
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chunxia Li | -- | 3132 | 2022-12-17 10:48:14 | | | |
| 2 | Jessie Wu | + 15 word(s) | 3147 | 2022-12-19 04:35:13 | | | | |
| 3 | Jessie Wu | + 4 word(s) | 3151 | 2022-12-19 04:38:18 | | | | |
| 4 | Jessie Wu | + 3 word(s) | 3154 | 2022-12-19 04:40:14 | | |
Video Upload Options
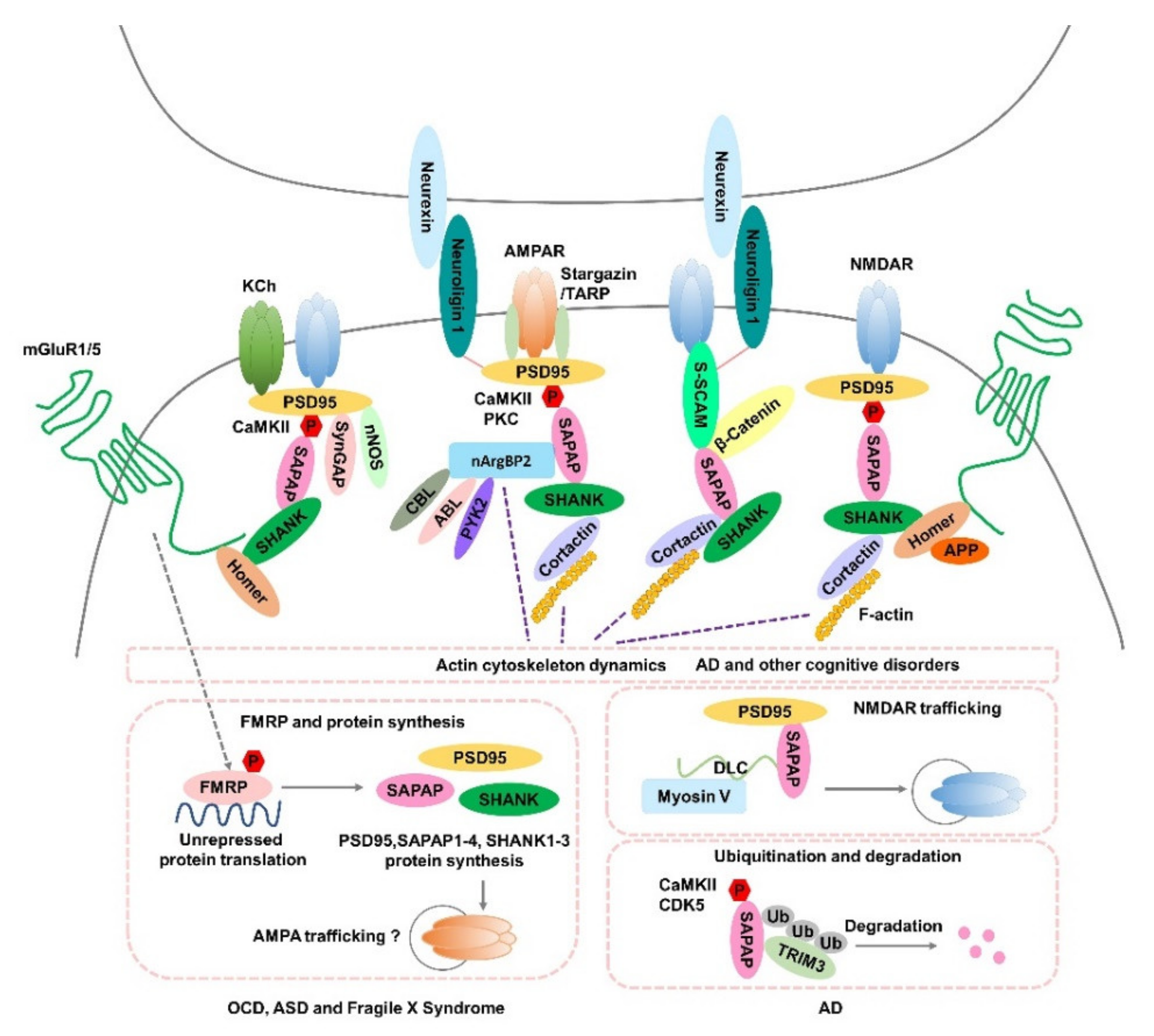
The SAPAP (SAP90/PSD-95-associated protein, also called discs-large-associated proteins (DLGAPs)) family is made up of key postsynaptic scaffold proteins that are highly concentrated in the PSD of excitatory synapses and are essential for synaptic structure and functions. Excitatory (glutamatergic) synaptic transmission underlies many aspects of brain activity and the genesis of normal human behavior. The postsynaptic scaffolding proteins SAP90/PSD-95-associated proteins (SAPAPs), which are abundant components of the postsynaptic density (PSD) at excitatory synapses, play critical roles in synaptic structure, formation, development, plasticity, and signaling. The convergence of human genetic data with recent in vitro and in vivo animal model data indicates that mutations in the genes encoding SAPAP1–4 are associated with neurological and psychiatric disorders, and that dysfunction of SAPAP scaffolding proteins may contribute to the pathogenesis of various neuropsychiatric disorders, such as schizophrenia, autism spectrum disorders, obsessive compulsive disorders, Alzheimer’s disease, and bipolar disorder.
