Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicole C. Riedel | -- | 2802 | 2022-12-16 10:21:11 | | | |
| 2 | Camila Xu | Meta information modification | 2802 | 2022-12-19 02:57:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Riedel, N.C.; Faria, F.W.D.; Alfert, A.; Bruder, J.M.; Kerl, K. Organoids in Pediatric Brain Tumor Precision Medicine. Encyclopedia. Available online: https://encyclopedia.pub/entry/38862 (accessed on 08 March 2026).
Riedel NC, Faria FWD, Alfert A, Bruder JM, Kerl K. Organoids in Pediatric Brain Tumor Precision Medicine. Encyclopedia. Available at: https://encyclopedia.pub/entry/38862. Accessed March 08, 2026.
Riedel, Nicole C., Flavia W. De Faria, Amelie Alfert, Jan M. Bruder, Kornelius Kerl. "Organoids in Pediatric Brain Tumor Precision Medicine" Encyclopedia, https://encyclopedia.pub/entry/38862 (accessed March 08, 2026).
Riedel, N.C., Faria, F.W.D., Alfert, A., Bruder, J.M., & Kerl, K. (2022, December 16). Organoids in Pediatric Brain Tumor Precision Medicine. In Encyclopedia. https://encyclopedia.pub/entry/38862
Riedel, Nicole C., et al. "Organoids in Pediatric Brain Tumor Precision Medicine." Encyclopedia. Web. 16 December, 2022.
Copy Citation
Malignant brain neoplasms are a heterogeneous group of tumors, including glioma, ependymoma, embryonal tumors, and many other (rare) entities and subentities, affecting patients from birth to adulthood. Organoids emerged as three-dimensional (3D) cell culture systems for modeling healthy and diseased tissues. These organoids potentially model development, diseases, and drug responses [13]. They are self-organizing three-dimensional structures that closely mimic an organ or tissue at a morphological, cellular, and functional level.
organoids
precision medicine
pediatric
Medulloblastoma
pediatric glioma
ETMR
ATRT
pediatric brain tumors
1. Introduction
Malignant brain neoplasms are a heterogeneous group of tumors, including glioma, ependymoma, embryonal tumors, and many other (rare) entities and subentities, affecting patients from birth to adulthood. Despite intensive treatment protocols, including surgery, chemotherapy, and radiotherapy, the prognosis for many high-grade brain tumor patients remains poor [1][2][3][4][5][6]. Although extensive research in this field has resulted in a detailed molecular classification of brain tumors and led to various new insights into their biology, numerous recent clinical trials have failed to significantly improve the prognosis for these patients, especially those suffering a relapse [7]. Overall, there is a noticeable gap between recent preclinical achievements and the clinical improvements in patient outcomes. This dichotomy may stem from current preclinical studies frequently being conducted in two-dimensional (2D) cell culture, which neither sufficiently recapitulates inter- nor intratumoral heterogeneity nor the cellular diversity of the tumor microenvironment (TME) [8]. Importantly, heterogeneous in vivo-like tumor cell populations respond differently to drug treatment than 2D in vitro entities, and intratumoral diversity results in a higher risk of treatment resistance and tumor recurrence [9][10]. Furthermore, the interplay of tumor cells with their TME, including endothelial cells, immune cells, and neuronal cells, affects the treatment response [11][12]. Therefore, it has become paramount to establish models which include these key characteristics and allow tumor–TME interactions, thus potentially generating more accurate predictions of tumor biology, drug efficacy, and immune response.
In the past few years, organoids emerged as three-dimensional (3D) cell culture systems for modeling healthy and diseased tissues. These organoids potentially model development, diseases, and drug responses [13]. They are self-organizing three-dimensional structures that closely mimic an organ or tissue at a morphological, cellular, and functional level. They can be divided into two major groups: organoids that mirror healthy tissue, including brain organoids, and those that simulate diseased tissue, including tumor organoids [13]. Initial organoid models utilized the intrinsic self-patterning abilities of human pluripotent stem cells in appropriate conditions to generate small aggregates with optic cups [14] or even tissues representing a wide gamut of brain regions, forming the so-called cerebral organoids [15]. Later on, other groups investigated organoids resembling specific brain regions, including the forebrain, the midbrain, the hypothalamus, or the cerebellum [16][17][18]. Pioneering the use of organoids for tumor research, Sato et al. generated 3D in vitro models from primary colon carcinoma samples [19]. They were followed by other groups who developed tumor organoids from various entities, including prostatic, pancreatic, and liver cancers, as well as glioblastoma [20][21][22][23].
These tumor organoids can then be used for high-throughput drug and toxicity screenings to uncover new personalized therapeutics [13][24][25][26]. Additionally, healthy tissue 3D cell culture systems, such as organoids or organ-on-a-chip models, can be further used to test the side-effects of drugs. These technologies may identify drugs with a high efficacy against the tumor and a low burden of side effects on healthy tissues [24][27][28]. Recapitulating parental tumors, cancer organoids have proven to be capable of predicting cancer treatment efficiency in vivo [23][29][30], thus ringing in the era of organoid-based in vitro anti-cancer drug tests.
