 500 persons [5]. Vestibular schwannoma can cause various symptoms, including dizziness due to its effect on the vestibular nerve, and sensorineural hearing loss due to damage to the cochlear nerves. These damages lead to hearing loss caused by the secreted molecules of the schwannoma [6][7] or the schwannoma-associated inflammatory response [8], as well as direct compression of the tumor and inner ear dysfunction [9]. It can also cause facial paralysis due to compression of the facial nerve, which runs parallel to the vestibulocochlear nerve in the inner ear canal. In addition, as the tumor grows, it can cause pressure on the brain stem, which can be fatal.
2. The Natural Course of Hearing Levels in Vestibular Schwannoma Patients
Hearing loss caused by vestibular schwannoma is not always severe or permanent. This hearing loss can be mild to moderate [10][11][12][13] and reversible in several patients [14][15]. An estimated 10–30% of patients diagnosed with vestibular schwannoma develop sudden sensorineural hearing loss [16][17][18] and 3–5% of patients diagnosed with sudden sensorineural hearing loss exhibit vestibular schwannomas [19][20]. This hearing loss caused by sporadic vestibular schwannomas is often treated with corticosteroids according to the treatment protocols used for idiopathic sudden sensorineural hearing loss. Several earlier reports have shown that hearing recovers with corticosteroid treatment [21][22][23]. This heterogeneity of hearing loss caused by vestibular schwannoma makes decision making in terms of when interventions should be carried out difficult.
Thus far, Stangerup et al. reported that, while 53% of patients had good hearing and speech discrimination upon diagnosis, this became 31% after ten years of observation [24]. Recently, Wasano et al. reported that the recovery rate of vestibular schwannoma-associated sudden sensorineural hearing loss decreases with increasing episodes of hearing loss [14]. They also estimated that the recurrence rate of hearing loss within one year was 25%. This suggests that patients with vestibular schwannoma who apparently recovered from hearing loss should be considered to undergo further surgical interventions due to a high risk of hearing loss recurrence.
3. Importance of Hearing Preservation after Vestibular Schwannoma Surgery
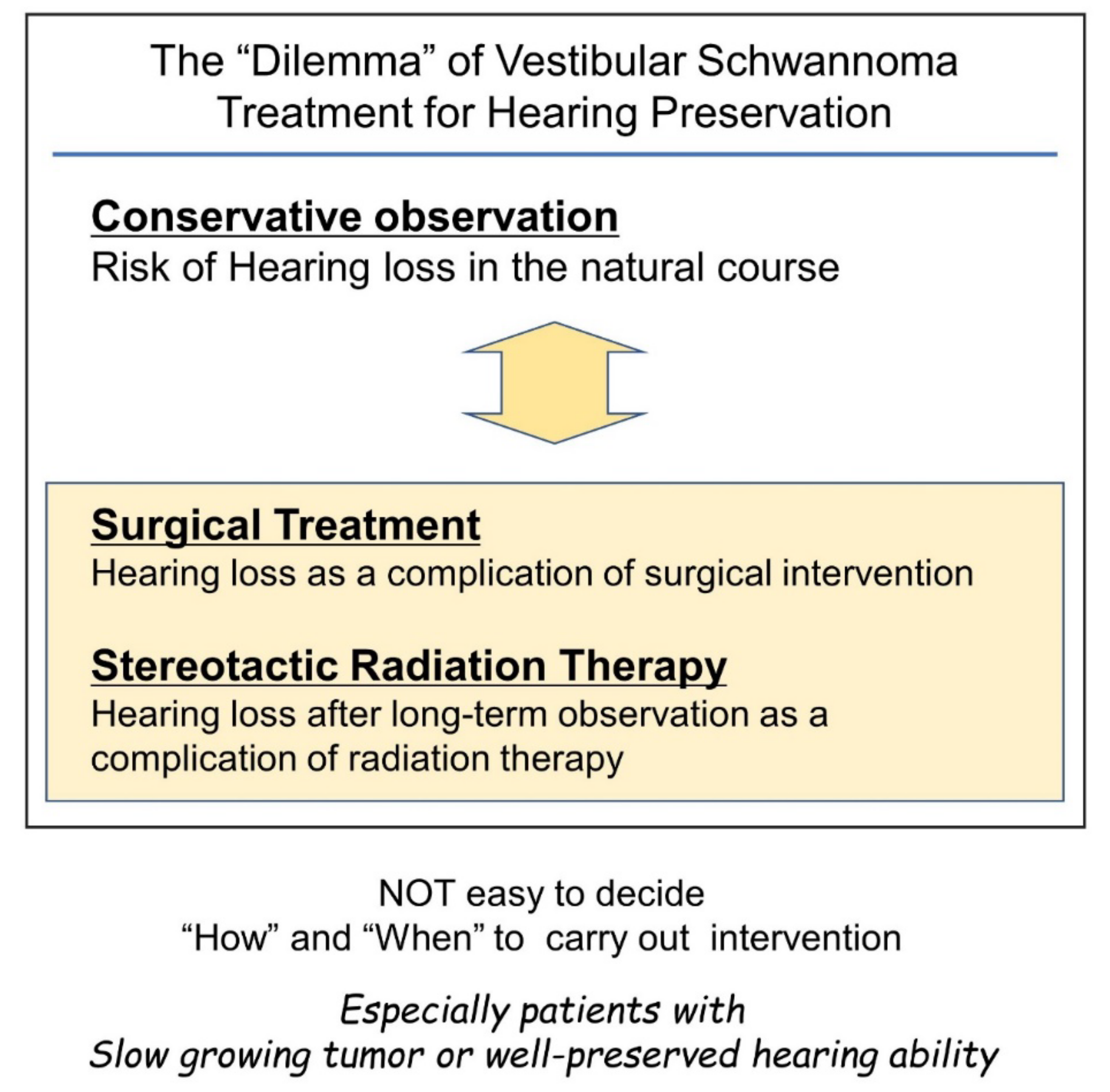
Considering the risk of gradual or sudden hearing loss in the natural course of disease for patients with vestibular schwannoma [14][25], hearing preservation through the surgical removal of the tumor would be ideal. However, surgical intervention itself is a risk factor for hearing loss, and there is no ideal surgical approach to ensure hearing preservation. Hence, surgeons are faced with a dilemma (Figure 1).