1. Expression of SAP90/PSD-95-Associated Proteins in the Central Nervous System
2. SAP90/PSD-95-Associated Proteins’ Expression, Fragile X Mental Retardation Protein, and Neuropsychiatric Disorders
| Gene | Regulation | Expression | Brain Regions | Animal Models/Patients | References |
|---|---|---|---|---|---|
| SAPAP1 | Upregulation | Protein | Cortex or hippocampus | Fmr1-KO mice | [27] |
| Upregulation | mRNA or protein | Rats: NAc and hippocampus; patients: NAc | Schizophrenia rat model and patients | [25] | |
| SAPAP2 | Upregulation | Protein | Cortex or hippocampus | Fmr1-KO mice | [27] |
| SAPAP3 | Upregulation | Protein | Cortex or hippocampus | Fmr1-KO mice | [27] |
| Upregulation | mRNAor Protein | NAc | Syrian hamsters after aggressive experience | [29] | |
| Downregulation | Protein | OFC and mPFC | Fmr1-KO mice | [28] | |
| Upregulation | Protein | Mice: cortex and hippocampus; patients: cortex | Epilepsy patients and murine models | [26] | |
| SAPAP4 | Upregulation in the hippocampus |
mRNA | Hippocampus | Fmr1-KO mice | [33] |
| Downregulation | mRNA | Amygdala | Taste-aversion-resistant rats after cocaine exposure | [38] |
| Gene | Regulation | Expression | Brain Regions/Cells |
Animal Models/Patients |
References |
|---|---|---|---|---|---|
| SAPAP2 | Upregulation (with lower DNA methylation) | mRNA | Hippocampus | PTSD rat model | [22] |
| Upregulation (with lower DNA methylation) | mRNA | dlPFC or NAc |
Patients with alcohol dependence | [19] | |
| Downregulation (related to DNA methylation) | mRNA protein |
dlPFC | AD patients | [20] | |
| SAPAP3 | Downregulation (by absence of histone deacetylases) | mRNA | Frontal cortex and striatum | Mice deficient in histone deacetylases 1 and 2 | [41] |
| SAPAP4 | Disruption of SAPAP4 by the chromosome translocation results in monoallelic hypermethylation of the truncated SAPAP4 promoter CpG island | N/A | Cerebellum | Early-onset cerebellar ataxia patients | [21] |
| Identified as a target of the BD-associated microRNA miR-1908-5p | N/A | Neural progenitor cells | BD patients | [40] |
3. Human Molecular Genetic Analysis of Neuropsychiatric Disorders Linked to SAP90/PSD-95-Associated Protein
3.1. SAPAP1 and Neuropsychiatric Disorders
3.2. SAPAP2 and Neuropsychiatric Disorders
| Gene | Schizophrenia | ASD | OCD and Related Disorders | AD and Other Cognitive Disorders | ADHD and MDD |
|---|---|---|---|---|---|
| SAPAP1 | Rare missense mutation c.1922A > G (a benign variant) [50] | Associated with the IGAP (International Genomics of Alzheimer’s Project) SNP rs8093731 in Desmoglein-2/DSG2; underexpressed in the entorhinal cortex, hippocampus, and frontal and temporal cortex of AD cases [48] | SNPs rs1116345 and rs34248 [66] | c.1397A > G, p. Asp466Gly, exon 8 in subcortical heterotopias [3] | SNPs rs2049161and rs16946051 associated with cognitive flexibility in ADHD [47] SNP rs12455524 in recurrent MDD [52] |