2. Organoids Are Superior to Prior 2D In Vitro Models in Recapitulating the Primary Tumor Characteristics
Despite making progress on exploring the mechanisms leading to tumor initiation by identifying, for example, (I) the mutational burden of tumors, (II) malignancies’ cells of origin, and (III) the impact of the TME on tumor cells [31][32][33][34], many key scientific questions to finally improve brain tumor patients’ survival remain unanswered. This lack of fundamental insight may partly stem from in vitro models insufficiently recapitulating the core characteristics of the primary tumors.
For decades, brain tumor research has been based on 2D and 3D cell cultures in mono-layers and spheroids, respectively. Traditional 2D in vitro cultures of tumor models rely on cell propagation in standard petri dishes. These 2D cell culture models undergo clonal selection for fast-growing and cell-culture-compatible cell populations, thereby losing cellular diversity and often resulting in a homogeneous cell population that are no longer recapitulating the tumors’ original heterogeneity [8][35][36]. Due to their mono-layer arrangement, these cell cultures are adapted to conditions of 20% oxygen, which exceeds the usual oxygen level of about 5% in in vivo tumors [8][35]. Spheroids consist of mostly uniform aggregates of a mixture of desired and relevant cell types for a given disease model assembled in an essentially random three-dimensional arrangement. On the other hand, organoids self-arrange their cell types into micro-moieties that more closely approximate organ tissue structure and function. With the advent of these self-organizing organoid tissues, a plethora of more complex three-dimensional model systems have addressed brain tumors in recent years, including patient-derived tumor organoids (PDO), patient-derived explants (PDEs), tumor-brain organoids (TBOs), neoplastic cerebral organoids (neoCORs), and, lastly, bioprinted tumor models (Figure 1). These organoid models can be divided into two major groups: (I) tumoroids starting only from a tumor tissue, including PDOs and PDEs, and (II) organoids composed of a tumor and a non-tumor compartment, including TBOs and neoCORs.
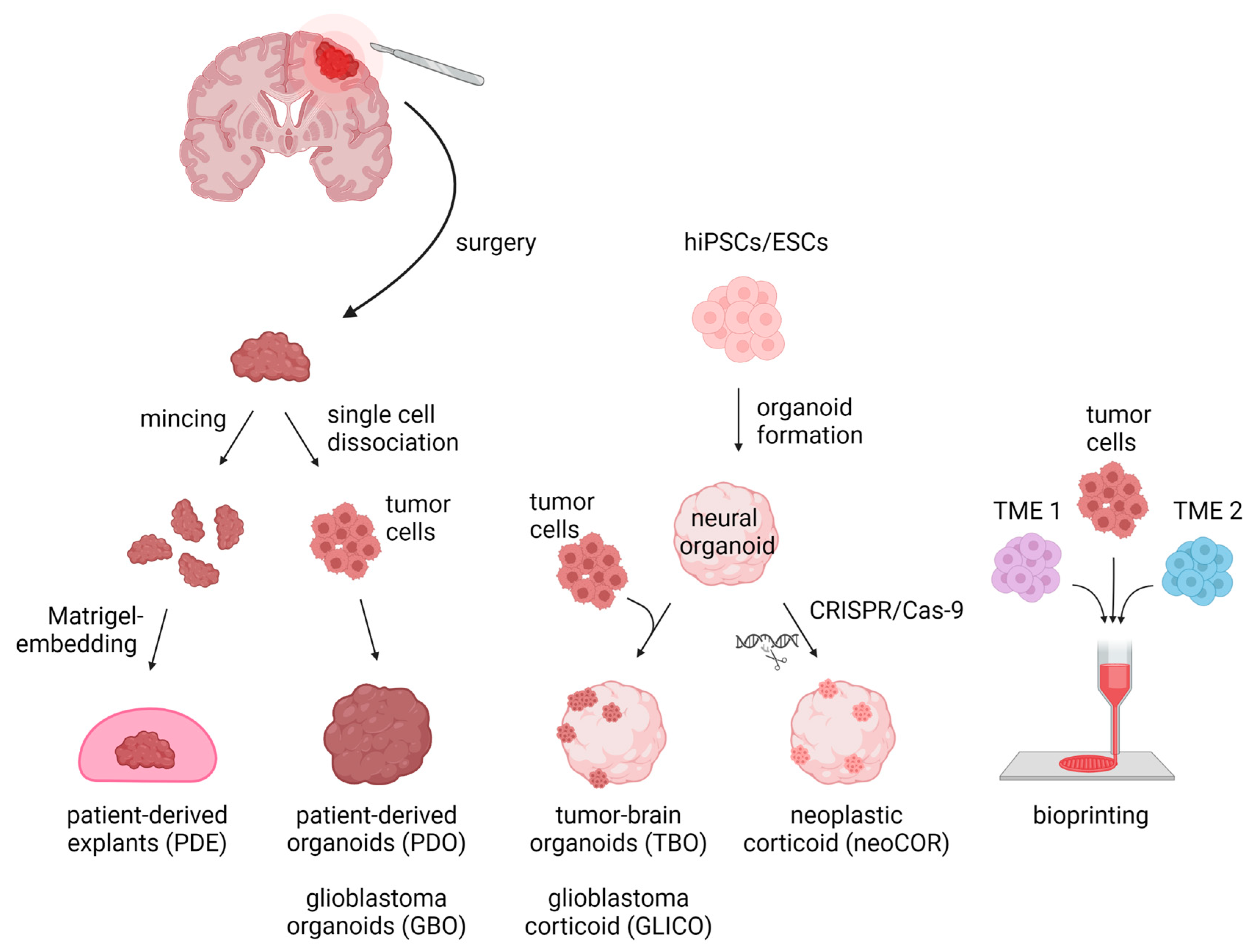
