
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raghad AL-Ishaq | -- | 3528 | 2022-12-17 12:07:28 | | | |
| 2 | Raghad AL-Ishaq | -475 word(s) | 3053 | 2022-12-19 08:28:18 | | | | |
| 3 | Amina Yu | -24 word(s) | 3029 | 2022-12-19 09:32:30 | | |
Video Upload Options
Diabetes and gastrointestinal cancers (GI) are global health conditions with a massive burden on patients’ lives worldwide. The development of both conditions is influenced by several factors, such as diet, genetics, environment, and infection, which shows a potential link between them. Flavonoids are naturally occurring phenolic compounds present in fruits and vegetables. Once ingested, unabsorbed flavonoids reaching the colon undergo enzymatic modification by the gut microbiome to facilitate absorption and produce ring fission products. The metabolized flavonoids exert antidiabetic and anti-GI cancer properties, targeting major impaired pathways such as apoptosis and cellular proliferation in both conditions, suggesting the potentially dual effects of flavonoids on diabetes and GI cancers. Herein, the knowledge on the impact of flavonoids on diabetes and GI cancers in four significant pathways is summarized. It also addresses the synergistic effects of selected flavonoids on both conditions. While this is an intriguing approach, more studies are required to better understand the mechanism of how flavonoids can influence the same impaired pathways with different outcomes depending on the disease.
1. Introduction
1.1. Diabetes
1.2. Gastrointestinal Cancer
1.3. Flavonoids Influence Diabetes and GI Cancer
1.4. Gut Microbiota: The Role in Diabetes and GI Cancer
2. Flavonoids and Apoptosis in Diabetes and GI Cancers
