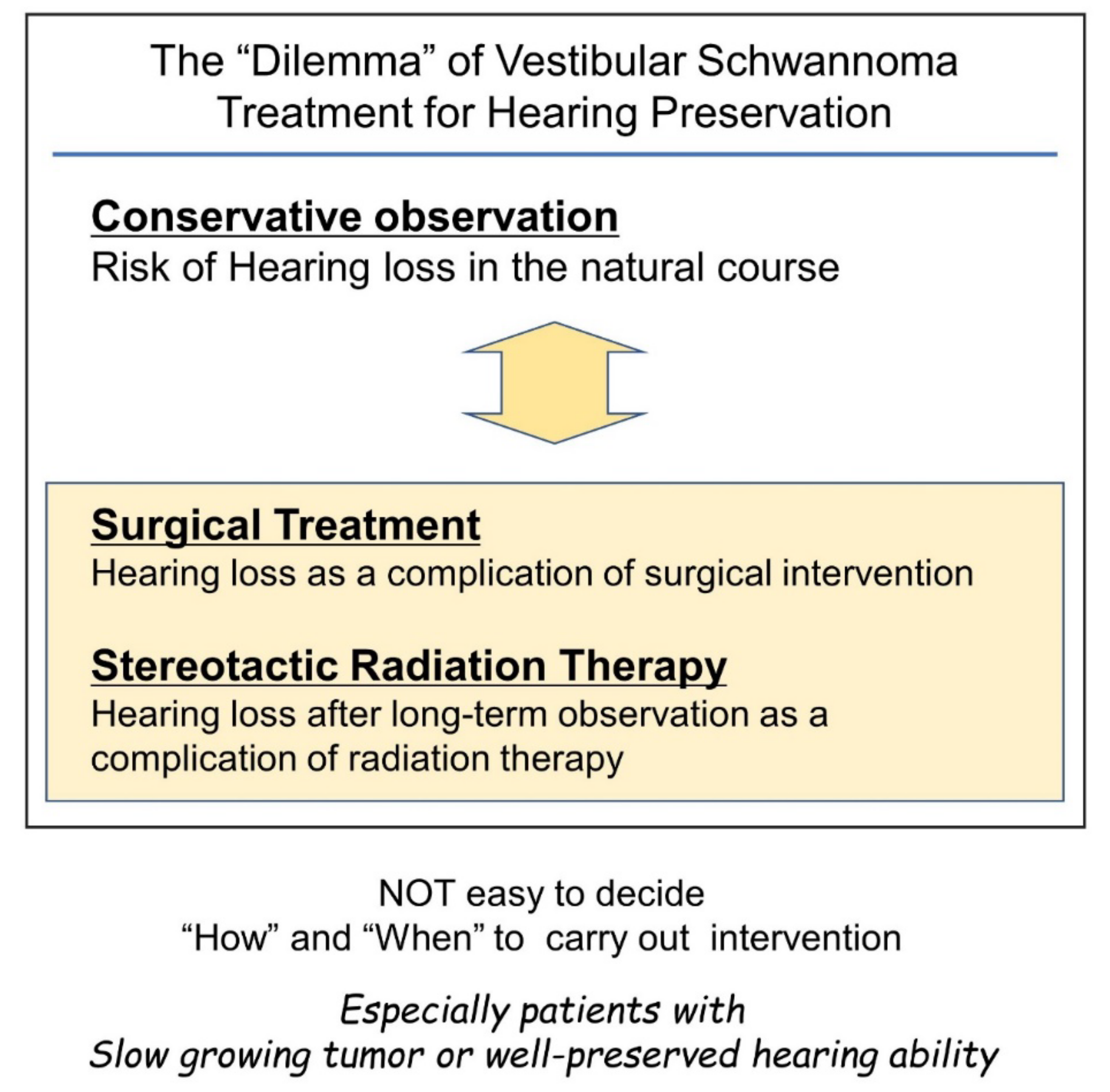
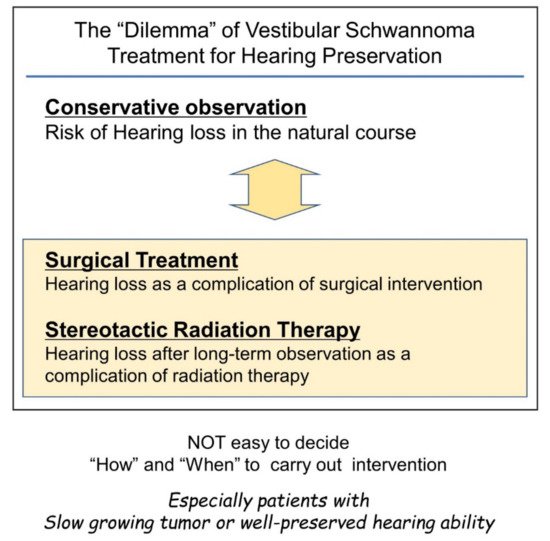
Figure 1. Difficulties in timing considerations in vestibular schwannoma intervention with the goal of preserving hearing. Conservative observation and intervention with surgery or stereotactic radiotherapy can cause hearing loss. Therefore, the decision to intervene can be a difficult decision for the surgeon.