Figure 1. Three-dimensional in vitro tumor models can be derived from either primary patient materials or generated de novo from pluripotent stem cells. PDEs and PDOs are derived from patients’ resected tumor tissue, which is minced and Matrigel-embedded for PDE generation or is single-cell dissociated followed by re-aggregation for PDOs/GBOs. TBOs/GLICOs and neoCORs are generated by seeding hiPSCs/ESCs for organoid generation, followed by co-culture with tumor cells for TBOs/GLICOs or CRISPR-Cas9-based gene editing for neoCORs. To create 3D models via bioprinting, tumor cells are loaded together with TME cells into a bioink and spatially printed. Abbreviations: PDE: patient-derived explant; PDO: patient-derived tumor organoid; GBO; glioblastoma organoid; TBO: tumor-brain organoid; GLICO: glioblastoma corticoid; neoCOR: neoplastic corticoid; 3D: three-dimensional; hiPSC: human induced pluripotent stem cell; ESC: embryonic stem cell; TME1/2: tumor microenvironment cell type 1 or 2. Created with BioRender.com (accessed on 7 November 2022).
In contrast to traditional mono-layer and spheroid cultures, organoids across all models can preserve intra- and intertumoral heterogeneity [23][29][37] and establish diffusion-limited oxygen gradients within the organoids similar to that in early primary tumors, which may aid in maintaining a diverse tumor cell pool [23][38].
This might be a significant advantage in drug screens, as intratumoral heterogeneity plays an important role in intrinsic and acquired therapy resistance [39]. Two-dimensional cell lines, spheroids, and organoids respond differently to treatment, with organoids more accurately recapitulating the biological response of the parenteral tumor [8][40][41][42]. These findings are driving the hopes for organoid-based drug screens for improved clinical relevance [23][29][40][43].
3. Current Limitations of Organoids
While 3D model systems have improved over recent years, they still face several limitations. To properly mimic a tumor in vitro, current organoid models are missing vascularization, TME cells (immune cells and neuronal TME), and protocols resulting in more reproducible and scalable organoid generation (Figure 2).
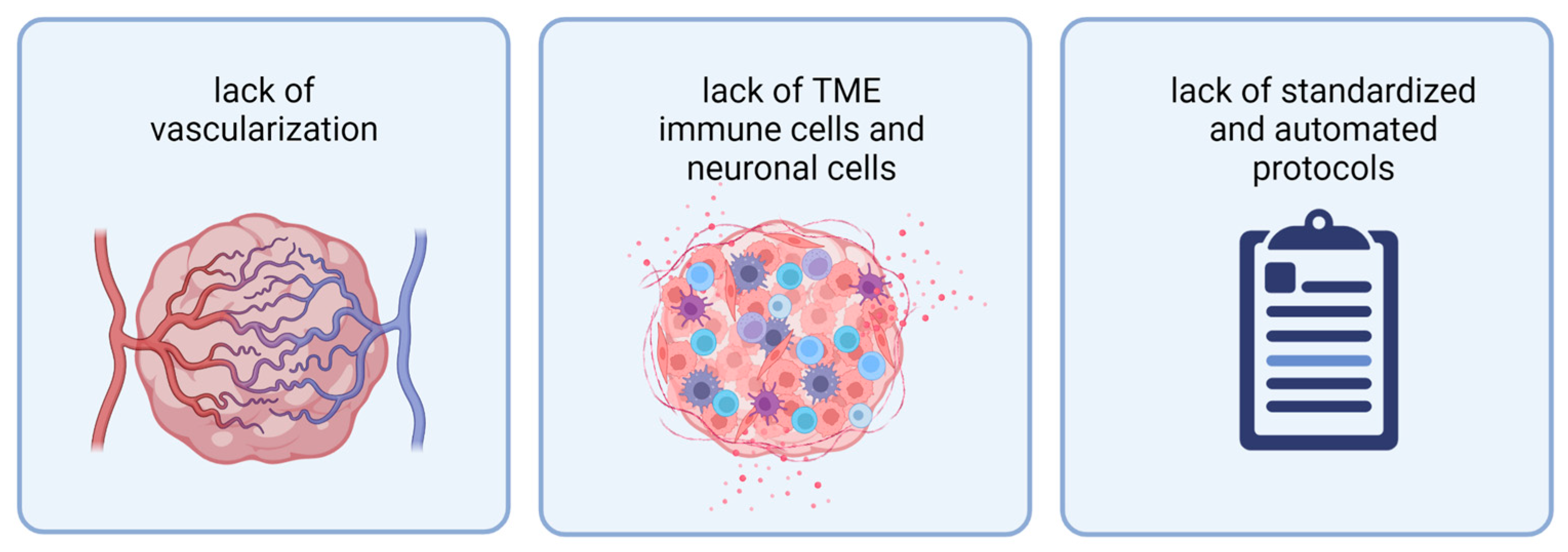
Figure 2. Current organoids face three limitations: from left to right: lack of vascularization, lack of TME immune cells and neuronal cells, and lack of standardized and automated protocols for organoid generation. Abbreviations: TME: tumor microenvironment. Created with BioRender.com (accessed on 7 November 2022).
