
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katharina Miller | -- | 4640 | 2022-10-24 10:29:08 | | | |
| 2 | Lindsay Dong | -11 word(s) | 4629 | 2022-12-15 02:37:38 | | | | |
| 3 | Lindsay Dong | Meta information modification | 4629 | 2022-12-15 02:40:16 | | | | |
| 4 | Lindsay Dong | Meta information modification | 4629 | 2022-12-16 07:26:35 | | |
Video Upload Options
There are various physical, chemical, and biochemical modifications approaches for potato constituents. Physical modifications to alter the physicochemical properties of powders including starches and proteins are e.g., hydrothermal treatment, irradiation, ultrasonication and high-pressure treatment. Starches and proteins, which have been modified via physical methods do not have to be claimed as “modified”. Physical modification is also viewed as cost-efficient and environmentally friendly, because no hazardous substances (chemicals) are used. Chemical modifications refer to the substitution, cross-linking or degradation of a polymer via chemical reaction. Starch contains a large number of hydroxyl groups, and proteins contain a variety of different functional groups (hydroxyl-, carboxyl-, amine groups, etc.). These functional groups can be used as reactive sides for chemical modification reactions such as acylation, esterification, etherification, cross-linking, grafting, acid hydrolysis and oxidation. Biochemical modifications of starches and proteins including enzymatic substitution, cross-linking or hydrolysis are usually regarded as a clean or green alternative to chemical modification. Throughout the different biochemical modification methods, substrate specific enzymes can be used such as in enzymatic de-/branching modification, where the branched structure of potato starch can be altered to effect starch crystallinity and thus its properties.