| De novo CNV deletion [44] | Identified as an ASD-associated gene in a genome-wide network analysis [51] | Two SNPs located within an intron of SAPAP1 [45] | N/A | N/A | |
| N/A | N/A | Rare CNVs (62 kb duplication) [46] | N/A | N/A | |
| SAPAP2 | Damaging missense [69] | CNV 8:1626547:G:C [58] | SNPs rs6558484 and rs7014992 associated with OFC white matter volume [66] | 8p23.3 deletion in DD/mental retardation [67] | N/A |
| CNV duplication [68] | Rare de novo CNV duplications(704383-1521910) [57] | Rare CNVs (16 kb deletion) [46] | SNP rs34130287C within the first intron [71] | N/A | |
| SNV c.-69+9C.T, c.-69+13C.T, c.-69+47C.T, c.-69+55C.T [56] | SNP rs2906569 at intron 1, rs2301963 (P384Q) at exon 3 and several nonsynonymous variants [59] | N/A | SNP rs6992443 [72] | N/A | |
| N/A | Rare de novo CMV duplication 8:704383-1521910 [60] | N/A | SNP rs2957061 SNP chr8:1316870; minor allele frequency (a locus within SAPAP2) [20] | N/A | |
| Gene | Schizophrenia | ASD | OCD and Related Disorders | AD and Other Cognitive Disorders | |
| SAPAP2 | CNV deletion, 8p 23.2 and 8p 23.1 [61] | CNV deletion 8p 23.3 [73] | N/A | N/A | |
| N/A | CNV deletion 8p 23.2 and 8p 23.1 [61] | N/A | N/A | ||
| CNV deletion, 8p23.3-p23.1 [70] | CNV duplication 8p23.3 (patients with de novo rearrangements) [62] | N/A | N/A | ||
| N/A | CNV duplication,8p23.3 (1, 499, 963–1, 854, 917) [63] | N/A | N/A | ||
| SAPAP3 | SNVs c.1141G > A; c.1759G > C; c.2309G > T; c.2578-11C > T [56] |
CNV 1:35365700:G:A [58] | Increased frequency of rare nonsynonymous coding variants (in OCD and TTM) [74] | N/A | |
| N/A | N/A | SNPs rs6662980–rs4652867 in grooming disorder [75] | N/A | ||
| N/A | N/A | SNP rs11264126 and two haplotypes containing rs11264126 and rs12141243 in TS [76] |
N/A | ||
| N/A | N/A | SNPs rs11583978 and rs6682829 in early-onset OCD [77] | N/A | ||
| SAPAP4 | N/A | SNP located at the 20q11.21–q13.12 locus comprising the Sapap4 gene [78] | N/A | De novo frameshift SNVs (c.2714_2715insCAGCTGG) insertion, N905Qfs, exon 12; c.2893T > G, p. Ser965Ala, exon 13 in subcortical heterotopias [3] | |
3.3. SAPAP3 and Neuropsychiatric Disorders
3.4. SAPAP4 and Neuropsychiatric Disorders
References
- Kindler, S.; Rehbein, M.; Classen, B.; Richter, D.; Bockers, T.M. Distinct spatiotemporal expression of SAPAP transcripts in the developing rat brain: A novel dendritically localized mRNA. Brain Res. Mol. Brain Res. 2004, 126, 14–21.
- Chua, J.J.; Schob, C.; Rehbein, M.; Gkogkas, C.G.; Richter, D.; Kindler, S. Synthesis of two SAPAP3 isoforms from a single mRNA is mediated via alternative translational initiation. Sci. Rep. 2012, 2, 484.
- Romero, D.M.; Poirier, K.; Belvindrah, R.; Moutkine, I.; Houllier, A.; LeMoing, A.G.; Petit, F.; Boland, A.; Collins, S.C.; Soiza-Reilly, M.; et al. Novel role of the synaptic scaffold protein Dlgap4 in ventricular surface integrity and neuronal migration during cortical development. Nat. Commun. 2022, 13, 2746.