In current brain tumor organoid models, the lack of vascularization impairs their growth, further tumor development, and long-term culture [44]. In brain tumors, the vasculature and blood–brain barrier (BBB) play an essential role in metastasis and selective drug delivery [45]. Despite recent achievements in modeling a BBB in cerebral organoids, vasculature has yet to be introduced into 3D brain tumor models, and this challenge is a very active field of research [46][47][48].
In recent years, several groups have started elucidating the essential role of the TME in brain tumor progression, immune escape, and chemoresistance [11][12][49][50][51]. Therefore, in vitro models should ideally recapitulate the rich gamut of cells, extracellular matrix, and signaling of the TME. However, current organoid models only host single TME cell populations. Primary tissue-derived tumor organoids can retain tumor-resident immune cells for a short time but progressively lose them [29][37]. Recent insights, especially into the importance of neuronal activity and neuron–glioma interactions in glioma proliferation, highlight the need for a neuronal TME in brain tumor models [51]. In TBOs and neoCORs, which are based on cerebral organoids, tumor cells interact with their neural surrounding [40][52][53][54].
Despite their advantages as the next-generation in vitro models, both TBOs and neoCORs are derived from cerebral organoids and, thus, share their challenges. They are difficult to standardize, with a high variance from one sample to the next, likely due to their reliance on self-organization. Currently, the field needs comprehensive strategies to incorporate key cell types, including microglia and near-native levels of astrocytes. These organoids self-arrest at the fetal levels of cellular maturity, peaking at the equivalent of weeks 17–24 of pregnancy [55][56]. Many researchers believe that further maturation requires a functional, perfusable vascular bed and a blood–brain barrier, which has not been demonstrated yet [57]. In this manner, organoids mimic early embryonal brain development (and thus a basic TME) and not mature brain tissue [58].
3. Organoid Models in Pediatric Brain Tumors
Pediatric brain tumors are much rarer than brain tumors in adults but belong to the most frequent tumor entities in children. Besides pediatric glioma and ependymoma, children are affected by embryonal tumors, including medulloblastoma, atypical teratoid and rhabdoid tumor (ATRT), and embryonal tumor with multilayered rosettes (ETMR), most of which are associated with a poor prognosis [59]. Despite recent achievements in establishing organoid models for adult brain tumors, such as glioblastoma or LGG [29][40][54][60], a similar body of work for pediatric brain tumors is still missing (Table 1). Unfortunately, the results gathered from in vitro tumor models cannot necessarily be transferred from adult to pediatric entities as tumor location and molecular characteristics of the tumor and the developmental state of the brain, as a key player of the TME, differ. Recent studies have shown that drugs that work perfectly in adults may cause major side effects on the developing brain of children [61]. Nevertheless, drugs are primarily established for adults and then transferred to pediatric patients [61]. Using 3D cell culture techniques might help to overcome this problem and directly establish drugs for this age group. Thus, one major challenge is the development of individual model systems for each pediatric brain tumor entity and subentity. In the last few years, some groups started testing PDO or neoCOR models for some of these entities [52][62][63][64]. Due to the scarcity of publications, the next section will summarize recent achievements by entity and not by model.
Table 1. Established three-dimensional brain tumor models.