Figure 1. Difficulties in timing considerations in vestibular schwannoma intervention with the goal of preserving hearing. Conservative observation and intervention with surgery or stereotactic radiotherapy can cause hearing loss. Therefore, the decision to intervene can be a difficult decision for the surgeon.
Therefore, if the tumor size is not life-threatening, non-hearing preservation surgery can be chosen once hearing loss sets in by natural course. This type of surgical decision making has been widely applied. However, the importance of hearing preservation has been underestimated in this context.
Preserving residual hearing in vestibular schwannoma patients is necessary for several reasons. Hearing loss on the affected side due to either the natural course of the disease or the side effects of the interventions usually results in impaired sound localization and difficulty in understanding speech in the presence of background noise [26][27]. However, if serviceable hearing can be spared, hearing aid use can be considered. Recently, Kitamura et al. reported the possible importance of residual hearing after hearing preservation surgery for the treatment of tinnitus [28]. It has also been reported that tinnitus negatively affects the quality of life of patients with vestibular schwannomas [29][30][31]. These previous studies suggest that hearing preservation is desirable in tinnitus management and it improves the patients’ quality of life.
4. Interventions for the Vestibular Schwannoma
There are several management methods for vestibular schwannomas. A “wait-and-scan” policy, in which patients’ tumors are observed once or twice by MRI per year, is well applied to patients with non-growing tumors [32][33][34][35]. Typically, tumors smaller than 1.5 cm in diameter located on the cerebellopontine angle are considered for a wait-and-scan approach. Once tumor growth has been detected with or without cystic changes, interventions should be considered, and, especially in patients who are candidates for hearing preservation, surgery should be considered [36]. Because of the potential side effects of treatments and the fact that the tumor is benign and non-invasive, in several cases, such as elderly patients who do not want surgery, or patients with very slow-growing tumors, the “wait-and-scan” policy may still be preferable.
There are several interventions available for a growing vestibular schwannoma, including radiotherapy and surgical resection. Stereotactic radiosurgery, such as Gamma-Knife [37][38], has been widely used in radiation therapy for vestibular schwannoma. Stereotactic radiosurgery aims to prevent tumor growth but without expectation of a radiological cure, similar to other radiation therapies for malignant tumors. Stereotactic radiosurgery showed a high long-term local control rate [39] and had merit that it can be applied for the relatively large tumor that cannot be surgically removed [40]. Stereotactic radiosurgery is an effective treatment option for small- to medium-sized (<3 cm) vestibular schwannomas without significant mass effect, while large tumors causing brainstem compression or symptoms of mass effect require surgical intervention. Stereotactic radiosurgery has similar local control rates compared to surgery in appropriately selected patients, typically those without significant mass effect or brain compression [41]. In some instances, stereotactic radiosurgery provides better functional outcomes [41].
Although stereotactic radiosurgery has several merits and is widely used, it still has the disadvantage of hearing loss. It is believed that stereotactic radiosurgery has a high hearing preservation rate. However, recent long-term observations showed that patients’ hearing after stereotactic radiosurgery gradually decreased [42][43][44][45], and serviceable hearing levels are lost in most patients in a span of ten years [43]. This result suggests that stereotactic radiosurgery can only achieve temporary hearing preservation; therefore, this intervention may not be suitable for younger patients.
Neurofibromatosis type II is often treated by a neurosurgeon and less often by an otolaryngologist, requiring a different treatment plan. In neurofibromatosis type II, special consideration should be given to the possibility of bilateral hearing loss due to bilateral vestibular schwannomas. Treatment strategies for sporadic vestibular schwannomas rarely apply to neurofibromatosis type II cases, as contralateral hearing levels and the possible presence of other brain tumors such as meningiomas affect the treatment strategy for neurofibromatosis type II.
5. Surgical Approach for the Vestibular Schwannoma
Surgical resection can be performed for all tumor sizes. Usually, large tumors, such as those associated with brainstem compression, are resected by neurosurgeons, and otologists treat relatively smaller tumors that are limited to the inner ear canal or those that slightly reach the brainstem. The middle fossa approach, translabyrinthine approach, and retrosigmoid approach are thought to be the three primary microsurgical approaches; however, other approaches are also used to remove vestibular schwannomas, each with benefits and limitations.