1. Potato Tubers
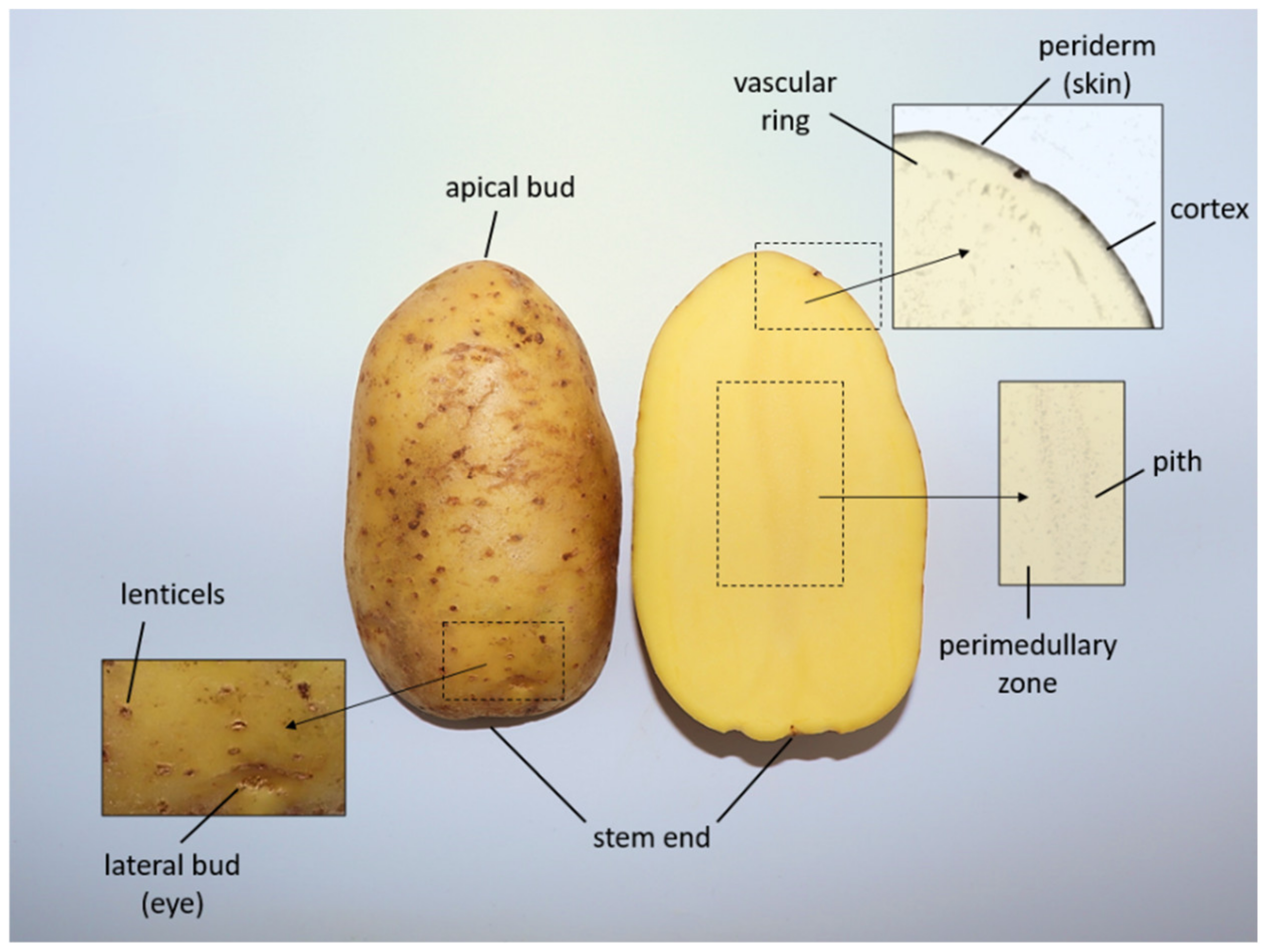
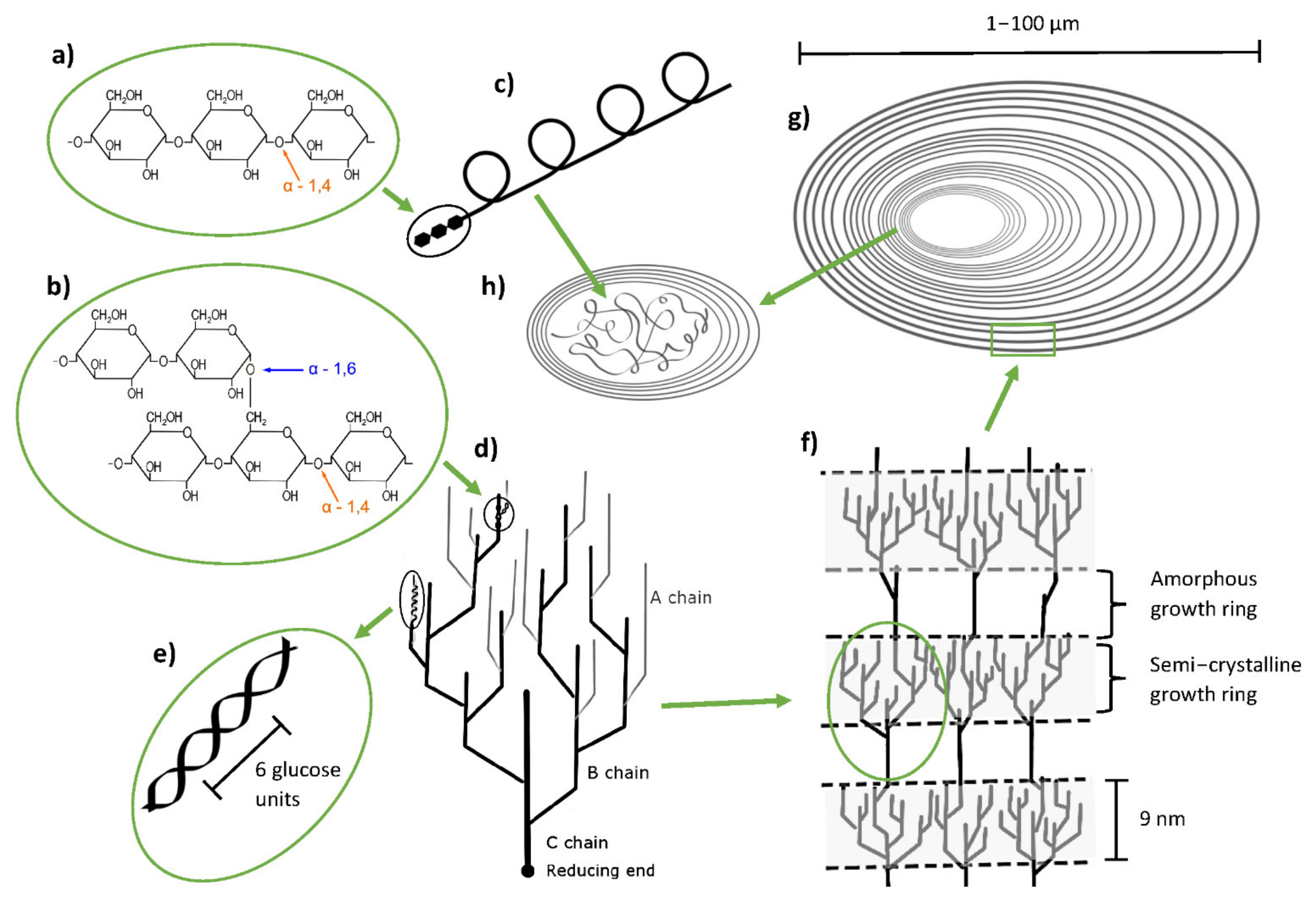
1.1. Potato Starch
1.2. Potato Protein
2. Modifications
2.1. Physical Modification
2.1.1. Thermal Physical Modification
Annealing
Microwave Treatment
Radio Frequency Treatment
Others
2.1.2. Non-Thermal Physical Modification
High Pressure Treatment
Ultrasonication
Ionizing Irradiation
Others
2.2. Chemical Modification
2.2.1. Chemical Substitution
Acetylation
Phosphorylation
Fatty Acid Esterification
Octenyl Succinylation
Citric Acid Esterification
2.2.2. Chemical Cross-Linking
2.2.3. Degradation
Acid Hydrolysis
Oxidation
2.3. Biochemical Modification
2.3.1. Enzymatic Substitution
One example of enzymatic substitution is the esterification/transesterification of fatty acids by lipases. Based on fatty acid solubility, organic solvents or ionic liquids need to be used. Dependent on the lipase used, degree of esterification and esterification site can vary [73].
2.3.2. Enzymatic Cross-Linking
2.3.3. Enzymatic De-/Branching
2.3.4. Enzymatic Hydrolysis
2.4. Dual Modification
2.4.1. Physical–Physical
As demonstrated by Wang et al. [87] and Cao and Gao [88], the order in which the two different physical treatment methods are performed can also influence the resulting qualitative and quantitative properties of the dual-modified samples, compared to native samples. For instance, ANN