| Reference | Entity | Model-Type | Method |
|---|---|---|---|
| Hubert et al., 2016 [23] | glioblastoma | GBO | Tumor cells embedded in Matrigel |
| Jacob et al., 2020 [29] | glioblastoma | GBO | Tumor pieces on an orbital shaker |
| Loong et al., 2020 [65] | glioblastoma | GBO | Tumor cells embedded in Matrigel |
| Chen et al., 2022 [43] | glioblastoma | GBO | Tumor pieces on an orbital shaker |
| LeBlanc et al., 2022 [37] | glioblastoma | PDE | Tumor pieces in Matrigel |
| da Silva et al., 2018 [66] | glioblastoma | GLICO | Murine brain organoids, GBM cells |
| Linkous et al., 2019 [40] | glioblastoma | GLICO | Brain organoids + GBM cells |
| Krieger et al., 2020 [54] | glioblastoma | GLICO | Brain organoids + GBM cells |
| Gorancia-Buzhala et al., 2020 [67] | glioblastoma | GLICO | Brain organoids + GBM cells |
| Azzarelli et al., 2021 [68] | glioblastoma | GLICO | Brain organoids + GBM cells |
| Ogawa et al., 2018 [69] | glioblastoma | neoCOR | HRas, TP53 mutations |
| Bian et al., 2018 [53] | glioblastoma | neoCOR | Several different mutations in combination and alone as PTEN, Myc, and EGFR |
| Yi et al., 2019 [70] | glioblastoma | bioprinting | GBM cells + endothelial cells + HUVECs |
| Heinrich et al., 2019 [71] | glioblastoma | bioprinting | GBM cells + macrophages |
| Tang et al., 2020 [72] | glioblastoma | bioprinting | GBM cells + neuronal progenitor cells + astrocytes + macrophages |
| Abdullah et al., 2022 [60] | LGG | PDO | Tumor pieces on an orbital shaker; 5% O2 |
| Sundar et al., 2022 [62] | pediatric HGG | PDO | Tumor cells embedded in Matrigel |
| Frisira et al., 2019 [64] | medulloblastoma | PDO | Tumor cells embedded in Matrigel |
| Ballabio et al., 2020 [63] | medulloblastoma | neoCOR | Different mutations, e.g., Otx-2 or c-Myc |
| Parisian et al., 2020 [52] | ATRT | neoCOR | SMARCB1-KD |
3.1. Pediatric High-Grade Glioma
Pediatric high-grade gliomas (HGGs) share many features of HGGs in adults but are genetically different. Therefore, models addressing the specialties of this age group merit their own disease models [73]. Recently, Sundar et al. generated organoids from pediatric HGG patients [62]. Here, organoids were formed by embedding single cells into Matrigel, followed by a shaking culture [62]. Distinct proliferative phenotypes were observed in the organoids pre- and post-treatment and evaluated via immunohistochemistry microarrays of the organoids. By testing the effects of the clinical standard of care (temozolomide and radiotherapy) on the proliferation of glioma sphere cultures and organoids, Sundar et al. found the organoids were resistant to this therapy, while the glioma spheroids stayed sensitive [62].
3.2. Medulloblastoma
Medulloblastoma is the most frequent malignant brain tumor of the cerebellum in children [74] and is divided into four consensus molecular subgroups: WNT, SHH, Group 3, and Group 4 [75]. Despite recent progress in the establishment of in vitro and in vivo medulloblastoma models, current models mainly cover SHH and Group 3 medulloblastoma and models for WNT and Group 4 are clearly underrepresented [76][77]. Therefore, a majority of patient tumors are not represented in current preclinical studies and the resulting information might only be relevant for a small group of patients [77].
Ivanov et al. pioneered a co-culture model of neuronal stem cells with tumor cells in spheroids to mimic tumor–host interactions for a cytotoxicity screen [42]. They generated medulloblastoma-neuronal stem cell (NSC) spheroids by seeding the same amount of medulloblastoma cells and NSCs, both labeled with distinct fluorescent dyes, followed by seven days of culture. For the cytotoxicity screen, spheroids were treated with different concentrations of Etoposide on day 3. Due to the presence of both tumor and healthy stem cells in the spheroid, they were able to simultaneously assess the toxicity in both compartments by dissociation, followed by flow cytometry on day 7 [42]. Later, other groups used organoids to model medulloblastoma in vitro, using either organoids derived from patient cells [64] or neoplastic cerebellar organoids [63]. Medulloblastoma organoids can be derived from single cells and similarly cultured in Matrigel as glioblastoma organoids [23][64]. Ballabio et al. used another approach: they first pre-differentiated cerebellar organoids until day 35, when all progenitors were present, and this was followed by transfection of the intact organoid with several potential oncogenic mutations derived from a patient-specific screening [63]. This approach proved the ability of Oct-2 and c-Myc mutations to elicit a medulloblastoma-like phenotype in the organoids [63]. These pioneering studies display an important starting point of 3D medulloblastoma modeling which might help establish model systems that can represent the whole molecular diversity of medulloblastoma, via direct patient-derived organoids, or help establish organoid modeling for all entities in future studies.
3.3. Atypical Teratoid Rhabdoid Tumors
ATRTs, belonging to the group of embryonal brain tumors, are much less prevalent than medulloblastoma. ATRTs are characterized by the loss of the SMARCB1 or SMARCA4 gene, which encodes for a subunit of the SWI/SNF chromatin-remodeling complex [52][78]. Through the inactivation of SMARCB1 with CRISPR-Cas9 in neoCORs, Parisian et al. uncovered the effects of its knockdown (KD) on neuronal development [52]. The impact of SMARCB1 KD on the cells depended on the organoids’ developmental stage. Interestingly, the KD blocked differentiation termination only in neuronal progenitor cells, while mature neuroblasts stayed unaffected, and human-induced pluripotent stem cells (hiPSCs) died [52]. This might indicate that malignancy initiation is only possible in a specific developmental window. In this model, only early neuronal progenitor cells had the capacity to transform into tumor cells [52], which hinted at the progenitor cells being the potential cells of origin for this tumor entity. This finding in organoids recapitulates current findings in mice, in which only defined cells of origin and differentiation states give rise to ATRT development when Smarcb1 or Smarca4 is lost [31][79][80][81].
