Diabetes and gastrointestinal cancers (GI) are global health conditions with a massive burden on patients’ lives worldwide. The development of both conditions is influenced by several factors, such as diet, genetics, environment, and infection, which shows a potential link between them. Flavonoids are naturally occurring phenolic compounds present in fruits and vegetables. Once ingested, unabsorbed flavonoids reaching the colon undergo enzymatic modification by the gut microbiome to facilitate absorption and produce ring fission products. The metabolized flavonoids exert antidiabetic and anti-GI cancer properties, targeting major impaired pathways such as apoptosis and cellular proliferation in both conditions, suggesting the potentially dual effects of flavonoids on diabetes and GI cancers. This review summarizes the current knowledge on the impact of flavonoids on diabetes and GI cancers in four significant pathways. It also addresses the synergistic effects of selected flavonoids on both conditions. While this is an intriguing approach, more studies are required to better understand the mechanism of how flavonoids can influence the same impaired pathways with different outcomes depending on the disease.
- flavonoid
- diabetes
- GI cancer
- dual
- synergetic
Introduction
Diabetes
Gastrointestinal Cancer
Flavonoids Influence Diabetes and GI Cancer
Gut Microbiota: The Role in Diabetes and GI Cancer
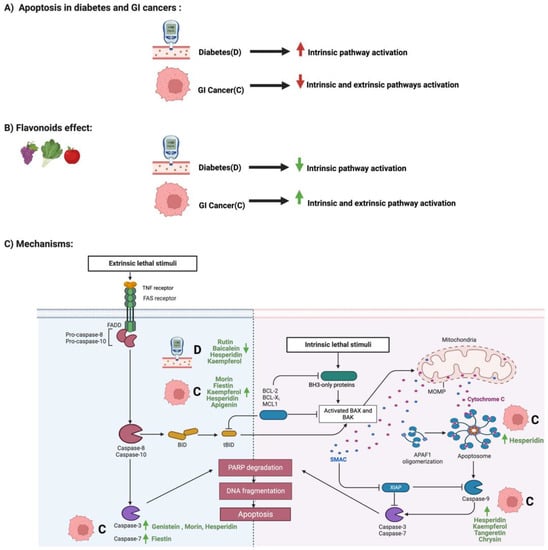
Flavonoids and Apoptosis in Diabetes and GI Cancers
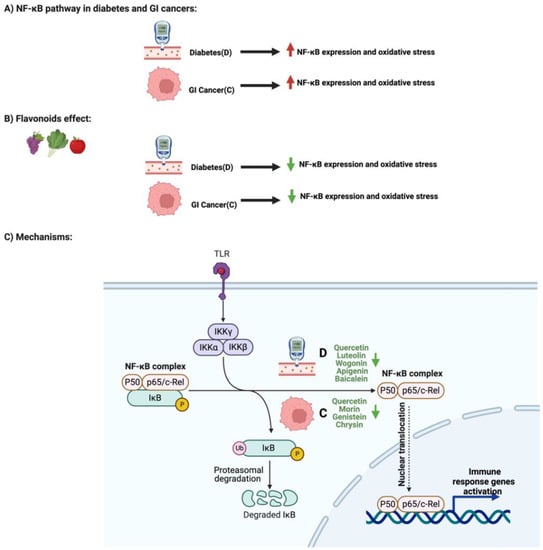
Flavonoids and NF-κB Pathway in Diabetes and GI Cancers
The paper also address the influence of Flavonoids on AMPK Pathway and Enzymatic Activities in Diabetes and GI Cancers.
Discussion
Targeting Multiple Pathways with Multiple Flavonoids That Act Synergistically?
Gut Microbiome and Flavonoids’ Effects
Challenges with Studying the Field
GI Cancers and Diabetes: Their Relationship?
Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cancers14246073
References
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Tripp-Reimer, T.; Parker, E.; Muller, B.; Laroche, H. Factors Influencing Diabetes Self-Management Among Medically Underserved Patients with Type II Diabetes. Glob. Qual. Nurs. Res. 2017, 4, 2333393617713097. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A. Know the signs and symptoms of diabetes. Indian J. Med. Res. 2014, 140, 579–581. [Google Scholar]
- Dhatariya, K. Diabetes: The place of new therapies. Adv. Endocrinol. Metab. 2019, 10, 2042018818807599. [Google Scholar] [CrossRef]
- Blaslov, K.; Naranda, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef]
- Hassanzade, J.; Molavi, E.V.H.; Farahmand, M.; Rajaiifard, A.R. Incidence and Mortality Rate of Common Gastrointestinal Cancers in South of Iran, a Population Based Study. Iran. J. Cancer Prev. 2011, 4, 163–169. [Google Scholar] [PubMed]
- Bordry, N.; Astaras, C.; Ongaro, M.; Goossens, N.; Frossard, J.L.; Koessler, T. Recent advances in gastrointestinal cancers. World J. Gastroenterol. 2021, 27, 4493–4503. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zali, H.; Rezaei-Tavirani, M.; Azodi, M. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench. 2011, 4, 175–185. [Google Scholar]
- Han, C.J.; Reding, K.; Cooper, B.A.; Paul, S.M.; Conley, Y.P.; Hammer, M.; Miaskowski, C. Symptom Clusters in PatientsWith Gastrointestinal Cancers Using Different Dimensions of the Symptom Experience. J. Pain Symptom Manag. 2019, 58, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2020, 42, 211–217. [Google Scholar]
- Matsuoka, T.; Yashiro, M. Precision medicine for gastrointestinal cancer: Recent progress and future perspective. World J. Gastrointest Oncol. 2020, 12, 1–20. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids—food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Juca, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Vasconcelos, S.M.M. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Sok Yen, F.; Shu Qin, C.; Tan Shi Xuan, S.; Jia Ying, P.; Yi Le, H.; Darmarajan, T.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid Based Complement. Altern. Med. 2021, 2057333. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Vitelli Storelli, F.; Molina, A.J.; Zamora-Ros, R.; Fernandez-Villa, T.; Roussou, V.; Romaguera, D.; Martin, V. Flavonoids and the Risk of Gastric Cancer: An Exploratory Case-Control Study in the MCC-Spain Study. Nutrients 2019, 11, 967. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. (Encinitas) 2014, 13, 17–22. [Google Scholar]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Busselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Pedersen, O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Voortman, T. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Busselberg, D. Flavonoids and Their Antidiabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar]
- Al-Ishaq, R.K.; Overy, A.J.; Busselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Aachoui, Y.; Sagulenko, V.; Miao, E.A.; Stacey, K.J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr. Opin. Microbiol. 2013, 16, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharm. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Naudi, A.; Jove, M.; Ayala, V.; Cassanye, A.; Serrano, J.; Gonzalo, H.; Pamplona, R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp. Diabetes Res. 2012, 696215. [Google Scholar] [CrossRef]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Mao, X.Y.; Li, X.L.; Ma, Y.; Qiao, Y.D.; Liu, Z.Q.; Cao, Y.G. Baicalin alleviates diabetes associated cognitive deficits via modulation of mitogen-activated protein kinase signaling, brain derived neurotrophic factor and apoptosis. Mol. Med. Rep. 2015, 12, 6377–6383. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xing, Y.; Sun, J.; Sun, W.; Xu, Y.; Quan, C. Baicalein Alleviates Liver Oxidative Stress and Apoptosis Induced by High-Level Glucose through the Activation of the PERK/Nrf2 Signaling Pathway. Molecules 2020, 25, 599. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.S.; Syed, I.; Gray, J.P.; Ray, S.D. Curcumin, Hesperidin, and Rutin Selectively Interfere with Apoptosis Signaling and Attenuate Streptozotocin-Induced Oxidative Stress-Mediated Hyperglycemia. Curr. Neurovasc. Res. 2015, 12, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Han, Y.B.; Zhao, C.C.; Liu, L.; Zhang, F.L. Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol. Metab. Syndr. 2021, 13, 50. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharm. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Li, J.; Yao, Z. Rutin alleviates cardiomyocyte injury induced by high glucose through inhibiting apoptosis and endoplasmic reticulum stress. Exp. Med. 2021, 22, 944. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Maeda, Y.; Takahashi, H.; Nakai, N.; Yanagita, T.; Ando, N.; Okubo, T.; Takiguchi, S. Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int. J. Oncol. 2018, 52, 1661–1673. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, C.; Hu, Z.; Chen, W.; Qi, W.; Li, A. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-kappaB/slug/E-cadherin pathway. BMC Cancer 2017, 17, 813. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.; Baharara, J.; Amini, E. Anticancer Properties of Chrysin on Colon Cancer Cells, In vitro and In vivo with Modulation of Caspase-3, -9, Bax and Sall4. Iran. J. Biotechnol. 2016, 14, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharm. 2017, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, D.; Vikash, S.J.; Wang, J.; Yi, J.; Dong, W. Hesperetin Induces the Apoptosis of Gastric Cancer Cells via Activating Mitochondrial Pathway by Increasing Reactive Oxygen Species. Dig. Dis Sci. 2015, 60, 2985–2995. [Google Scholar] [CrossRef]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-kappaB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharm. 2017, 8, 798. [Google Scholar] [CrossRef]
- Chaithongyot, S.; Jantaree, P.; Sokolova, O.; Naumann, M. NF-kappaB in Gastric Cancer Development and Therapy. Biomedicines 2021, 9, 870. [Google Scholar]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids Regulate Inflammation and Oxidative Stress in Cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Huang, L.; Ouyang, T.; Chen, L. Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-kappaB signaling pathway. Int. Immunopharmacol. 2018, 55, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-kappaB-TNF-alpha and TGF-beta1-MAPK-fibronectin pathways. Am. J. Physiol. Ren. Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: Effect on low grade inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lian, S.; Khoi, P.N.; Yoon, H.J.; Han, J.Y.; Chay, K.O.; Jung, Y.D. Chrysin inhibits cell invasion by inhibition of Recepteur d’origine Nantais via suppressing early growth response-1 and NF-kappaB transcription factor activities in gastric cancer cells. Int. J. Oncol. 2015, 46, 1835–1843. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L. Morin inhibits colorectal tumor growth through inhibition of NF-kappaB signaling pathway. Immunopharmacol. Immunotoxicol. 2019, 41, 622–629. [Google Scholar] [CrossRef]
- Li, Y.S.; Wu, L.P.; Li, K.H.; Liu, Y.P.; Xiang, R.; Zhang, S.B.; Zhang, L.Y. Involvement of nuclear factor kappaB (NF-kappaB) in the downregulation of cyclooxygenase-2 (COX-2) by genistein in gastric cancer cells. J. Int. Med. Res. 2011, 39, 2141–2150. [Google Scholar] [CrossRef]
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-kappab, PKC-delta, ERK1/2, and AMPKalpha. Integr. Cancer 2018, 17, 511–523. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Gamblin, S.J. AMP-activated protein kinase: Also regulated by ADP? Trends Biochem. Sci. 2011, 36, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, P.; Zou, M.H. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin. Sci. 2012, 122, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharm. 2017, 8, 336. [Google Scholar] [CrossRef]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef]
- Varshney, R.; Gupta, S.; Roy, P. Cytoprotective effect of Kaempferol against palmitic acid-induced pancreatic beta-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol. Cell Endocrinol. 2017, 448, 1–20. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Zallot, R.; Oberg, N.; Gerlt, J.A. Discovery of new enzymatic functions and metabolic pathways using genomic enzymology web tools. Curr. Opin. Biotechnol. 2021, 69, 77–90. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Hexokinase-2 Glycolytic Overload in Diabetes and Ischemia-Reperfusion Injury. Trends Endocrinol. Metab. 2019, 30, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Pari, L.; Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharm. 2010, 64, 477–481. [Google Scholar] [CrossRef]
- Patel, S.S.; Shah, R.S.; Goyal, R.K. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J. Exp. Biol. 2009, 47, 564–570. [Google Scholar]
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Ramkumar, K.M. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and beta-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharm. 2014, 37, 326–335. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules 2021, 26, 5333. [Google Scholar] [CrossRef] [PubMed]