Surgical interventions for vestibular schwannoma can be categorized into two types depending on the expected postoperative hearing results: non-hearing preservation surgery and hearing preservation surgery. Most non-hearing preservation surgeries are performed using the translabyrinthine approach [46]. The transotic approach can also be used [47][48]. Recently, the exclusive endoscopic transcanal transpromontorial approach has been reported as a non-hearing preservation surgery [49][50]. Several surgical methods can be used for hearing preservation, including the retrosigmoid approach, the middle temporal fossa approach, and the retrolabyrinthine approach. Recently, the retrolabyrinthine meatotomy technique combined with the retrosigmoid approach has been reported [51].
While the surgical approach can be easily divided into two groups, it is difficult to define “what is good and serviceable hearing” or “what is hearing worth to be preserved”. Therefore, several criteria have been used. Historically, Wade and House described the “50/50 rule”, which defined serviceable hearing as a pure tone average (PTA) of ≤50 dB with a speech discrimination score (SDS) of 50% or better [52]. In 1988, the Gardner–Robertson Scale was developed, in which Grade I (Good: PTA ≤ 30 dB with SDS ≥ 70%) and Grade II (Serviceable: PTA of 30–50 dB with SDS ≥ 50%) are considered “useful hearing” [53]. In 1995, the American Academy of Otolaryngology Head and Neck Surgery guideline was established for reporting hearing outcomes after hearing preservation surgery for lateral skull base surgery, in which all patients with SDS < 50% are considered as having non-serviceable hearing [54]. In 2003, the Tokyo classification was developed [55]. These criteria help report the results of surgical interventions and are usually also used for selecting the candidate for hearing preservation surgery.
Based on these previous criteria, candidates for hearing preservation surgery have often been defined as patients with PTA ≤ 30 dB with SDS ≥ 70% [56][57] or with PTA of 30–50 dB with SDS ≥ 50% [58][59]. However, as mentioned above, it was suggested that any level of residual hearing might be helpful for tinnitus control or the improvement of patients’ quality of life. Moreover, the residual hearing, even if it was at an “unserviceable level”, might broaden the possibility of hearing rehabilitation after vestibular schwannoma surgery [27].
The selection of the surgical approach is dependent on the degree of residual hearing, tumor size, tumor location, and whether hearing preservation is preferred. If hearing preservation surgery is selected, the central focus of the procedure is the preservation of auditory function. However, irrespective of which surgical category is selected, the surgeon must attempt to prevent facial nerve palsy, particularly in cases with no evident preoperative deficit. Intraoperative neural monitoring is a widely used method to facilitate postoperative hearing and prevent facial nerve function deficits.
6. Intraoperative Electrophysiological Monitoring for Vestibular Schwannoma Surgery
Since the first report of intraoperative ABR monitoring in vestibular schwannoma surgery in 1982 [60], it has become a standard monitoring method, and its clinical value is now broadly accepted in the field. Since then, other modalities have been introduced into clinical practice, including direct eighth cranial nerve monitoring via cochlear nerve action potentials (CNAP) [61][62][63][64] or dorsal nucleus action potential (DNAP) [65][66][67], electrocochleography [68][69], and OAE [70].
However, intraoperative ABR monitoring has two major limitations. First, ABR detection is time-consuming. ABR measurement requires an average of 500–2000 stimulations and a duration of more than one minute. Although this time period is acceptable when applying ABR as a hearing test in an outpatient setting, it is problematic in intraoperative monitoring. Second, vestibular schwannomas impede acoustic neuron conduction. As a result, the amplitude of the ABR decreases and, in some cases, intraoperative ABR waveforms cannot be obtained. In the 2010s, a novel form of intraoperative monitoring for hearing function was developed, i.e., DNAP monitoring [71]. In DNAP monitoring, the detection probe is set on the dorsal cochlear nucleus of the brainstem. The action potential of the dorsal cochlear nucleus is detected using this probe. DNAP has high sensitivity in detecting electrical signals and can detect signals that ABR overlooks. Moreover, the DNAP system requires an average of only 100–200 stimulations. Intraoperative DNAP signals can thus be obtained every 10 s. In contrast, when using the DNAP, putting the detection probe on the brainstem is required, which is not needed for ABR monitoring. Therefore, DNAP monitoring cannot be applicable in the middle fossa approach, although ABR monitoring can be used in all hearing preservation surgeries.
Electromyographic monitoring systems, such as the Medtronic NIM system, are widely used [72][73][74][75]. However, this system involves the detection of sporadic firing of the facial nerve via intrinsic physiological stimulation. Thus, this system cannot be used for continuous intraoperative monitoring. Advanced monitoring systems with continuous monitoring have been developed to overcome this limitation [71][76][77]. The monitoring system using facial nerve root exit zone-elicited compound muscle action potential (FREMAP) is one such system that has been recently introduced. Although the FREMAP monitoring system needs to set an electric stimulation probe to the main trunk of the facial nerve, this probe enables continuous intraoperative stimulation of the facial nerve, followed by detection of a facial muscle action potential. FREMAP monitoring enables stimulation of the facial nerve and the detection of facial muscle action potentials to be performed continuously throughout the surgical procedure [78]. Thus, the surgeon can obtain continuous quantitative data concerning facial nerve status during tumor resection. In addition, the surgeon can abandon the procedure or select an alternative resection route, if necessary. While the electrode for FREMAP monitoring is designed to be put on the main trunk of the facial nerve in the surgical view of the retrolabyrinthine approach or the retrosigmoid approach, it could be applied to other approaches, including non-hearing preservation surgeries.