treatment prior to HP treatment resulted in a decrease in potato starch relative crystallinity, whereas an increase was reported when physical treatments were applied vice versa [87]. In potato starch, solubility and swelling power decreased, and gel hardness increased, when treated with US prior to electric field treatment, compared to untreated native potato starch. Treating potato starch first with an electric field and then with US resulted in reverse effects meaning increase in solubility and swelling power. Interestingly, the simultaneous treatment of electric field and US resulted in similar changes as the electric field prior to US treatment, except for a decrease in swelling power and increase in gel adhesiveness [88]. Some additional general information on dual-modification of starch can be found elsewhere [89].
2.4.2. Physical-Chemical
2.4.3. Physical–Biochemical
2.4.4. Chemical–Chemical
References
- Singh, J.; Kaur, L. Chemistry, processing, and nutritional attributes of potatoes–An introduction. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 23–26. ISBN 9780128000021.
- Leonel, M.; do Carmo, E.L.; Fernandes, A.M.; Soratto, R.P.; Ebúrneo, J.A.M.; Garcia, É.L.; Dos Santos, T.P.R. Chemical composition of potato tubers: The effect of cultivars and growth conditions. J. Food Sci. Technol. 2017, 54, 2372–2378.
- Lieberei, R.; Reisdorff, C. Nutzpflanzen, 8th ed.; Thieme: Stuttgart, Germany, 2012; ISBN 9783131516381.
- Karki, D.B.; KC, Y.; Khanal, H.; Bhattarai, P.; Koirala, B.; Khatri, S.B. Analysis of biodegradable films of starch from potato waste. Asian Food Sci. J. 2020, 14, 28–40.