- Polioudakis, D.; de la Torre-Ubieta, L.; Langerman, J.; Elkins, A.G.; Shi, X.; Stein, J.L.; Vuong, C.K.; Nichterwitz, S.; Gevorgian, M.; Opland, C.K.; et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron 2019, 103, 785–801.e788.
- Welch, J.M.; Wang, D.; Feng, G. Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse. J. Comp. Neurol. 2004, 472, 24–39.
- Welch, J.M.; Lu, J.; Rodriguiz, R.M.; Trotta, N.C.; Peca, J.; Ding, J.D.; Feliciano, C.; Chen, M.; Adams, J.P.; Luo, J.; et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 2007, 448, 894–900.
- Hanus, C.; Schuman, E.M. Proteostasis in complex dendrites. Nat. Rev. Neurosci. 2013, 14, 638–648.
- Cajigas, I.J.; Tushev, G.; Will, T.J.; tom Dieck, S.; Fuerst, N.; Schuman, E.M. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 2012, 74, 453–466.
- Kang, H.; Schuman, E.M. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 1996, 273, 1402–1406.
- Aakalu, G.; Smith, W.B.; Nguyen, N.; Jiang, C.; Schuman, E.M. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 2001, 30, 489–502.
- Hegde, A.N.; Goldberg, A.L.; Schwartz, J.H. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: A molecular mechanism underlying long-term synaptic plasticity. Proc. Natl. Acad. Sci. USA 1993, 90, 7436–7440.
- Steward, O.; Schuman, E.M. Compartmentalized synthesis and degradation of proteins in neurons. Neuron 2003, 40, 347–359.
- Cajigas, I.J.; Will, T.; Schuman, E.M. Protein homeostasis and synaptic plasticity. EMBO J. 2010, 29, 2746–2752.
- Mabb, A.M.; Ehlers, M.D. Ubiquitination in postsynaptic function and plasticity. Annu. Rev. Cell Dev. Biol. 2010, 26, 179–210.
- Ehlers, M.D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 2003, 6, 231–242.
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767.
- Wang, T.; Bai, Y.; Zheng, X.; Liu, X.; Xing, S.; Wang, L.; Wang, H.; Feng, G.; Li, C. Sapap4 deficiency leads to postsynaptic defects and abnormal behaviors relevant to hyperkinetic neuropsychiatric disorder in mice. Cereb Cortex 2022.
- Wan, Y.; Ade, K.K.; Caffall, Z.; Ilcim Ozlu, M.; Eroglu, C.; Feng, G.; Calakos, N. Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biol. Psychiatry 2014, 75, 623–630.
- Meng, W.; Sjoholm, L.K.; Kononenko, O.; Tay, N.; Zhang, D.; Sarkisyan, D.; Geske, J.R.; Ing, A.; Qiu, W.; Watanabe, H.; et al. Genotype-dependent epigenetic regulation of DLGAP2 in alcohol use and dependence. Mol. Psychiatry 2021, 26, 4367–4382.
- Ouellette, A.R.; Neuner, S.M.; Dumitrescu, L.; Anderson, L.C.; Gatti, D.M.; Mahoney, E.R.; Bubier, J.A.; Churchill, G.; Peters, L.; Huentelman, M.J.; et al. Cross-species analyses identify dlgap2 as a regulator of age-related cognitive decline and alzheimer’s dementia. Cell Rep. 2020, 32, 108091.
- Minocherhomji, S.; Hansen, C.; Kim, H.G.; Mang, Y.; Bak, M.; Guldberg, P.; Papadopoulos, N.; Eiberg, H.; Doh, G.D.; Mollgard, K.; et al. Epigenetic remodelling and dysregulation of DLGAP4 is linked with early-onset cerebellar ataxia. Hum. Mol. Genet. 2014, 23, 6163–6176.
- Chertkow-Deutsher, Y.; Cohen, H.; Klein, E.; Ben-Shachar, D. DNA methylation in vulnerability to post-traumatic stress in rats: Evidence for the role of the post-synaptic density protein Dlgap2. Int. J. Neuropsychopharmacol. 2010, 13, 347–359.
- Li, Y.; Wang, K.; Zhang, P.; Huang, J.; An, H.; Wang, N.; De Yang, F.; Wang, Z.; Tan, S.; Chen, S.; et al. Quantitative DNA methylation analysis of DLGAP2 gene using pyrosequencing in schizophrenia with tardive dyskinesia: A linear mixed model approach. Sci. Rep. 2018, 8, 17466.