7. Retrolabyrinthine Approach under Reinforced Continuous Intraoperative Monitoring with FREMAP and DNAP
The retrolabyrinthine approach is a surgical approach for vestibular schwannomas that can preserve patients’ hearing [79][80][81][82][83][84]. In the retrolabyrinthine approach, the tumors are approached through the area posterior to the posterior semicircular canal and prior to the sigmoid sinus [83]. This approach has several advantages compared with other approaches that can preserve hearing. The first advantage is that this approach does not require craniotomy, which is required by the retrosigmoid and middle fossa approaches. Therefore, less pressure is applied to the temporal lobe or cerebellum. The second advantage of the retrolabyrinthine approach is that the risk of encountering the facial nerve first in the inner ear canal is relatively lower compared with the middle fossa approach. The third advantage of this approach is that DNAP and FREMAP electrodes, which cannot be used in the middle fossa approach, can be used because the brain stem is visible.
However, there are several disadvantages of the retrolabyrinthine approach as well. First is that this approach cannot be applied to some patients because of certain anatomical features of the temporal bones. Patients with one or more of the following anatomical features may be poor candidates for this approach: high jugular bulb, less developed bony cells posterior to the labyrinth, less developed mastoid air cells, and less developed sigmoid sinus on the opposite side of the surgical site. The second disadvantage of the retrolabyrinthine approach is that the surgical field is relatively narrower than other approaches, and the fundus of the inner ear canal cannot be visualized by surgical microscopy alone. Although the use of the endoscope can overcome this limitation to some extent [85][86][87], this approach is more suitable for smaller tumors (Koos classification I or II) with tumor-free space on the fundus of the inner ear canal.
References
- Komatsuzaki, A.; Tsunoda, A. Nerve origin of the acoustic neuroma. J. Laryngol. Otol. 2001, 115, 376–379.
- Khrais, T.; Romano, G.; Sanna, M. Nerve origin of vestibular schwannoma: A prospective study. J. Laryngol. Otol. 2008, 122, 128–131.
- Moffat, D.A.; Ballagh, R.H. Rare tumours of the cerebellopontine angle. Clin. Oncol. 1995, 7, 28–41.
- Stangerup, S.E.; Caye-Thomasen, P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol. Clin. N. Am. 2012, 45, 257–268.
- Marinelli, J.P.; Grossardt, B.R.; Lohse, C.M.; Carlson, M.L. Prevalence of sporadic vestibular schwannoma: Reconciling temporal bone, radiologic, and population-based studies. Otol. Neurotol. 2019, 40, 384–390.
- Soares, V.Y.R.; Atai, N.A.; Fujita, T.; Dilwali, S.; Sivaraman, S.; Landegger, L.D.; Hochberg, F.H.; Oliveira, C.A.P.C.; Bahmad, F.; Breakefield, X.O.; et al. Extracellular vesicles derived from human vestibular schwannomas associated with poor hearing damage cochlear cells. Neuro-Oncology 2016, 18, 1498–1507.
- Ren, Y.; Stankovic, K.M. The role of tumor necrosis factor alpha (tnfα) in hearing loss and vestibular schwannomas. Curr. Otorhinolaryngol. Rep. 2018, 6, 15–23.
- Sagers, J.E.; Sahin, M.I.; Moon, I.; Ahmed, S.G.; Stemmer-Rachamimov, A.; Brenner, G.J.; Stankovic, K.M. Nlrp3 inflammasome activation in human vestibular schwannoma: Implications for tumor-induced hearing loss. Hear. Res. 2019, 381, 107770.
- Ogawa, K.; Kanzaki, J.; Ogawa, S.; Tsuchihashi, N.; Ikeda, S. Progression of hearing loss in acoustic neuromas. Acta Oto-Laryngol. 1991, 111, 133–137.
- Ogawa, K.; Kanzaki, J.; Ogawa, S.; Tsuchihashi, N.; Inoue, Y. Acoustic neuromas presenting as sudden hearing loss. Acta Otolaryngol. Suppl. 1991, 487, 138–143.
- Johnson, E.W. Auditory test results in 500 cases of acoustic neuroma. Arch. Otolaryngol. 1977, 103, 152–158.
- Selesnick, S.H.; Jackler, R.K. Atypical hearing loss in acoustic neuroma patients. Laryngoscope 1993, 103, 437–441.
- Yang, W.; Mei, X.; Li, X.; Zhou, Y.; Zhong, J.; Liu, H.; Li, L.; Hu, H. The prevalence and clinical characteristics of vestibular schwannoma among patients treated as sudden sensorineural hearing loss: A 10-year retrospective study in southern china. Am. J. Otolaryngol. 2020, 41, 102452.