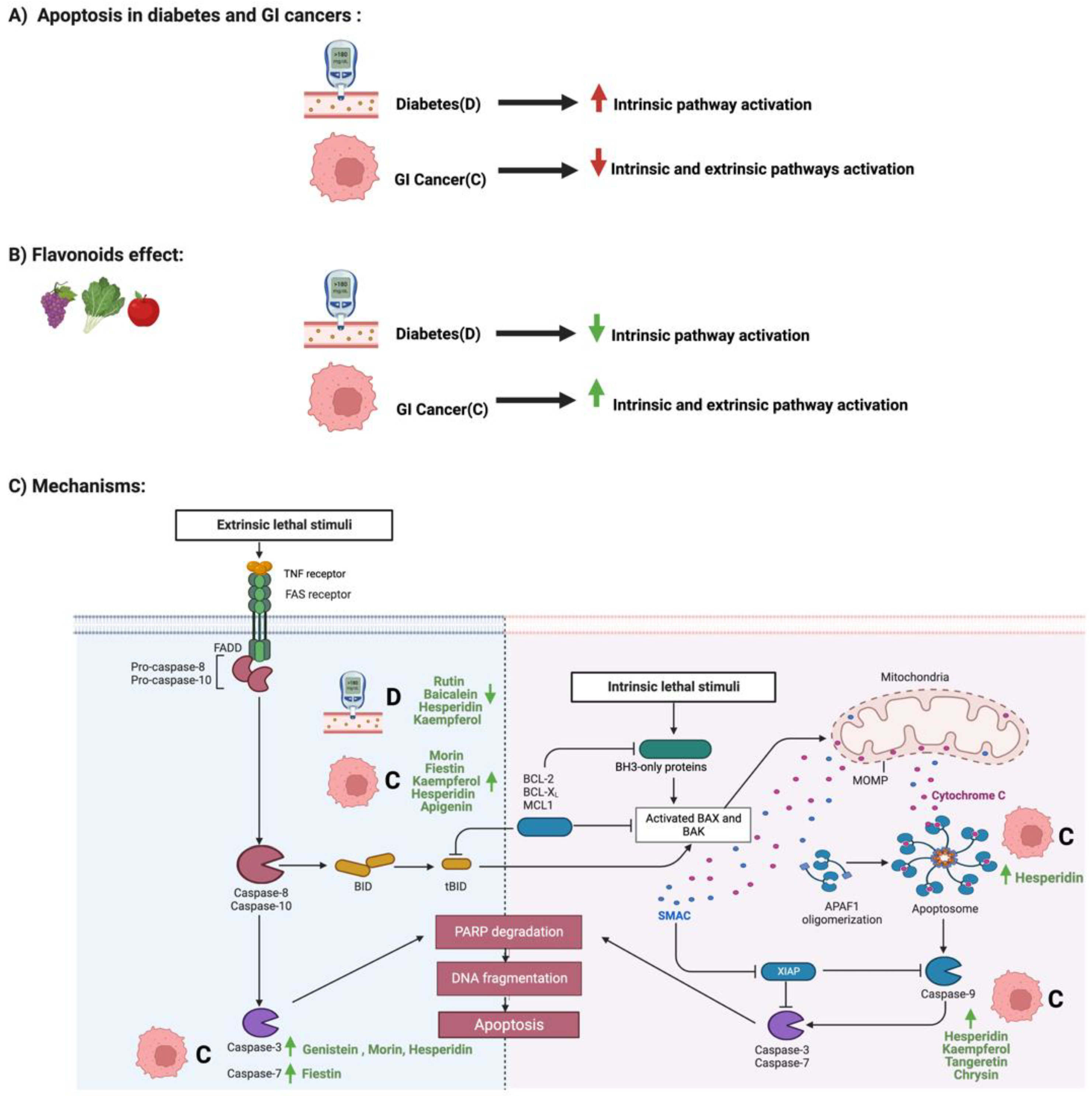
3. Flavonoids and NF-κB Pathway in Diabetes and GI Cancers
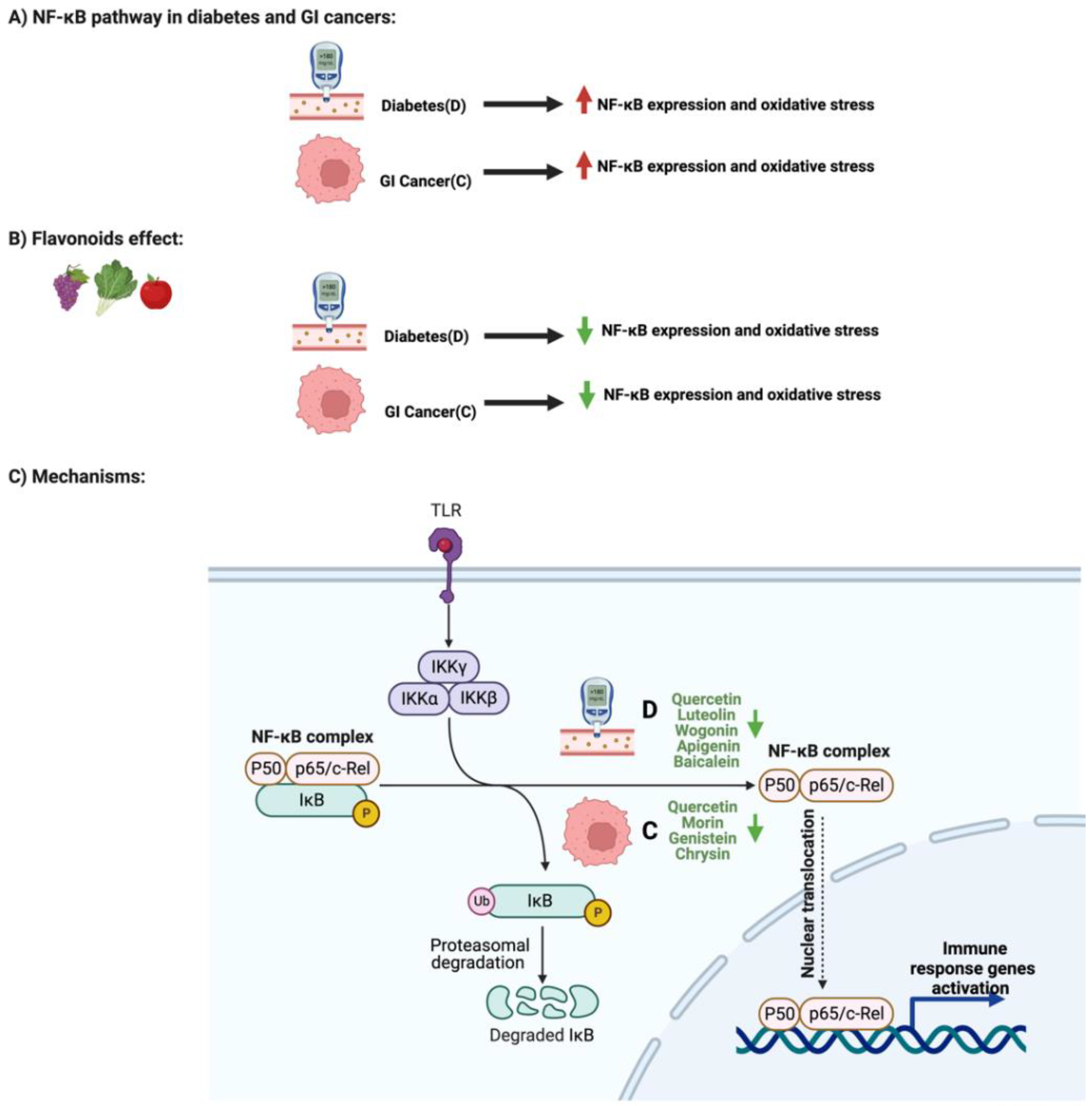
The paper also address the influence of Flavonoids on AMPK Pathway and Enzymatic Activities in Diabetes and GI Cancers.
4. Discussion
4.1. Targeting Multiple Pathways with Multiple Flavonoids That Act Synergistically?
4.2. Gut Microbiome and Flavonoids’ Effects
4.3. Challenges with Studying the Field
4.4. GI Cancers and Diabetes: Their Relationship?
5. Conclusions
References
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420.
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188.
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867.
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236.
- Reyes, J.; Tripp-Reimer, T.; Parker, E.; Muller, B.; Laroche, H. Factors Influencing Diabetes Self-Management Among Medically Underserved Patients with Type II Diabetes. Glob. Qual. Nurs. Res. 2017, 4, 2333393617713097.
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551.
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790.
- Ramachandran, A. Know the signs and symptoms of diabetes. Indian J. Med. Res. 2014, 140, 579–581.
- Dhatariya, K. Diabetes: The place of new therapies. Adv. Endocrinol. Metab. 2019, 10, 2042018818807599.
- Blaslov, K.; Naranda, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219.
- Hassanzade, J.; Molavi, E.V.H.; Farahmand, M.; Rajaiifard, A.R. Incidence and Mortality Rate of Common Gastrointestinal Cancers in South of Iran, a Population Based Study. Iran. J. Cancer Prev. 2011, 4, 163–169.