- Schrott, R.; Acharya, K.; Itchon-Ramos, N.; Hawkey, A.B.; Pippen, E.; Mitchell, J.T.; Kollins, S.H.; Levin, E.D.; Murphy, S.K. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 2020, 15, 161–173.
- Kajimoto, Y.; Shirakawa, O.; Lin, X.H.; Hashimoto, T.; Kitamura, N.; Murakami, N.; Takumi, T.; Maeda, K. Synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP) is expressed differentially in phencyclidine-treated rats and is increased in the nucleus accumbens of patients with schizophrenia. Neuropsychopharmacology 2003, 28, 1831–1839.
- Zhang, Y.; Wu, J.; Yan, Y.; Gu, Y.; Ma, Y.; Wang, M.; Zhang, H.; Tao, K.; Lu, Y.; Yu, W.; et al. SAPAP3 regulates epileptic seizures involving GluN2A in post-synaptic densities. Cell Death Dis. 2022, 13, 437.
- Schutt, J.; Falley, K.; Richter, D.; Kreienkamp, H.J.; Kindler, S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J. Biol. Chem. 2009, 284, 25479–25487.
- Krueger, D.D.; Osterweil, E.K.; Chen, S.P.; Tye, L.D.; Bear, M.F. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 2587–2592.
- Been, L.E.; Moore, K.M.; Kennedy, B.C.; Meisel, R.L. Metabotropic glutamate receptor and fragile X signaling in a female model of escalated aggression. Biol. Psychiatry 2016, 79, 685–692.
- Brown, V.; Jin, P.; Ceman, S.; Darnell, J.C.; O’Donnell, W.T.; Tenenbaum, S.A.; Jin, X.; Feng, Y.; Wilkinson, K.D.; Keene, J.D.; et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 2001, 107, 477–487.
- Darnell, J.C.; Klann, E. The translation of translational control by FMRP: Therapeutic targets for FXS. Nat. Neurosci. 2013, 16, 1530–1536.
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214.
- Dictenberg, J.B.; Swanger, S.A.; Antar, L.N.; Singer, R.H.; Bassell, G.J. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev. Cell 2008, 14, 926–939.
- Wan, Y.; Feng, G.; Calakos, N. Sapap3 deletion causes mGluR5-dependent silencing of AMPAR synapses. J. Neurosci. 2011, 31, 16685–16691.
- Ade, K.K.; Wan, Y.; Hamann, H.C.; O’Hare, J.K.; Guo, W.; Quian, A.; Kumar, S.; Bhagat, S.; Rodriguiz, R.M.; Wetsel, W.C.; et al. Increased metabotropic glutamate receptor 5 signaling underlies obsessive-compulsive disorder-like behavioral and striatal circuit abnormalities in mice. Biol. Psychiatry 2016, 80, 522–533.
- Nakamoto, M.; Nalavadi, V.; Epstein, M.P.; Narayanan, U.; Bassell, G.J.; Warren, S.T. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 15537–15542.
- Wang, X.; Bey, A.L.; Katz, B.M.; Badea, A.; Kim, N.; David, L.K.; Duffney, L.J.; Kumar, S.; Mague, S.D.; Hulbert, S.W.; et al. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat. Commun. 2016, 7, 11459.
- Elkins, R.L.; Orr, T.E.; Rausch, J.L.; Fei, Y.J.; Carl, G.F.; Hobbs, S.H.; Buccafusco, J.J.; Edwards, G.L. Cocaine-induced expression differences in PSD-95/SAP-90-associated protein 4 and in Ca2+/calmodulin-dependent protein kinase subunits in amygdalae of taste aversion-prone and taste aversion-resistant rats. Ann. N. Y. Acad. Sci. 2003, 1003, 386–390.
- Wu, B.; Li, C.; Lei, H. SAPAP4 deletion causes synaptic dysfunction in the nucleus accumbens. Biochem. Biophys. Res. Commun. 2018, 505, 1223–1227.
- Kim, Y.; Zhang, Y.; Pang, K.; Kang, H.; Park, H.; Lee, Y.; Lee, B.; Lee, H.J.; Kim, W.K.; Geum, D.; et al. Bipolar disorder associated microRNA, miR-1908-5p, regulates the expression of genes functioning in neuronal glutamatergic synapses. Exp. Neurobiol. 2016, 25, 296–306.