- Wasano, K.; Oishi, N.; Noguchi, M.; Hentona, K.; Shinden, S.; Kitama, T.; Tsuzuki, N.; Kawasaki, T.; Hiraga, Y.; Takei, Y.; et al. Sudden sensorineural hearing loss in patients with vestibular schwannoma. Sci. Rep. 2021, 11, 1624.
- Inoue, Y.; Kanzaki, J.; Ogawa, K. Vestibular schwannoma presenting as sudden deafness. J. Laryngol. Otol. 2000, 114, 589–592.
- Moffat, D.A.; Baguley, D.M.; von Blumenthal, H.; Irving, R.M.; Hardy, D.G. Sudden deafness in vestibular schwannoma. J. Laryngol. Otol. 1994, 108, 116–119.
- Higgs, W.A. Sudden deafness as the presenting symptom of acoustic neurinoma. Arch. Otolaryngol. 1973, 98, 73–76.
- Sauvaget, E.; Kici, S.; Kania, R.; Herman, P.; Tran Ba Huy, P. Sudden sensorineural hearing loss as a revealing symptom of vestibular schwannoma. Acta Otolaryngol. 2005, 125, 592–595.
- Fujita, T.; Saito, K.; Kashiwagi, N.; Sato, M.; Seo, T.; Doi, K. The prevalence of vestibular schwannoma among patients treated as sudden sensorineural hearing loss. Auris Nasus Larynx 2019, 46, 78–82.
- Tsuzuki, N.; Wasano, K.; Oishi, N.; Hentona, K.; Shimanuki, M.; Nishiyama, T.; Hiraga, Y.; Shinden, S.; Ogawa, K. Severe sudden sensorineural hearing loss related to risk of stroke and atherosclerosis. Sci. Rep. 2021, 11, 20204.
- Lin, C.; Gong, Q.; Zuo, W.; Zhang, R.; Zhou, A. The clinical characteristics and treatment for sudden sensorineural hearing loss with vestibular schwannoma. Eur. Arch. Otorhinolaryngol. 2015, 272, 839–842.
- Aronzon, A.; Ruckenstein, M.J.; Bigelow, D.C. The efficacy of corticosteroids in restoring hearing in patients undergoing conservative management of acoustic neuromas. Otol. Neurotol. 2003, 24, 465–468.
- Puccinelli, C.; Carlson, M.L. Improvement or recovery from sudden sensorineural hearing loss with steroid therapy does not preclude the need for mri to rule out vestibular schwannoma. Otol. Neurotol. 2019, 40, 674–680.
- Stangerup, S.E.; Caye-Thomasen, P.; Tos, M.; Thomsen, J. Change in hearing during ‘wait and scan’ management of patients with vestibular schwannoma. J. Laryngol. Otol. 2008, 122, 673–681.
- Carlson, M.L.; Dowling, E.M.; Lohse, C.M.; O’Connell, B.P.; Driscoll, C.L.W.; Haynes, D.S.; Link, M.J.; Hunter, J.B. Rate of initial hearing loss during early observation predicts time to non-serviceable hearing in patients with conservatively managed sporadic vestibular schwannoma. Otol. Neurotol. 2019, 40, e1012–e1017.
- Tveiten, O.V.; Carlson, M.L.; Link, M.J.; Lund-Johansen, M. Audiovestibular handicap and quality of life in patients with vestibular schwannoma and “excellent” hearing. Neurosurgery 2017, 80, 386–392.
- Drusin, M.A.; Lubor, B.; Losenegger, T.; Selesnick, S. Trends in hearing rehabilitation use among vestibular schwannoma patients. Laryngoscope 2020, 130, 1558–1564.
- Kitamura, M.; Oishi, N.; Suzuki, N.; Kojima, T.; Nishiyama, T.; Noguchi, M.; Hosoya, M.; Ogawa, K. Management of tinnitus in patients with vestibular schwannoma who underwent surgical resection. Eur. Arch. Otorhinolaryngol. 2021, 278, 4243–4249.
- Kojima, T.; Oishi, N.; Nishiyama, T.; Ogawa, K. Severity of tinnitus distress negatively impacts quality of life in patients with vestibular schwannoma and mimics primary tinnitus. Front. Neurol. 2019, 10, 389.
- Nishiyama, T.; Oishi, N.; Kojima, T.; Kasuya, K.; Noguchi, M.; Ishikawa, T.; Hosoya, M.; Ogawa, K. Validation and multidimensional analysis of the japanese penn acoustic neuroma quality-of-life scale. Laryngoscope 2020, 130, 2885–2890.
- Peris-Celda, M.; Graffeo, C.S.; Perry, A.; Kleinstern, G.; Kerezoudis, P.; Driscoll, C.L.W.; Carlson, M.L.; Link, M.J. Beyond the abcs: Hearing loss and quality of life in vestibular schwannoma. Mayo Clin. Proc. 2020, 95, 2420–2428.
- Van de Langenberg, R.; de Bondt, B.J.; Nelemans, P.J.; Dohmen, A.J.; Baumert, B.G.; Stokroos, R.J. Predictors of volumetric growth and auditory deterioration in vestibular schwannomas followed in a wait and scan policy. Otol. Neurotol. 2011, 32, 338–344.