Therefore, if the tumor size is not life-threatening, non-hearing preservation surgery can be chosen once hearing loss sets in by natural course. This type of surgical decision making has been widely applied. However, the importance of hearing preservation has been underestimated in this context.
Preserving residual hearing in vestibular schwannoma patients is necessary for several reasons. Hearing loss on the affected side due to either the natural course of the disease or the side effects of the interventions usually results in impaired sound localization and difficulty in understanding speech in the presence of background noise
[26][27][26,27]. However, if serviceable hearing can be spared, hearing aid use can be considered. Recently, Kitamura et al. reported the possible importance of residual hearing after hearing preservation surgery for the treatment of tinnitus
[28]. It has also been reported that tinnitus negatively affects the quality of life of patients with vestibular schwannomas
[29][30][31][29,30,31]. These previous studies suggest that hearing preservation is desirable in tinnitus management and it improves the patients’ quality of life.
4. Interventions for the Vestibular Schwannoma
There are several management methods for vestibular schwannomas. A “wait-and-scan” policy, in which patients’ tumors are observed once or twice by MRI per year, is well applied to patients with non-growing tumors
[32][33][34][35][32,33,34,35]. Typically, tumors smaller than 1.5 cm in diameter located on the cerebellopontine angle are considered for a wait-and-scan approach. Once tumor growth has been detected with or without cystic changes, interventions should be considered, and, especially in patients who are candidates for hearing preservation, surgery should be considered
[36]. Because of the potential side effects of treatments and the fact that the tumor is benign and non-invasive, in several cases, such as elderly patients who do not want surgery, or patients with very slow-growing tumors, the “wait-and-scan” policy may still be preferable.
There are several interventions available for a growing vestibular schwannoma, including radiotherapy and surgical resection. Stereotactic radiosurgery, such as Gamma-Knife
[37][38][37,38], has been widely used in radiation therapy for vestibular schwannoma. Stereotactic radiosurgery aims to prevent tumor growth but without expectation of a radiological cure, similar to other radiation therapies for malignant tumors. Stereotactic radiosurgery showed a high long-term local control rate
[39] and had merit that it can be applied for the relatively large tumor that cannot be surgically removed
[40]. Stereotactic radiosurgery is an effective treatment option for small- to medium-sized (<3 cm) vestibular schwannomas without significant mass effect, while large tumors causing brainstem compression or symptoms of mass effect require surgical intervention. Stereotactic radiosurgery has similar local control rates compared to surgery in appropriately selected patients, typically those without significant mass effect or brain compression
[41]. In some instances, stereotactic radiosurgery provides better functional outcomes
[41].
Although stereotactic radiosurgery has several merits and is widely used, it still has the disadvantage of hearing loss. It is believed that stereotactic radiosurgery has a high hearing preservation rate. However, recent long-term observations showed that patients’ hearing after stereotactic radiosurgery gradually decreased
[42][43][44][45][42,43,44,45], and serviceable hearing levels are lost in most patients in a span of ten years
[43]. This result suggests that stereotactic radiosurgery can only achieve temporary hearing preservation; therefore, this intervention may not be suitable for younger patients.