- Subroto, E.; Indiarto, R.; Marta, H.; Shalihah, S. Effect of heat-moisture treatment on functional and pasting properties of potato (Solanum tuberosum L. var. Granola) starch. Food Res. 2018, 3, 469–476.
- Awokoya, K.N.; Odeleye, I.E.; Muhammed, Y.A.; Ndukwe, N.A.; Ibikunle, A.A. Impact of microwave irradiation energy levels on molecular rotation, structural, physicochemical, proximate and functional properties of potato (Ipomoea batatas) starch. Ghana J. Sci. 2021, 61, 57–72.
- Van Koningsveld, G.A.; Gruppen, H.; Jongh, H.H.; de Wijngaards, G.; van Boekel, M.A.; Walstra, P.; Voragen, A.G. The solubility of potato proteins from industrial potato fruit juice as influenced by pH and various additives. J. Sci. Food Agric. 2002, 82, 134–142.
- Løkra, S.; Strætkvern, K.O. Industrial proteins from potato juice. A review. Food 2009, 3, 88–95.
- Lee, C.H. A simple outline of methods for protein isolation and purification. Endocrinol. Metab. 2017, 32, 18–22.
- Li, H.; Zeng, X.; Shi, W.; Zhang, H.; Huang, S.; Zhou, R.; Qin, X. Recovery and purification of potato proteins from potato starch wastewater by hollow fiber separation membrane integrated process. Innov. Food Sci. Emerg. Technol. 2020, 63, 102380.
- Knorr, D.; Kohler, G.O.; Betschart, A.A. Potato protein concentrates: The influence of various methods of recovery upon yield, compositional and functional characteristics. J. Food Process. Preserv. 1977, 1, 235–247.
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382.
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA J. Food 2018, 16, 1003–1017.
- Bertoft, E.; Blennow, A. Structure of Potato Starch. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–73. ISBN 9780128000021.
- Raigond, P.; Singh, B.; Dutt, S.; Chakrabarti, S.K. Potato; Springer: Singapore, 2020; ISBN 978-981-15-7661-4.
- Waglay, A.; Karboune, S. Potato proteins: Functional food ingredients. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 75–104. ISBN 9780128000021.
- Fonseca, L.M.; Halal, S.L.M.E.; Dias, A.R.G.; Zavareze, E.D.R. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohyd. Polym. 2021, 274, 118665.
- Zhang, B.; Saleh, A.S.M.; Su, C.; Gong, B.; Zhao, K.; Zhang, G.; Li, W.; Yan, W. The molecular structure, morphology, and physicochemical property and digestibility of potato starch after repeated and continuous heat-moisture treatment. J. Food Sci. 2020, 85, 4215–4224.
- Shi, M.; Gao, Q.; Liu, Y. Corn, potato, and wrinkled pea starches with heat-moisture treatment: Structure and digestibility. Cereal Chem. 2018, 95, 603–614.
- Lin, C.-L.; Lin, J.-H.; Lin, J.-J.; Chang, Y.-H. Progressive alterations in crystalline structure of starches during heat-moisture treatment with varying iterations and holding times. Int. J. Biol. Macromol. 2019, 135, 472–480.
- Oliveira, C.S.; de Bet, C.D.; Bisinella, R.Z.B.; Waiga, L.H.; Colman, T.A.D.; Schnitzler, E. Heat-moisture treatment (HMT) on blends from potato starch (PS) and sweet potato starch (SPS). J. Therm. Anal. Calorim. 2018, 133, 1491–1498.
- Bartz, J.; da Rosa Zavareze, E.; Dias, A.R.G. Study of heat-moisture treatment of potato starch granules by chemical surface gelatinization. J. Sci. Food Agric. 2017, 97, 3114–3123.
- Chen, X.; Luo, J.; Liang, Z.; Zhu, J.; Li, L.; Wang, Q. Structural and physicochemical/digestion characteristics of potato starch-amino acid complexes prepared under hydrothermal conditions. Int. J. Biol. Macromol. 2020, 145, 1091–1098.
- Wang, S.; Wang, J.; Wang, S.; Wang, S. Annealing improves paste viscosity and stability of starch. Food Hydrocoll. 2017, 62, 203–211.