- Bordry, N.; Astaras, C.; Ongaro, M.; Goossens, N.; Frossard, J.L.; Koessler, T. Recent advances in gastrointestinal cancers. World J. Gastroenterol. 2021, 27, 4493–4503.
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28.
- Zali, H.; Rezaei-Tavirani, M.; Azodi, M. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench. 2011, 4, 175–185.
- Han, C.J.; Reding, K.; Cooper, B.A.; Paul, S.M.; Conley, Y.P.; Hammer, M.; Miaskowski, C. Symptom Clusters in PatientsWith Gastrointestinal Cancers Using Different Dimensions of the Symptom Experience. J. Pain Symptom Manag. 2019, 58, 224–234.
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012.
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2020, 42, 211–217.
- Matsuoka, T.; Yashiro, M. Precision medicine for gastrointestinal cancer: Recent progress and future perspective. World J. Gastrointest Oncol. 2020, 12, 1–20.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids—food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85.
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243.
- Juca, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Vasconcelos, S.M.M. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705.
- Sok Yen, F.; Shu Qin, C.; Tan Shi Xuan, S.; Jia Ying, P.; Yi Le, H.; Darmarajan, T.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid Based Complement. Altern. Med. 2021, 2057333.
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients 2016, 8, 310.
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137.
- Vitelli Storelli, F.; Molina, A.J.; Zamora-Ros, R.; Fernandez-Villa, T.; Roussou, V.; Romaguera, D.; Martin, V. Flavonoids and the Risk of Gastric Cancer: An Exploratory Case-Control Study in the MCC-Spain Study. Nutrients 2019, 11, 967.