- Somers, T.; Kania, R.; Waterval, J.; Van Havenbergh, T. What is the required frequency of mri scanning in the wait and scan management? J. Int. Adv. Otol. 2018, 14, 85–89.
- Ferri, G.G.; Modugno, G.C.; Pirodda, A.; Fioravanti, A.; Calbucci, F.; Ceroni, A.R. Conservative management of vestibular schwannomas: An effective strategy. Laryngoscope 2008, 118, 951–957.
- Hentschel, M.A.; Hannink, G.; Steens, S.C.A.; Mulder, J.J.S.; Rovers, M.M.; Kunst, H.P.M. Development of a model to predict vestibular schwannoma growth: An opportunity to introduce new wait and scan strategies. Clin. Otolaryngol. 2021, 46, 273–283.
- Charabi, S.; Thomsen, J.; Mantoni, M.; Charabi, B.; Jorgensen, B.; Borgesen, S.E.; Gyldensted, C.; Tos, M. Acoustic neuroma (vestibular schwannoma): Growth and surgical and nonsurgical consequences of the wait-and-see policy. Otolaryngol. Head Neck Surg. 1995, 113, 5–14.
- Mahboubi, H.; Sahyouni, R.; Moshtaghi, O.; Tadokoro, K.; Ghavami, Y.; Ziai, K.; Lin, H.W.; Djalilian, H.R. Cyberknife for treatment of vestibular schwannoma: A meta-analysis. Otolaryngol. Head Neck Surg. 2017, 157, 7–15.
- Hafez, R.F.A.; Morgan, M.S.; Fahmy, O.M.; Hassan, H.T. Outcomes of gamma knife surgery retreatment for growing vestibular schwannoma and review of the literature. Clin. Neurol. Neurosurg. 2020, 198, 106171.
- Johnson, S.; Kano, H.; Faramand, A.; Pease, M.; Nakamura, A.; Hassib, M.; Spencer, D.; Sisterson, N.; Faraji, A.H.; Arai, Y.; et al. Long term results of primary radiosurgery for vestibular schwannomas. J. Neurooncol. 2019, 145, 247–255.
- Conley, G.S.; Hirsch, B.E. Stereotactic radiation treatment of vestibular schwannoma: Indications, limitations, and outcomes. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 351–356.
- Buss, E.J.; Wang, T.J.C.; Sisti, M.B. Stereotactic radiosurgery for management of vestibular schwannoma: A short review. Neurosurg. Rev. 2021, 44, 901–904.
- Hasegawa, T.; Kato, T.; Yamamoto, T.; Naito, T.; Kato, N.; Torii, J.; Ishii, K. Long-term hearing outcomes after gamma knife surgery in patients with vestibular schwannoma with hearing preservation: Evaluation in 92 patients with serial audiograms. J. Neuro-Oncol. 2018, 138, 283–290.
- Carlson, M.L.; Jacob, J.T.; Pollock, B.E.; Neff, B.A.; Tombers, N.M.; Driscoll, C.L.; Link, M.J. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: Patterns of hearing loss and variables influencing audiometric decline. J. Neurosurg. 2013, 118, 579–587.
- Van Linge, A.; Van Os, R.; Hoekstra, N.; Heijmen, B.; Stienstra, L.; Dallenga, A.; Wolbers, J.; Mendez Romero, A. Progression of hearing loss after linac-based stereotactic radiotherapy for vestibular schwannoma is associated with cochlear dose, not with pre-treatment hearing level. Radiat. Oncol. 2018, 13, 253.
- Patel, K.S.; Ng, E.; Kaur, T.; Miao, T.; Kaprealian, T.; Lee, P.; Pouratian, N.; Selch, M.T.; De Salles, A.A.F.; Gopen, Q.; et al. Increased cochlear radiation dose predicts delayed hearing loss following both stereotactic radiosurgery and fractionated stereotactic radiotherapy for vestibular schwannoma. J. Neuro-Oncol. 2019, 145, 329–337.
- Day, J.D.; Chen, D.A.; Arriaga, M. Translabyrinthine approach for acoustic neuroma. Neurosurgery 2004, 54, 391–395; discussion 395–396.
- Browne, J.D.; Fisch, U. Transotic approach to the cerebellopontine angle. 1992. Neurosurg. Clin. N. Am. 2008, 19, 265–278.
- Xia, Y.; Zhang, W.; Li, Y.; Ma, X.; Liu, Q.; Shi, J. The transotic approach for vestibular schwannoma: Indications and results. Eur. Arch. Otorhinolaryngol. 2017, 274, 3041–3047.
- Marchioni, D.; Alicandri-Ciufelli, M.; Rubini, A.; Masotto, B.; Pavesi, G.; Presutti, L. Exclusive endoscopic transcanal transpromontorial approach: A new perspective for internal auditory canal vestibular schwannoma treatment. J. Neurosurg. 2017, 126, 98–105.
- Moon, I.; Cha, D.; Nam, S.-I.; Lee, H.-J.; Choi, J. The feasibility of a modified exclusive endoscopic transcanal transpromontorial approach for vestibular schwannomas. J. Neurol. Surg. Part B Skull Base 2019, 80, 082–087.