Neurofibromatosis type II is often treated by a neurosurgeon and less often by an otolaryngologist, requiring a different treatment plan. In neurofibromatosis type II, special consideration should be given to the possibility of bilateral hearing loss due to bilateral vestibular schwannomas. Treatment strategies for sporadic vestibular schwannomas rarely apply to neurofibromatosis type II cases, as contralateral hearing levels and the possible presence of other brain tumors such as meningiomas affect the treatment strategy for neurofibromatosis type II.
5. Surgical Approach for the Vestibular Schwannoma
Surgical resection can be performed for all tumor sizes. Usually, large tumors, such as those associated with brainstem compression, are resected by neurosurgeons, and otologists treat relatively smaller tumors that are limited to the inner ear canal or those that slightly reach the brainstem. The middle fossa approach, translabyrinthine approach, and retrosigmoid approach are thought to be the three primary microsurgical approaches; however, other approaches are also used to remove vestibular schwannomas, each with benefits and limitations.
Surgical interventions for vestibular schwannoma can be categorized into two types depending on the expected postoperative hearing results: non-hearing preservation surgery and hearing preservation surgery. Most non-hearing preservation surgeries are performed using the translabyrinthine approach
[46]. The transotic approach can also be used
[47][48][47,48]. Recently, the exclusive endoscopic transcanal transpromontorial approach has been reported as a non-hearing preservation surgery
[49][50][49,50]. Several surgical methods can be used for hearing preservation, including the retrosigmoid approach, the middle temporal fossa approach, and the retrolabyrinthine approach. Recently, the retrolabyrinthine meatotomy technique combined with the retrosigmoid approach has been reported
[51].
While the surgical approach can be easily divided into two groups, it is difficult to define “what is good and serviceable hearing” or “what is hearing worth to be preserved”. Therefore, several criteria have been used. Historically, Wade and House described the “50/50 rule”, which defined serviceable hearing as a pure tone average (PTA) of ≤50 dB with a speech discrimination score (SDS) of 50% or better
[52]. In 1988, the Gardner–Robertson Scale was developed, in which Grade I (Good: PTA ≤ 30 dB with SDS ≥ 70%) and Grade II (Serviceable: PTA of 30–50 dB with SDS ≥ 50%) are considered “useful hearing”
[53]. In 1995, the American Academy of Otolaryngology Head and Neck Surgery guideline was established for reporting hearing outcomes after hearing preservation surgery for lateral skull base surgery, in which all patients with SDS < 50% are considered as having non-serviceable hearing
[54]. In 2003, the Tokyo classification was developed
[55]. These criteria help report the results of surgical interventions and are usually also used for selecting the candidate for hearing preservation surgery.
Based on these previous criteria, candidates for hearing preservation surgery have often been defined as patients with PTA ≤ 30 dB with SDS ≥ 70%
[56][57][56,57] or with PTA of 30–50 dB with SDS ≥ 50%
[58][59][58,59]. However, as mentioned above, it was suggested that any level of residual hearing might be helpful for tinnitus control or the improvement of patients’ quality of life. Moreover, the residual hearing, even if it was at an “unserviceable level”, might broaden the possibility of hearing rehabilitation after vestibular schwannoma surgery
[27].
The selection of the surgical approach is dependent on the degree of residual hearing, tumor size, tumor location, and whether hearing preservation is preferred. If hearing preservation surgery is selected, the central focus of the procedure is the preservation of auditory function. However, irrespective of which surgical category is selected, the surgeon must attempt to prevent facial nerve palsy, particularly in cases with no evident preoperative deficit. Intraoperative neural monitoring is a widely used method to facilitate postoperative hearing and prevent facial nerve function deficits.
6. Intraoperative Electrophysiological Monitoring for Vestibular Schwannoma Surgery
Since the first report of intraoperative ABR monitoring in vestibular schwannoma surgery in 1982
[60][61], it has become a standard monitoring method, and its clinical value is now broadly accepted in the field. Since then, other modalities have been introduced into clinical practice, including direct eighth cranial nerve monitoring via cochlear nerve action potentials (CNAP)
[61][62][63][64][62,63,64,65] or dorsal nucleus action potential (DNAP)
[65][66][67][66,67,68], electrocochleography
[68][69][69,70], and OAE
[70][71].