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. (Encinitas) 2014, 13, 17–22.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836.
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Busselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934.
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Pedersen, O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266.
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27.
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Voortman, T. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811.
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876.
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Aachoui, Y.; Sagulenko, V.; Miao, E.A.; Stacey, K.J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr. Opin. Microbiol. 2013, 16, 319–326.
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193.
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharm. 2022, 146, 112442.
- Naudi, A.; Jove, M.; Ayala, V.; Cassanye, A.; Serrano, J.; Gonzalo, H.; Pamplona, R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp. Diabetes Res. 2012, 696215.
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550.
- Ma, P.; Mao, X.Y.; Li, X.L.; Ma, Y.; Qiao, Y.D.; Liu, Z.Q.; Cao, Y.G. Baicalin alleviates diabetes associated cognitive deficits via modulation of mitogen-activated protein kinase signaling, brain derived neurotrophic factor and apoptosis. Mol. Med. Rep. 2015, 12, 6377–6383.
- Dong, Y.; Xing, Y.; Sun, J.; Sun, W.; Xu, Y.; Quan, C. Baicalein Alleviates Liver Oxidative Stress and Apoptosis Induced by High-Level Glucose through the Activation of the PERK/Nrf2 Signaling Pathway. Molecules 2020, 25, 599.
- Parmar, M.S.; Syed, I.; Gray, J.P.; Ray, S.D. Curcumin, Hesperidin, and Rutin Selectively Interfere with Apoptosis Signaling and Attenuate Streptozotocin-Induced Oxidative Stress-Mediated Hyperglycemia. Curr. Neurovasc. Res. 2015, 12, 363–374.
- Tian, M.; Han, Y.B.; Zhao, C.C.; Liu, L.; Zhang, F.L. Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol. Metab. Syndr. 2021, 13, 50.
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharm. 2017, 96, 305–312.
- Wang, J.; Wang, R.; Li, J.; Yao, Z. Rutin alleviates cardiomyocyte injury induced by high glucose through inhibiting apoptosis and endoplasmic reticulum stress. Exp. Med. 2021, 22, 944.
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448.
- Maeda, Y.; Takahashi, H.; Nakai, N.; Yanagita, T.; Ando, N.; Okubo, T.; Takiguchi, S. Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int. J. Oncol. 2018, 52, 1661–1673.
- Zhou, P.; Wang, C.; Hu, Z.; Chen, W.; Qi, W.; Li, A. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-kappaB/slug/E-cadherin pathway. BMC Cancer 2017, 17, 813.
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875.
- Bahadori, M.; Baharara, J.; Amini, E. Anticancer Properties of Chrysin on Colon Cancer Cells, In vitro and In vivo with Modulation of Caspase-3, -9, Bax and Sall4. Iran. J. Biotechnol. 2016, 14, 177–184.
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharm. 2017, 8, 640.
- Zhang, J.; Wu, D.; Vikash, S.J.; Wang, J.; Yi, J.; Dong, W. Hesperetin Induces the Apoptosis of Gastric Cancer Cells via Activating Mitochondrial Pathway by Increasing Reactive Oxygen Species. Dig. Dis Sci. 2015, 60, 2985–2995.
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144.
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468.
- Park, M.H.; Hong, J.T. Roles of NF-kappaB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15.
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443.
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharm. 2017, 8, 798.
- Chaithongyot, S.; Jantaree, P.; Sokolova, O.; Naumann, M. NF-kappaB in Gastric Cancer Development and Therapy. Biomedicines 2021, 9, 870.
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628.
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094.
- Yin, H.; Huang, L.; Ouyang, T.; Chen, L. Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-kappaB signaling pathway. Int. Immunopharmacol. 2018, 55, 55–62.
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-kappaB-TNF-alpha and TGF-beta1-MAPK-fibronectin pathways. Am. J. Physiol. Ren. Physiol. 2017, 313, F414–F422.
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774.
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: Effect on low grade inflammation. PLoS ONE 2013, 8, e63784.