- Xu, M.; Saleh, A.S.M.; Gong, B.; Li, B.; Jing, L.; Gou, M.; Jiang, H.; Li, W. The effect of repeated versus continuous annealing on structural, physicochemical, and digestive properties of potato starch. Food Res. Int. 2018, 111, 324–333.
- Zhu, Z.; Guo, W. Frequency, moisture content, and temperature dependent dielectric properties of potato starch related to drying with radio-frequency/microwave energy. Sci. Rep. 2017, 7, 9311.
- Shen, G.; Zhang, L.; Hu, T.; Li, Z.; Chen, A.; Zhang, Z.; Wu, H.; Li, S.; Hou, X. Preparation of potato flour by freeze-thaw pretreatment: Effect of different thawing methods on hot-air drying process and physicochemical properties. LWT 2020, 133, 110157.
- Ozturk, S.; Kong, F.; Trabelsi, S.; Singh, R.K. Dielectric properties of dried vegetable powders and their temperature profile during radio frequency heating. J. Food Eng. 2016, 169, 91–100.
- Fan, D.; Wang, L.; Shen, H.; Huang, L.; Zhao, J.; Zhang, H. Ultrastructure of potato starch granules as affected by microwave treatment. Int. J. Food Prop. 2017, 20, S3189–S3194.
- Zhu, H.; Li, D.; Li, S.; Wang, S. A novel method to improve heating uniformity in mid-high moisture potato starch with radio frequency assisted treatment. J. Food Eng. 2017, 206, 23–36.
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of microwave treatment (low power and varying time) on potato starch: Microstructure, thermo-functional, pasting and rheological properties. Int. J. Biol. Macromol. 2020, 155, 27–35.
- Przetaczek-Rożnowska, I.; Fortuna, T.; Wodniak, M.; Łabanowska, M.; Pająk, P.; Królikowska, K. Properties of potato starch treated with microwave radiation and enriched with mineral additives. Int. J. Biol. Macromol. 2019, 124, 229–234.
- Xu, X.; Chen, Y.; Luo, Z.; Lu, X. Different variations in structures of A- and B-type starches subjected to microwave treatment and their relationships with digestibility. LWT 2019, 99, 179–187.
- Zhang, C.; Lim, S.-T.; Chung, H.-J. Physical modification of potato starch using mild heating and freezing with minor addition of gums. Food Hydrocoll. 2019, 94, 294–303.
- Li, J.; Shen, C.; Ge, B.; Wang, L.; Wang, R.; Luo, X.; Chen, Z. Preparation and application of potato flour with low gelatinization degree using flash drying. Dry. Technol. 2018, 36, 374–382.
- Hu, X.; Guo, B.; Liu, C.; Yan, X.; Chen, J.; Luo, S.; Liu, Y.; Wang, H.; Yang, R.; Zhong, Y.; et al. Modification of potato starch by using superheated steam. Carbohyd. Polym. 2018, 198, 375–384.
- Liu, Y.; Chao, C.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. New insights into starch gelatinization by high pressure: Comparison with heat-gelatinization. Food Chem. 2020, 318, 126493.
- Kunugi, S.; Tanaka, N. Cold denaturation of proteins under high pressure. Biochim. Biophys. Acta 2002, 1595, 329–344.
- Wang, J.; Zhu, H.; Li, S.; Wang, S.; Wang, S.; Copeland, L. Insights into structure and function of high pressure-modified starches with different crystalline polymorphs. Int. J. Biol. Macromol. 2017, 102, 414–424.
- Baier, A.K.; Knorr, D. Influence of high isostatic pressure on structural and functional characteristics of potato protein. Food Res. Int. 2015, 77, 753–761.
- Słomińska, L.; Zielonka, R.; Jarosławski, L.; Krupska, A.; Szlaferek, A.; Kowalski, W.; Tomaszewska-Gras, J.; Nowicki, M. High pressure impact on changes in potato starch granules. Pol. J. Chem. Technol. 2015, 17, 65–73.
- Dos Santos Aguilar, J.G.; Cristianini, M.; Sato, H.H. Modification of enzymes by use of high-pressure homogenization. Food Res. Int. 2018, 109, 120–125.