3.4. Conclusions for 3D Models of Pediatric Brain Tumors
In summary, few organoid models have been established for pediatric brain tumors, and published work only exists for a subset of the overall tumor entities [52][62][63][64]. This may be partially due to the overall rarity of these diseases, and, thereby, limited sample availability. Examples of organoid models in pediatric brain tumors have shown that the same techniques used for adult glioblastoma can be transferred to pediatric brain tumors, opening similar opportunities for a wide range of potential applications in cancer research and personalized medicine. Special difficulties include how to model the variety of different entities and associated microenvironments. Differences in the componence of the tumor microenvironment, especially in the immune microenvironment, need to be considered when designing and establishing a new model. For instance, the immune infiltration in embryonal tumors, such as ATRT, medulloblastoma, and ETMR, is much lower than that of gliomas [82]. Additionally, within one entity, such as medulloblastoma, the infiltrating immune cell populations and numbers are significantly differing between distinct subgroups [82]. This makes the development of 3D models, which include also immune cells, even more challenging. Moreover, in this field, further research is needed to establish organoid models for entities that have not yet been addressed, including ependymoma and ETMR.
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Al, E. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Cohen, K.J.; Heideman, R.L.; Zhou, T.; Holmes, E.J.; Lavey, R.S.; Bouffet, E.; Pollack, I.F. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children’s Oncology Group. Neuro-Oncol. 2011, 13, 410–416.
- Ginn, K.F.; Gajjar, A. Atypical teratoid rhabdoid tumor: Current therapy and future directions. Front. Oncol. 2012, 2, 114.
- Rasmussen, B.K.; Hansen, S.; Laursen, R.J.; Kosteljanetz, M.; Schultz, H.; Nørgård, B.M.; Guldberg, R.; Gradel, K.O. Epidemiology of glioma: Clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the the Danish Neuro-Oncology Registry. J. Neurooncol. 2017, 135, 571–579.
- Eriksson, M.; Kahari, J.; Vestman, A.; Hallmans, M.; Johansson, M.; Bergenheim, A.T.; Sandström, M. Improved treatment of glioblastoma–changes in survival over two decades at a single regional Centre. Acta Oncol. 2019, 58, 334–341.
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622.
- Bagley, S.J.; Kothari, S.; Rahman, R.; Lee, E.Q.; Dunn, G.P.; Galanis, E.; Chang, S.M.; Nabors, L.B.; Ahluwalia, M.S.; Stupp, R.; et al. Glioblastoma Clinical Trials: Current Landscape and Opportunities for Improvement. Clin. Cancer Res. 2022, 28, 594–602.
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat. Mater. 2021, 21, 143–159.
- Pribluda, A.; De La Cruz, C.C.; Jackson, E.L. Intratumoral heterogeneity: From diversity comes resistance. Clin. Cancer Res. 2015, 21, 2916–2923.
- Kim, J.H.; An, G.H.; Kim, J.Y.; Rasaei, R.; Kim, W.J.; Jin, X.; Woo, D.H.; Han, C.; Yang, S.R.; Kim, J.H.; et al. Human pluripotent stem-cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov. 2021, 7, 48.
- Dapash, M.; Hou, D.; Castro, B.; Lee-Chang, C.; Lesniak, M.S. The interplay between glioblastoma and its microenvironment. Cells 2021, 10, 2257.
- Melcher, V.; Kerl, K. The Growing Relevance of Immunoregulation in Pediatric Brain Tumors. Cancers 2021, 13, 5601.
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597.
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–58.
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379.
- Sloan, S.A.; Andersen, J.; Pașca, A.M.; Birey, F.; Pașca, S.P. Generation and assembly of human brain region–specific three-dimensional cultures. Nat. Protoc. 2018, 13, 2062–2085.
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254.
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015, 10, 537–550.
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772.
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187.
- Boj, S.F.; Hwang, C.I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338.
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435.
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 2016, 76, 2465–2477.
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418.
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941.
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687.
- Nii, T.; Makino, K.; Tabata, Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers 2020, 12, 2754.
- Toh, Y.C.; Lim, T.C.; Tai, D.; Xiao, G.; Van Noort, D.; Yu, H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 2009, 9, 2026–2035.
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204.e22.
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409.
- Graf, M.; Interlandi, M.; Moreno, N.; Holdhof, D.; Göbel, C.; Melcher, V.; Mertins, J.; Albert, T.K.; Kastrati, D.; Alfert, A.; et al. Single-cell transcriptomics identifies potential cells of origin of MYC rhabdoid tumors. Nat. Commun. 2022, 13, 1544.
- Kim, H.J.; Park, J.W.; Lee, J.H. Genetic Architectures and Cell-of-Origin in Glioblastoma. Front. Oncol. 2021, 10, 615400.
- Melcher, V.; Graf, M.; Interlandi, M.; Moreno, N.; de Faria, F.W.; Kim, S.N.; Kastrati, D.; Korbanka, S.; Alfert, A.; Gerß, J.; et al. Macrophage-tumor cell interaction promotes ATRT progression and chemoresistance. Acta Neuropathol. 2020, 139, 913–936.