- Mazzoni, A.; Zanoletti, E.; Denaro, L.; Martini, A.; Avella, D. Retrolabyrinthine meatotomy as part of retrosigmoid approach to expose the whole internal auditory canal: Rationale, technique, and outcome in hearing preservation surgery for vestibular schwannoma. Oper. Neurosurg. 2018, 14, 36–44.
- Wade, P.J.; House, W. Hearing preservation in patients with acoustic neuromas via the middle fossa approach. Otolaryngol. Head Neck Surg. 1984, 92, 184–193.
- Gardner, G.; Robertson, J.H. Hearing preservation in unilateral acoustic neuroma surgery. Ann. Otol. Rhinol. Laryngol. 1988, 97, 55–66.
- Monsell, E.M.; Balkany, T.A.; Gates, G.A.; Goldenberg, R.A.; Meyerhoff, W.L.; House, J.W. Committee on hearing and equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American academy of otolaryngology-head and neck surgery foundation, inc. Otolaryngol. Head Neck Surg. 1995, 113, 179–180.
- Kanzaki, J.; Tos, M.; Sanna, M.; Moffat, D.A.; Monsell, E.M.; Berliner, K.I. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol. Neurotol. 2003, 24, 642–648; discussion 648–649.
- Zanoletti, E.; Mazzoni, A.; Frigo, A.C.; Borsetto, D.; Cazzador, D. Hearing preservation outcomes and prognostic factors in acoustic neuroma surgery: Predicting cutoffs. Otol. Neurotol. 2020, 41, 686–693.
- Mazzoni, A.; Biroli, F.; Foresti, C.; Signorelli, A.; Sortino, C.; Zanoletti, E. Hearing preservation surgery in acoustic neuroma. Slow progress and new strategies. Acta Otorhinolaryngol. Ital. 2011, 31, 76–84.
- Mastronardi, L.; Di Scipio, E.; Cacciotti, G.; Roperto, R. Vestibular schwannoma and hearing preservation: Usefulness of level specific ce-chirp abr monitoring. A retrospective study on 25 cases with preoperative socially useful hearing. Clin. Neurol. Neurosurg. 2018, 165, 108–115.
- Mastronardi, L.; Di Scipio, E.; Cacciotti, G.; Roperto, R.; Scavo, C.G. Hearing preservation after removal of small vestibular schwannomas by retrosigmoid approach: Comparison of two different abr neuromonitoring techniques. Acta Neurochir. 2019, 161, 69–78.
- Raudzens, P.A. Intraoperative monitoring of evoked-potentials. Ann. N. Y. Acad. Sci. 1982, 388, 308–326.
- Moller, A.R.; Jannetta, P.J. Compound action potentials recorded intracranially from the auditory nerve in man. Exp. Neurol. 1981, 74, 862–874.
- Watanabe, N.; Ishii, T.; Fujitsu, K.; Kaku, S.; Ichikawa, T.; Miyahara, K.; Okada, T.; Tanino, S.; Uriu, Y.; Murayama, Y. Intraoperative cochlear nerve mapping with the mobile cochlear nerve compound action potential tracer in vestibular schwannoma surgery. J. Neurosurg. 2018, 130, 1568–1575.
- Sun, D.Q.; Sullivan, C.B.; Kung, R.W.; Asklof, M.; Hansen, M.R.; Gantz, B.J. How well does intraoperative audiologic monitoring predict hearing outcome during middle fossa vestibular schwannoma resection? Otol. Neurotol. 2018, 39, 908–915.
- Colletti, V.; Fiorino, F.G.; Carner, M.; Cumer, G.; Giarbini, N.; Sacchetto, L. Intraoperative monitoring for hearing preservation and restoration in acoustic neuroma surgery. Skull Base Surg. 2000, 10, 187–195.
- Magnan, J.; Parikh, B.; Miyazaki, H. Nerve monitoring for cerebellopontine angle surgery. In Functional Surgery of Cerebellopontine Angle by Minimally Invasive Retrosigmoid Approach; Jaypee Brothers Medical Pub: New Delhi, India, 2013.
- Miyazaki, H.; Caye-Thomasen, P. Intraoperative auditory system monitoring. Adv. Otorhinolaryngol. 2018, 81, 123–132.
- Sass, H.C.R.; Miyazaki, H.; West, N.; Hansen, S.; Moller, M.N.; Caye-Thomasen, P. Extended retrolabyrinthine approach: Results of hearing preservation surgery using a new system for continuous near real-time neuromonitoring in patients with growing vestibular schwannomas. Otol. Neurotol. 2019, 40, S72–S79.
- Gouveris, H.; Mann, W. Association between surgical steps and intraoperative auditory brainstem response and electrocochleography waveforms during hearing preservation vestibular schwannoma surgery. Eur. Arch. Otorhinolaryngol. 2009, 266, 225–229.
- Schlake, H.P.; Milewski, C.; Goldbrunner, R.H.; Kindgen, A.; Riemann, R.; Helms, J.; Roosen, K. Combined intra-operative monitoring of hearing by means of auditory brainstem responses (abr) and transtympanic electrocochleography (ecochg) during surgery of intra- and extrameatal acoustic neurinomas. Acta Neurochir. 2001, 143, 985–995; discussion 995–996.