- Xia, Y.; Lian, S.; Khoi, P.N.; Yoon, H.J.; Han, J.Y.; Chay, K.O.; Jung, Y.D. Chrysin inhibits cell invasion by inhibition of Recepteur d’origine Nantais via suppressing early growth response-1 and NF-kappaB transcription factor activities in gastric cancer cells. Int. J. Oncol. 2015, 46, 1835–1843.
- Chen, R.; Zhang, L. Morin inhibits colorectal tumor growth through inhibition of NF-kappaB signaling pathway. Immunopharmacol. Immunotoxicol. 2019, 41, 622–629.
- Li, Y.S.; Wu, L.P.; Li, K.H.; Liu, Y.P.; Xiang, R.; Zhang, S.B.; Zhang, L.Y. Involvement of nuclear factor kappaB (NF-kappaB) in the downregulation of cyclooxygenase-2 (COX-2) by genistein in gastric cancer cells. J. Int. Med. Res. 2011, 39, 2141–2150.
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-kappab, PKC-delta, ERK1/2, and AMPKalpha. Integr. Cancer 2018, 17, 511–523.
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The Potential Role of Phytonutrients Flavonoids Influencing Gut Microbiota in the Prophylaxis and Treatment of Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 798038.
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159.
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610.
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521.
- Li, W.Z.; Stirling, K.; Yang, J.J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293–308.
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103.
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front. Immunol. 2021, 12, 807648.
- Liu, Y.; Baba, Y.; Ishimoto, T.; Gu, X.; Zhang, J.; Nomoto, D.; Qiu, P. Gut microbiome in gastrointestinal cancer: A friend or foe? Int. J. Biol Sci. 2022, 18, 4101–4117.
- Tremmel, M.; Paetz, C.; Heilmann, J. In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules 2021, 26, 6632.
- Wang, L.; Huang, G.; Hou, R.; Qi, D.; Wu, Q.; Nie, Y.; Wei, F. Multi-omics reveals the positive leverage of plant secondary metabolites on the gut microbiota in a non-model mammal. Microbiome 2021, 9, 192.
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420.
- Boker, K.L.; Van der Schouw, Y.T.; De Kleijn, M.J.; Jacques, P.F.; Grobbee, D.E.; Peeters, P.H. Intake of Dietary Phytoestrogens by Dutch Women. J. Nutr. 2002, 132, 1319.
- Arts, I.C.; Hollman, P.C.; Feskens, E.J.; Bueno de Mesquita, H.B.; Kromhout, D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 2001, 55, 76–81.
- Rodriguez De Luna, S.L.; Ramirez-Garza, R.E.; Serna Saldivar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 6792069.
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387.
- Roderburg, C.; Loosen, S.H.; Hoyer, L.; Luedde, T.; Kostev, K. Prevalence of diabetes mellitus among 80,193 gastrointestinal cancer patients in five European and three Asian countries. J. Cancer Res. Clin. Oncol. 2022, 148, 1057–1062.
- Tseng, C.H. The Relationship between Diabetes Mellitus and Gastric Cancer and the Potential Benefits of Metformin: An Extensive Review of the Literature. Biomolecules 2021, 11, 1022.
- Tseng, C.H.; Tseng, F.H. Diabetes and gastric cancer: The potential links. World J. Gastroenterol. 2014, 20, 1701–1711.
- Samec, M.; Liskova, A.; Koklesova, L.; Mersakova, S.; Strnadel, J.; Kajo, K.; Kubatka, P. Flavonoids Targeting HIF-1: Implications on Cancer Metabolism. Cancers 2021, 13, 559–587.
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Golubnitschaja, O. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587.
- Zhang, S.; Jin, S.; Zhang, S.; Li, Y.Y.; Wang, H.; Chen, Y.; Lu, H. Vitexin protects against high glucose-induced endothelial cell apoptosis and oxidative stress via Wnt/beta-catenin and Nrf2 signalling pathway. Arch. Physiol. Biochem. 2022, 1–10.
- Ling, J.Y.; Wang, Q.L.; Liang, H.N.; Liu, Q.B.; Yin, D.H.; Lin, L. Flavonoid-Rich Extract of Oldenlandia diffusa (Willd.) Roxb. Inhibits Gastric Cancer by Activation of Caspase-Dependent Mitochondrial Apoptosis. Chin. J. Integr. Med. 2022, 1–11.