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812.
- Li, Z.; Langhans, S.A. In Vivo and Ex Vivo Pediatric Brain Tumor Models: An Overview. Front. Oncol. 2021, 11.
- Rybin, M.J.; Ivan, M.E.; Ayad, N.G.; Zeier, Z. Organoid Models of Glioblastoma and Their Role in Drug Discovery. Front. Cell. Neurosci. 2021, 15, 605255.
- LeBlanc, V.G.; Trinh, D.L.; Aslanpour, S.; Hughes, M.; Livingstone, D.; Jin, D.; Ahn, B.Y.; Blough, M.D.; Cairncross, J.G.; Chan, J.A.; et al. Single-cell landscapes of primary glioblastomas and matched explants and cell lines show variable retention of inter- and intratumor heterogeneity. Cancer Cell 2022, 40, 379–392.e9.
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part C Methods 2015, 22, 221–249.
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017, 28, 1448–1456.
- Linkous, A.; Balamatsias, D.; Snuderl, M.; Edwards, L.; Miyaguchi, K.; Milner, T.; Reich, B.; Cohen-Gould, L.; Storaska, A.; Nakayama, Y.; et al. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 2019, 26, 3203–3211.e5.
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. Multiplexing spheroid volume, resazurin and acid phosphatase viability assays for high-throughput screening of tumour spheroids and stem cell neurospheres. PLoS ONE 2014, 9, e103817.
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. In vitro co-culture model of medulloblastoma and human neural stem cells for drug delivery assessment. J. Biotechnol. 2015, 205, 3–13.
- Chen, C.-C.; Li, H.-W.; Wang, Y.-L.; Lee, C.-C.; Shen, Y.-C.; Hsieh, C.-Y.; Lin, H.-L.; Chen, X.-X.; Cho, D.-Y.; Hsieh, C.-L.; et al. Patient-derived tumor organoids as a platform of precision treatment for malignant brain tumors. Sci. Rep. 2022, 12, 16399.
- Ma, X.; Li, H.; Zhu, S.; Hong, Z.; Kong, W.; Yuan, Q.; Wu, R.; Pan, Z.; Zhang, J.; Chen, Y.; et al. Angiorganoid: Vitalizing the organoid with blood vessels. Vasc. Biol. 2022, 4, R44–R57.
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41.
- Pham, M.T.; Pollock, K.M.; Rose, M.D.; Cary, W.A.; Stewart, H.R.; Zhou, P.; Nolta, J.A.; Waldau, B. Generation of human vascularized brain organoids. Neuroreport 2018, 29, 588–593.
- Shi, Y.; Sun, L.; Wang, M.; Liu, J.; Zhong, S.; Li, R.; Li, P.; Guo, L.; Fang, A.; Chen, R.; et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020, 18, e3000705.
- Ahn, Y.; An, J.H.; Yang, H.J.; Lee, D.G.; Kim, J.; Koh, H.; Park, Y.H.; Song, B.S.; Sim, B.W.; Lee, H.J.; et al. Human blood vessel organoids penetrate human cerebral organoids and form a vessel-like system. Cells 2021, 10, 2036.
- Henrik Heiland, D.; Ravi, V.M.; Behringer, S.P.; Frenking, J.H.; Wurm, J.; Joseph, K.; Garrelfs, N.W.C.; Strähle, J.; Heynckes, S.; Grauvogel, J.; et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 2019, 10, 2541.
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 2015, 161, 803–816.
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545.
- Parisian, A.D.; Koga, T.; Miki, S.; Johann, P.D.; Kool, M.; Crawford, J.R.; Furnari, F.B. SMARCB1 loss interacts with neuronal differentiation state to block maturation and impact cell stability. Genes Dev. 2020, 34, 1316–1329.
- Bian, S.; Repic, M.; Guo, Z.; Kavirayani, A.; Burkard, T.; Bagley, J.A.; Krauditsch, C.; Knoblich, J.A. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 2018, 15, 631–639.
- Krieger, T.G.; Tirier, S.M.; Park, J.; Jechow, K.; Eisemann, T.; Peterziel, H.; Angel, P.; Eils, R.; Conrad, C. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro-Oncol. 2020, 22, 1138–1149.
- Chiaradia, I.; Lancaster, M.A. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat. Neurosci. 2020, 23, 1496–1508.
- Tanaka, Y.; Cakir, B.; Xiang, Y.; Sullivan, G.J.; Park, I.H. Synthetic Analyses of Single-Cell Transcriptomes from Multiple Brain Organoids and Fetal Brain. Cell Rep. 2020, 30, 1682–1689.e3.
- Benito-Kwiecinski, S.; Lancaster, M.A. Brain organoids: Human neurodevelopment in a dish. Cold Spring Harb. Perspect. Biol. 2020, 12, a035709.
- Quadrato, G.; Brown, J.; Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 2016, 22, 1220–1228.
- Partap, S.; Monje, M. Pediatric Brain Tumors. Continuum 2020, 26, 1553–1583.