- Mahgoub, M.; Adachi, M.; Suzuki, K.; Liu, X.; Kavalali, E.T.; Chahrour, M.H.; Monteggia, L.M. MeCP2 and histone deacetylases 1 and 2 in dorsal striatum collectively suppress repetitive behaviors. Nat. Neurosci. 2016, 19, 1506–1512.
- Saadatmand, F.; Gurdziel, K.; Jackson, L.; Kwabi-Addo, B.; Ruden, D.M. DNA methylation and exposure to violence among African American young adult males. Brain Behav. Immun. Health 2021, 14, 100247.
- Minocherhomji, S.; Seemann, S.; Mang, Y.; El-Schich, Z.; Bak, M.; Hansen, C.; Papadopoulos, N.; Josefsen, K.; Nielsen, H.; Gorodkin, J.; et al. Sequence and expression analysis of gaps in human chromosome 20. Nucleic Acids Res. 2012, 40, 6660–6672.
- Kirov, G.; Pocklington, A.J.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 2012, 17, 142–153.
- Stewart, S.E.; Yu, D.; Scharf, J.M.; Neale, B.M.; Fagerness, J.A.; Mathews, C.A.; Arnold, P.D.; Evans, P.D.; Gamazon, E.R.; Davis, L.K.; et al. Genome-wide association study of obsessive-compulsive disorder. Mol. Psychiatry 2013, 18, 788–798.
- Gazzellone, M.J.; Zarrei, M.; Burton, C.L.; Walker, S.; Uddin, M.; Shaheen, S.M.; Coste, J.; Rajendram, R.; Schachter, R.J.; Colasanto, M.; et al. Uncovering obsessive-compulsive disorder risk genes in a pediatric cohort by high-resolution analysis of copy number variation. J. Neurodev. Disord. 2016, 8, 36.
- Fan, Z.; Qian, Y.; Lu, Q.; Wang, Y.; Chang, S.; Yang, L. DLGAP1 and NMDA receptor-associated postsynaptic density protein genes influence executive function in attention deficit hyperactivity disorder. Brain Behav. 2018, 8, e00914.
- Katsumata, Y.; Nelson, P.T.; Estus, S.; Alzheimer’s Disease Neuroimaging, I.; Fardo, D.W. Translating Alzheimer’s disease-associated polymorphisms into functional candidates: A survey of IGAP genes and SNPs. Neurobiol. Aging 2019, 74, 135–146.
- Aoyama, S.; Shirakawa, O.; Ono, H.; Hashimoto, T.; Kajimoto, Y.; Maeda, K. Mutation and association analysis of the DAP-1 gene with schizophrenia. Psychiatry Clin. Neurosci. 2003, 57, 545–547.
- Li, J.M.; Lu, C.L.; Cheng, M.C.; Luu, S.U.; Hsu, S.H.; Chen, C.H. Genetic analysis of the DLGAP1 gene as a candidate gene for schizophrenia. Psychiatry Res. 2013, 205, 13–17.
- Li, J.; Shi, M.; Ma, Z.; Zhao, S.; Euskirchen, G.; Ziskin, J.; Urban, A.; Hallmayer, J.; Snyder, M. Integrated systems analysis reveals a molecular network underlying autism spectrum disorders. Mol. Syst. Biol. 2014, 10, 774.
- Mathias, S.R.; Knowles, E.E.; Kent, J.W., Jr.; McKay, D.R.; Curran, J.E.; de Almeida, M.A.; Dyer, T.D.; Goring, H.H.; Olvera, R.L.; Duggirala, R.; et al. Recurrent major depression and right hippocampal volume: A bivariate linkage and association study. Hum. Brain Mapp. 2016, 37, 191–202.
- Mukherjee, O.; Meera, P.; Ghosh, S.; Kubendran, S.; Kiran, K.; Manjunath, K.R.; Subhash, M.N.; Benegal, V.; Brahmachari, S.K.; Majumder, P.P.; et al. Evidence of linkage and association on 18p11.2 for psychosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 868–873.
- Schwab, S.G.; Hallmayer, J.; Lerer, B.; Albus, M.; Borrmann, M.; Honig, S.; Strauss, M.; Segman, R.; Lichtermann, D.; Knapp, M.; et al. Support for a chromosome 18p locus conferring susceptibility to functional psychoses in families with schizophrenia, by association and linkage analysis. Am. J. Hum. Genet. 1998, 63, 1139–1152.