However, intraoperative ABR monitoring has two major limitations. First, ABR detection is time-consuming. ABR measurement requires an average of 500–2000 stimulations and a duration of more than one minute. Although this time period is acceptable when applying ABR as a hearing test in an outpatient setting, it is problematic in intraoperative monitoring. Second, vestibular schwannomas impede acoustic neuron conduction. As a result, the amplitude of the ABR decreases and, in some cases, intraoperative ABR waveforms cannot be obtained. In the 2010s, a novel form of intraoperative monitoring for hearing function was developed, i.e., DNAP monitoring
[71][72]. In DNAP monitoring, the detection probe is set on the dorsal cochlear nucleus of the brainstem. The action potential of the dorsal cochlear nucleus is detected using this probe. DNAP has high sensitivity in detecting electrical signals and can detect signals that ABR overlooks. Moreover, the DNAP system requires an average of only 100–200 stimulations. Intraoperative DNAP signals can thus be obtained every 10 s. In contrast, when using the DNAP, putting the detection probe on the brainstem is required, which is not needed for ABR monitoring. Therefore, DNAP monitoring cannot be applicable in the middle fossa approach, although ABR monitoring can be used in all hearing preservation surgeries.
Electromyographic monitoring systems, such as the Medtronic NIM system, are widely used
[72][73][74][75][73,74,75,76]. However, this system involves the detection of sporadic firing of the facial nerve via intrinsic physiological stimulation. Thus, this system cannot be used for continuous intraoperative monitoring. Advanced monitoring systems with continuous monitoring have been developed to overcome this limitation
[71][76][77][72,77,78]. The monitoring system using facial nerve root exit zone-elicited compound muscle action potential (FREMAP) is one such system that has been recently introduced. Although the FREMAP monitoring system needs to set an electric stimulation probe to the main trunk of the facial nerve, this probe enables continuous intraoperative stimulation of the facial nerve, followed by detection of a facial muscle action potential. FREMAP monitoring enables stimulation of the facial nerve and the detection of facial muscle action potentials to be performed continuously throughout the surgical procedure
[78][79]. Thus, the surgeon can obtain continuous quantitative data concerning facial nerve status during tumor resection. In addition, the surgeon can abandon the procedure or select an alternative resection route, if necessary. While the electrode for FREMAP monitoring is designed to be put on the main trunk of the facial nerve in the surgical view of the retrolabyrinthine approach or the retrosigmoid approach, it could be applied to other approaches, including non-hearing preservation surgeries.
7. Retrolabyrinthine Approach under Reinforced Continuous Intraoperative Monitoring with FREMAP and DNAP
The retrolabyrinthine approach is a surgical approach for vestibular schwannomas that can preserve patients’ hearing
[79][80][81][82][83][84][60,80,81,82,83,84]. In the retrolabyrinthine approach, the tumors are approached through the area posterior to the posterior semicircular canal and prior to the sigmoid sinus
[83]. This approach has several advantages compared with other approaches that can preserve hearing. The first advantage is that this approach does not require craniotomy, which is required by the retrosigmoid and middle fossa approaches. Therefore, less pressure is applied to the temporal lobe or cerebellum. The second advantage of the retrolabyrinthine approach is that the risk of encountering the facial nerve first in the inner ear canal is relatively lower compared with the middle fossa approach. The third advantage of this approach is that DNAP and FREMAP electrodes, which cannot be used in the middle fossa approach, can be used because the brain stem is visible.
However, there are several disadvantages of the retrolabyrinthine approach as well. First is that this approach cannot be applied to some patients because of certain anatomical features of the temporal bones. Patients with one or more of the following anatomical features may be poor candidates for this approach: high jugular bulb, less developed bony cells posterior to the labyrinth, less developed mastoid air cells, and less developed sigmoid sinus on the opposite side of the surgical site. The second disadvantage of the retrolabyrinthine approach is that the surgical field is relatively narrower than other approaches, and the fundus of the inner ear canal cannot be visualized by surgical microscopy alone. Although the use of the endoscope can overcome this limitation to some extent
[85][86][87][85,86,87], this approach is more suitable for smaller tumors (Koos classification I or II) with tumor-free space on the fundus of the inner ear canal.