- Abdullah, K.G.; Bird, C.E.; Buehler, J.D.; Gattie, L.C.; Savani, M.R.; Sternisha, A.C.; Xiao, Y.; Levitt, M.M.; Hicks, W.H.; Li, W.; et al. Establishment of patient-derived organoid models of lower-grade glioma. Neuro-Oncol. 2022, 24, 612–623.
- Dobson, T.H.W.; Gopalakrishnan, V. Preclinical models of pediatric brain tumors—Forging ahead. Bioengineering 2018, 5, 81.
- Sundar, S.J.; Shakya, S.; Barnett, A.; Wallace, L.C.; Jeon, H.; Sloan, A.; Recinos, V.; Hubert, C.G. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Transl. Oncol. 2022, 15, 101251.
- Ballabio, C.; Anderle, M.; Gianesello, M.; Lago, C.; Miele, E.; Cardano, M.; Aiello, G.; Piazza, S.; Caron, D.; Gianno, F.; et al. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat. Commun. 2020, 11, 583.
- Frisira, E.; Rashid, F.; Varma, S.N.; Badodi, S.; Benjamin-Ombo, V.A.; Michod, D.; Niklison-Chirou, M.V. NPI-0052 and γ-radiation induce a synergistic apoptotic effect in medulloblastoma. Cell Death Dis. 2019, 10, 785.
- Loong, H.H.F.; Wong, A.M.; Chan, D.T.M.; Cheung, M.S.H.; Chow, C.; Ding, X.; Chan, A.K.Y.; Johnston, P.A.; Lau, J.Y.W.; Poon, W.S.; et al. Patient-derived tumor organoid predicts drugs response in glioblastoma: A step forward in personalized cancer therapy? J. Clin. Neurosci. 2020, 78, 400–402.
- da Silva, B.; Mathew, R.K.; Polson, E.S.; Williams, J.; Wurdak, H. Spontaneous Glioblastoma Spheroid Infiltration of Early-Stage Cerebral Organoids Models Brain Tumor Invasion. SLAS Discov. 2018, 23, 862–868.
- Goranci-Buzhala, G.; Mariappan, A.; Gabriel, E.; Ramani, A.; Ricci-Vitiani, L.; Buccarelli, M.; D’Alessandris, Q.G.; Pallini, R.; Gopalakrishnan, J. Rapid and Efficient Invasion Assay of Glioblastoma in Human Brain Organoids. Cell Rep. 2020, 31, 107738.
- Azzarelli, R.; Ori, M.; Philpott, A.; Simons, B.D. Three-dimensional model of glioblastoma by co-culturing tumor stem cells with human brain organoids. Biol. Open 2021, 10, bio056416.
- Ogawa, J.; Pao, G.M.; Shokhirev, M.N.; Verma, I.M. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229.
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H.; et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519.
- Heinrich, M.A.; Bansal, R.; Lammers, T.; Zhang, Y.S.; Michel Schiffelers, R.; Prakash, J. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv. Mater. 2019, 31, 1806590.
- Tang, M.; Xie, Q.; Gimple, R.C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R.L.; Wu, Q.; Prager, B.C.; Qiu, Z.; et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020, 30, 833–853.
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncol. 2017, 19, 153–161.
- Orr, B.A. Pathology, diagnostics, and classification of medulloblastoma. Brain Pathol. 2020, 30, 664–678.
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472.
- Neumann, J.E.; Swartling, F.J.; Schüller, U. Medulloblastoma: Experimental models and reality. Acta Neuropathol. 2017, 134, 679–689.
- Ivanov, D.P.; Coyle, B.; Walker, D.A.; Grabowska, A.M. In vitro models of medulloblastoma: Choosing the right tool for the job. J. Biotechnol. 2016, 236, 10–25.
- Johann, P.D.; Erkek, S.; Zapatka, M.; Kerl, K.; Buchhalter, I.; Hovestadt, V.; Jones, D.T.W.; Sturm, D.; Hermann, C.; Segura Wang, M.; et al. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell 2016, 29, 379–393.
- Moreno, N.; Schmidt, C.; Ahlfeld, J.; Pöschl, J.; Dittmar, S.; Pfister, S.M.; Kool, M.; Kerl, K.; Schüller, U. Loss of Smarc proteins impairs cerebellar development. J. Neurosci. 2014, 34, 13486–13491.
- Holdhof, D.; Schoof, M.; Al-Kershi, S.; Spohn, M.; Kresbach, C.; Göbel, C.; Hellwig, M.; Indenbirken, D.; Moreno, N.; Kerl, K.; et al. Brahma-related gene 1 has time-specific roles during brain and eye development. Development 2021, 148, dev196147.
- Han, Z.Y.; Richer, W.; Fréneaux, P.; Chauvin, C.; Lucchesi, C.; Guillemot, D.; Grison, C.; Lequin, D.; Pierron, G.; Masliah-Planchon, J.; et al. The occurrence of intracranial rhabdoid tumours in mice depends on temporal control of Smarcb1 inactivation. Nat. Commun. 2016, 7, 10421.
- Grabovska, Y.; Mackay, A.; O’Hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J.; et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324.
More
Information
Subjects:
Pediatrics; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
19 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