- JZ, A.L.; BinMowyna, M.N.; AlFaris, N.A.; Alagal, R.I.; El-Kott, A.F.; Al-Farga, A.M. Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R. Saudi Pharm. J. 2021, 29, 27–42. [Google Scholar]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Wu, X.D.; Davey, A.K.; Wang, J. Antihyperglycemic effects of baicalin on streptozotocin-nicotinamide induced diabetic rats. Phytother. Res. 2011, 25, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.C.; Zhu, W.; Lei, L.; Liu, X.Q.; Wu, Y.G. Wogonin Ameliorates Renal Inflammation and Fibrosis by Inhibiting NF-kappaB and TGF-beta1/Smad3 Signaling Pathways in Diabetic Nephropathy. Drug Des. Devel. 2020, 14, 4135–4148. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-kappaB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Med. 2020, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Antihyperglycemic effect of equol, a daidzein derivative, in cultured L6 myocytes and ob/ob mice. Mol. Nutr. Food Res. 2014, 58, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Bhuvaneswari, V.; Nagini, S. Antiperoxidative effects of lycopene during N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis. Fitoterapia 2002, 73, 604–611. [Google Scholar] [CrossRef]
- Hwang, J.T.; Ha, J.; Park, O.J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun 2005, 332, 433–440. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, X.; Chinnathambi, A.; Alharbi, S.; Wang, Q. Fisetin suppresses 1,2-dimethylhydrazine-induced colon tumorigenesis in Wistar rats. J. King Saud Univ. Sci. 2020, 32, 1959–1964. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Hanchang, W.; Khamchan, A.; Wongmanee, N.; Seedadee, C. Hesperidin ameliorates pancreatic beta-cell dysfunction and apoptosis in streptozotocin-induced diabetic rat model. Life Sci. 2019, 235, 116858. [Google Scholar] [CrossRef] [PubMed]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharm. 2011, 670, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharm. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The Potential Role of Phytonutrients Flavonoids Influencing Gut Microbiota in the Prophylaxis and Treatment of Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 798038. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Stirling, K.; Yang, J.J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front. Immunol. 2021, 12, 807648. [Google Scholar] [CrossRef]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Gu, X.; Zhang, J.; Nomoto, D.; Qiu, P. Gut microbiome in gastrointestinal cancer: A friend or foe? Int. J. Biol Sci. 2022, 18, 4101–4117. [Google Scholar] [CrossRef]
- Tremmel, M.; Paetz, C.; Heilmann, J. In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules 2021, 26, 6632. [Google Scholar] [CrossRef]
- Wang, L.; Huang, G.; Hou, R.; Qi, D.; Wu, Q.; Nie, Y.; Wei, F. Multi-omics reveals the positive leverage of plant secondary metabolites on the gut microbiota in a non-model mammal. Microbiome 2021, 9, 192. [Google Scholar] [CrossRef]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Boker, K.L.; Van der Schouw, Y.T.; De Kleijn, M.J.; Jacques, P.F.; Grobbee, D.E.; Peeters, P.H. Intake of Dietary Phytoestrogens by Dutch Women. J. Nutr. 2002, 132, 1319. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C.; Feskens, E.J.; Bueno de Mesquita, H.B.; Kromhout, D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 2001, 55, 76–81. [Google Scholar] [CrossRef]
- Rodriguez De Luna, S.L.; Ramirez-Garza, R.E.; Serna Saldivar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 6792069. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Loosen, S.H.; Hoyer, L.; Luedde, T.; Kostev, K. Prevalence of diabetes mellitus among 80,193 gastrointestinal cancer patients in five European and three Asian countries. J. Cancer Res. Clin. Oncol. 2022, 148, 1057–1062. [Google Scholar] [CrossRef]
- Tseng, C.H. The Relationship between Diabetes Mellitus and Gastric Cancer and the Potential Benefits of Metformin: An Extensive Review of the Literature. Biomolecules 2021, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Tseng, F.H. Diabetes and gastric cancer: The potential links. World J. Gastroenterol. 2014, 20, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Mersakova, S.; Strnadel, J.; Kajo, K.; Kubatka, P. Flavonoids Targeting HIF-1: Implications on Cancer Metabolism. Cancers 2021, 13, 559–587. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Golubnitschaja, O. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, S.; Zhang, S.; Li, Y.Y.; Wang, H.; Chen, Y.; Lu, H. Vitexin protects against high glucose-induced endothelial cell apoptosis and oxidative stress via Wnt/beta-catenin and Nrf2 signalling pathway. Arch. Physiol. Biochem. 2022, 1–10. [Google Scholar] [CrossRef]
- Ling, J.Y.; Wang, Q.L.; Liang, H.N.; Liu, Q.B.; Yin, D.H.; Lin, L. Flavonoid-Rich Extract of Oldenlandia diffusa (Willd.) Roxb. Inhibits Gastric Cancer by Activation of Caspase-Dependent Mitochondrial Apoptosis. Chin. J. Integr. Med. 2022, 1–11. [Google Scholar] [CrossRef]