- Soler, J.; Fananas, L.; Parellada, M.; Krebs, M.O.; Rouleau, G.A.; Fatjo-Vilas, M. Genetic variability in scaffolding proteins and risk for schizophrenia and autism-spectrum disorders: A systematic review. J. Psychiatry Neurosci. 2018, 43, 223–244.
- Li, J.M.; Lu, C.L.; Cheng, M.C.; Luu, S.U.; Hsu, S.H.; Hu, T.M.; Tsai, H.Y.; Chen, C.H. Role of the DLGAP2 gene encoding the SAP90/PSD-95-associated protein 2 in schizophrenia. PLoS ONE 2014, 9, e85373.
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372.
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221.
- Chien, W.H.; Gau, S.S.; Liao, H.M.; Chiu, Y.N.; Wu, Y.Y.; Huang, Y.S.; Tsai, W.C.; Tsai, H.M.; Chen, C.H. Deep exon resequencing of DLGAP2 as a candidate gene of autism spectrum disorders. Mol. Autism 2013, 4, 26.
- Pinto, D.; Delaby, E.; Merico, D.; Barbosa, M.; Merikangas, A.; Klei, L.; Thiruvahindrapuram, B.; Xu, X.; Ziman, R.; Wang, Z.; et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 2014, 94, 677–694.
- Autism Genome Project, C.; Szatmari, P.; Paterson, A.D.; Zwaigenbaum, L.; Roberts, W.; Brian, J.; Liu, X.Q.; Vincent, J.B.; Skaug, J.L.; Thompson, A.P.; et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007, 39, 319–328.
- Marshall, C.R.; Noor, A.; Vincent, J.B.; Lionel, A.C.; Feuk, L.; Skaug, J.; Shago, M.; Moessner, R.; Pinto, D.; Ren, Y.; et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008, 82, 477–488.
- Woodbury-Smith, M.; Zarrei, M.; Wei, J.; Thiruvahindrapuram, B.; O’Connor, I.; Paterson, A.D.; Yuen, R.K.C.; Dastan, J.; Stavropoulos, D.J.; Howe, J.L.; et al. Segregating patterns of copy number variations in extended autism spectrum disorder (ASD) pedigrees. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 268–276.
- Catusi, I.; Garzo, M.; Capra, A.P.; Briuglia, S.; Baldo, C.; Canevini, M.P.; Cantone, R.; Elia, F.; Forzano, F.; Galesi, O.; et al. 8p23.2-pter microdeletions: Seven new cases narrowing the candidate region and review of the literature. Genes 2021, 12, 652.
- Shi, S.; Lin, S.; Chen, B.; Zhou, Y. Isolated chromosome 8p23.2pter deletion: Novel evidence for developmental delay, intellectual disability, microcephaly and neurobehavioral disorders. Mol. Med. Rep. 2017, 16, 6837–6845.
- Wu, K.; Hanna, G.L.; Easter, P.; Kennedy, J.L.; Rosenberg, D.R.; Arnold, P.D. Glutamate system genes and brain volume alterations in pediatric obsessive-compulsive disorder: A preliminary study. Psychiatry Res. 2013, 211, 214–220.
- Wu, Y.; Ji, T.; Wang, J.; Xiao, J.; Wang, H.; Li, J.; Gao, Z.; Yang, Y.; Cai, B.; Wang, L.; et al. Submicroscopic subtelomeric aberrations in Chinese patients with unexplained developmental delay/mental retardation. BMC Med. Genet. 2010, 11, 72.
- Guilmatre, A.; Dubourg, C.; Mosca, A.L.; Legallic, S.; Goldenberg, A.; Drouin-Garraud, V.; Layet, V.; Rosier, A.; Briault, S.; Bonnet-Brilhault, F.; et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch. Gen. Psychiatry 2009, 66, 947–956.
- Purcell, S.M.; Moran, J.L.; Fromer, M.; Ruderfer, D.; Solovieff, N.; Roussos, P.; O’Dushlaine, C.; Chambert, K.; Bergen, S.E.; Kahler, A.; et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 2014, 506, 185–190.