- Cane, M.A.; O’Donoghue, G.M.; Lutman, M.E. The feasibility of using oto-acoustic emissions to monitor cochlear function during acoustic neuroma surgery. Scand. Audiol. 2009, 21, 173–176.
- Nakatomi, H.; Miyazaki, H.; Tanaka, M.; Kin, T.; Yoshino, M.; Oyama, H.; Usui, M.; Moriyama, H.; Kojima, H.; Kaga, K.; et al. Improved preservation of function during acoustic neuroma surgery. J. Neurosurg. 2015, 122, 24–33.
- Benecke, J.E., Jr.; Calder, H.B.; Chadwick, G. Facial nerve monitoring during acoustic neuroma removal. Laryngoscope 1987, 97, 697–700.
- Niparko, J.K.; Kileny, P.R.; Kemink, J.L.; Lee, H.M.; Graham, M.D. Neurophysiologic intraoperative monitoring: Ii. Facial nerve function. Am. J. Otol. 1989, 10, 55–61.
- Harper, C.M.; Daube, J.R. Facial nerve electromyography and other cranial nerve monitoring. J. Clin. Neurophysiol. 1998, 15, 206–216.
- Tawfik, K.O.; Walters, Z.A.; Kohlberg, G.D.; Lipschitz, N.; Breen, J.T.; O’Neal, K.; Zuccarello, M.; Samy, R.N. Impact of motor-evoked potential monitoring on facial nerve outcomes after vestibular schwannoma resection. Ann. Otol. Rhinol. Laryngol. 2018, 128, 56–61.
- Amano, M.; Kohno, M.; Nagata, O.; Taniguchi, M.; Sora, S.; Sato, H. Intraoperative continuous monitoring of evoked facial nerve electromyograms in acoustic neuroma surgery. Acta Neurochir. 2011, 153, 1059–1067; discussion 1067.
- Kohno, M.; Taniguchi, M. Intraoperative real-time continuous facial nerve monitoring in acoustic neuroma surgery. Acta Neurochir. 2011, 153, 2273–2274.
- Hosoya, M.; Oishi, N.; Noguchi, M.; Kasuya, K.; Nishiyama, T.; Ishikawa, T.; Kasahara, K.; Miyazaki, H.; Ogawa, K. Intraoperative facial nerve monitoring revealed the origin of rapidly progressing schwannoma in the cerebellopontine angle: A case of large intermediate nerve schwannoma. J. Int. Adv. Otol. 2018, 14, 488–492.
- Hosoya, M.; Oishi, N.; Nishiyama, T.; Noguchi, M.; Kasuya, K.; Suzuki, N.; Miyazaki, H.; Ogawa, K. Preoperative electrophysiological analysis predicts preservation of hearing and facial nerve function following vestibular schwannoma surgery with continuous intraoperative neural monitoring: Clinical outcomes of 22 cases. Clin. Otolaryngol. 2019, 44, 875–880.
- Brackmann, D.E.; Hitselberger, W.E. Retrolabyrinthine approach. Laryngoscope 1978, 88, 286–297.
- Hitselberger, W.E.; Pulec, J.L. Trigeminal nerve (posterior root) retrolabyrinthine selective section. Operative procedure for intractable pain. Arch. Otolaryngol. 1972, 96, 412–415.
- Wang, J.; Li, Y.; Wei, X.; Chen, J.; Zhang, L.; Hao, X.; Li, Y. Hearing preservation/rehabilitation surgery for small vestibular schwannoma: Preliminary experience with the presigmoid retrolabyrinthine approach. Acta Oto-Laryngol. 2021, 141, 608–614.
- Troude, L.; Baucher, G.; Lavieille, J.-P.; Roche, P.-H. The presigmoid retrolabyrinthine approach: Technical note. Neurochirurgie 2021, 67, 503–507.
- Bento, R.F.; Lopes, P.T. The transmastoid retrolabyrinthine approach in acoustic neuroma surgery: Our experience in 189 patients. Otol. Neurotol. 2020, 41, 972–977.
- Iacoangeli, M.; Salvinelli, F.; Di Rienzo, A.; Gladi, M.; Alvaro, L.; Greco, F.; Carassiti, M.; Scerrati, M. Microsurgical endoscopy-assisted presigmoid retrolabyrinthine approach as a minimally invasive surgical option for the treatment of medium to large vestibular schwannomas. Acta Neurochir. 2013, 155, 663–670.
- Muelleman, T.; Shew, M.; Alvi, S.; Shah, K.; Staecker, H.; Chamoun, R.; Lin, J. Endoscopically assisted drilling, exposure of the fundus through a presigmoid retrolabyrinthine approach: A cadaveric feasibility study. Otolaryngol. Head Neck Surg. 2018, 158, 155–157.
- Tan, H.-Y.; Yang, J.; Wang, Z.-Y.; Zhu, W.-D.; Chai, Y.-C.; Jia, H.; Wu, H. Simultaneous supervision by microscope of endoscope-assisted microsurgery via presigmoid retrolabyrinthine approach: A pilot study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, S103–S106.
More
Information
Subjects:
Otorhinolaryngology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
19 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