- Costain, G.; Lionel, A.C.; Merico, D.; Forsythe, P.; Russell, K.; Lowther, C.; Yuen, T.; Husted, J.; Stavropoulos, D.J.; Speevak, M.; et al. Pathogenic rare copy number variants in community-based schizophrenia suggest a potential role for clinical microarrays. Hum. Mol. Genet. 2013, 22, 4485–4501.
- White, C.C.; Yang, H.S.; Yu, L.; Chibnik, L.B.; Dawe, R.J.; Yang, J.; Klein, H.U.; Felsky, D.; Ramos-Miguel, A.; Arfanakis, K.; et al. Identification of genes associated with dissociation of cognitive performance and neuropathological burden: Multistep analysis of genetic, epigenetic, and transcriptional data. PLoS Med. 2017, 14, e1002287.
- Chaudhry, M.; Wang, X.; Bamne, M.N.; Hasnain, S.; Demirci, F.Y.; Lopez, O.L.; Kamboh, M.I. Genetic variation in imprinted genes is associated with risk of late-onset Alzheimer’s disease. J. Alzheimers Dis. 2015, 44, 989–994.
- Chien, W.H.; Gau, S.S.; Wu, Y.Y.; Huang, Y.S.; Fang, J.S.; Chen, Y.J.; Soong, W.T.; Chiu, Y.N.; Chen, C.H. Identification and molecular characterization of two novel chromosomal deletions associated with autism. Clin. Genet. 2010, 78, 449–456.
- Zuchner, S.; Wendland, J.R.; Ashley-Koch, A.E.; Collins, A.L.; Tran-Viet, K.N.; Quinn, K.; Timpano, K.C.; Cuccaro, M.L.; Pericak-Vance, M.A.; Steffens, D.C.; et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol. Psychiatry 2009, 14, 6–9.
- Bienvenu, O.J.; Wang, Y.; Shugart, Y.Y.; Welch, J.M.; Grados, M.A.; Fyer, A.J.; Rauch, S.L.; McCracken, J.T.; Rasmussen, S.A.; Murphy, D.L.; et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 710–720.
- Crane, J.; Fagerness, J.; Osiecki, L.; Gunnell, B.; Stewart, S.E.; Pauls, D.L.; Scharf, J.M.; Tourette Syndrome International Consortium for, G. Family-based genetic association study of DLGAP3 in Tourette Syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 108–114.
- Boardman, L.; van der Merwe, L.; Lochner, C.; Kinnear, C.J.; Seedat, S.; Stein, D.J.; Moolman-Smook, J.C.; Hemmings, S.M. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Compr. Psychiatry 2011, 52, 181–187.
- Allen-Brady, K.; Miller, J.; Matsunami, N.; Stevens, J.; Block, H.; Farley, M.; Krasny, L.; Pingree, C.; Lainhart, J.; Leppert, M.; et al. A high-density SNP genome-wide linkage scan in a large autism extended pedigree. Mol. Psychiatry 2009, 14, 590–600.
- Jiang-Xie, L.F.; Liao, H.M.; Chen, C.H.; Chen, Y.T.; Ho, S.Y.; Lu, D.H.; Lee, L.J.; Liou, H.H.; Fu, W.M.; Gau, S.S. Autism-associated gene Dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits. Mol. Autism 2014, 5, 32.
- Roselli, F.; Livrea, P.; Almeida, O.F. CDK5 is essential for soluble amyloid beta-induced degradation of GKAP and remodeling of the synaptic actin cytoskeleton. PLoS ONE 2011, 6, e23097.
- de Bartolomeis, A.; Barone, A.; Buonaguro, E.F.; Tomasetti, C.; Vellucci, L.; Iasevoli, F. The Homer1 family of proteins at the crossroad of dopamine-glutamate signaling: An emerging molecular “Lego” in the pathophysiology of psychiatric disorders. A systematic review and translational insight. Neurosci. Biobehav. Rev. 2022, 136, 104596.
- Monteiro, P.; Feng, G. Learning from animal models of obsessive-compulsive disorder. Biol. Psychiatry 2016, 79, 7–16.
- Pauls, D.L.; Abramovitch, A.; Rauch, S.L.; Geller, D.A. Obsessive-compulsive disorder: An integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014, 15, 410–424.
- Han, K.; Holder, J.L., Jr.; Schaaf, C.P.; Lu, H.; Chen, H.; Kang, H.; Tang, J.; Wu, Z.; Hao, S.; Cheung, S.W.; et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 2013, 503, 72–77.