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234.
- Castro, L.M.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Impact of high pressure on starch properties: A review. Food Hydrocoll. 2020, 106, 105877.
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Impact of pressure on physicochemical properties of starch dispersions. Food Hydrocoll. 2017, 68, 164–177.
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154.
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207.
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505.
- Wang, X.; Majzoobi, M.; Farahnaky, A. Ultrasound-assisted modification of functional properties and biological activity of biopolymers: A review. Ultrason. Sonochem. 2020, 65, 105057.
- Brodnjak, U.V. Influence of ultrasonic treatment on properties of bio-based coated paper. Prog. Org. Coat. 2017, 103, 93–100.
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523.
- Fu, Z.; Wu, M.; Zhang, H.; Wang, J. Retrogradation of partially gelatinised potato starch prepared by ball milling. Int. J. Food Sci. Technol. 2018, 53, 1065–1071.
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakis, E. The principles of high voltage electric field and its application in food processing: A review. Food Res. Int. 2016, 89, 48–62.
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Influence of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J. Food Eng. 2012, 108, 320–326.
- Zięba, T.; Kapelko, M.; Szumny, A. Effect of preparation method on the properties of potato starch acetates with an equal degree of substitution. Carbohyd. Polym. 2013, 94, 193–198.
- Miedzianka, J.; Pęksa, A.; Aniołowska, M. Properties of acetylated potato protein preparations. Food Chem. 2012, 133, 1283–1291.
- Rożnowski, J.; Juszczak, L.; Szwaja, B.; Przetaczek-Rożnowska, I. Effect of esterification conditions on the physicochemical properties of phosphorylated potato starch. Polymers 2021, 13, 2548.
- Vanmarcke, A.; Leroy, L.; Stoclet, G.; Duchatel-Crépy, L.; Lefebvre, J.-M.; Joly, N.; Gaucher, V. Influence of fatty chain length and starch composition on structure and properties of fully substituted fatty acid starch esters. Carbohyd. Polym. 2017, 164, 249–257.
- Wang, C.; Tang, C.-H.; Fu, X.; Huang, Q.; Zhang, B. Granular size of potato starch affects structural properties, octenylsuccinic anhydride modification and flowability. Food Chem. 2016, 212, 453–459.
- Won, C.; Jin, Y.I.; Chang, D.-C.; Kim, M.; Lee, Y.; Gansean, P.; Lee, Y.-K.; Chang, Y.H. Rheological, pasting, thermal and retrogradation properties of octenyl succinic anhydride modified potato starch. Food Sci. Technol. 2017, 37, 321–327.
- Won, C.; Jin, Y.; Kim, M.; Lee, Y.; Chang, Y.H. Structural and rheological properties of potato starch affected by degree of substitution by octenyl succinic anhydride. Int. J. Food Prop. 2017, 20, 3076–3089.
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohyd. Polym. 2018, 202, 29–38.
- Van Hung, P.; Vien, N.L.; Lan Phi, N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73.
- Martins, P.C.; Gutkoski, L.C.; Martins, V.G. Impact of acid hydrolysis and esterification process in rice and potato starch properties. Int. J. Biol. Macromol. 2018, 120, 959–965.
- Kou, T.; Gao, Q. New insight in crosslinking degree determination for crosslinked starch. Carbohyd. Res. 2018, 458–459, 13–18.
- Shulga, O.; Simurova, N.; Shulga, S.; Smirnova, J. Modification of potato starch by acetylmalic acid chloroanhydride and physicochemical research of the new product. Int. J. Polym. Sci. 2018, 2018, 1–7.
- Heo, H.; Lee, Y.-K.; Chang, Y.H. Rheaological, pasting, and structural properties of potato starch by cross-linking. Int. J. Food Prop. 2017, 2, 1–13.
- Zdanowicz, M.; Johansson, C. Mechanical and barrier properties of starch-based films plasticized with two- or three component deep eutectic solvents. Carbohyd. Polym. 2016, 151, 103–112.
- Celikci, N.; Dolaz, M.; Colakoglu, A.S. An environmentally friendly carton adhesive from acidic hydrolysis of waste potato starch. Int. J. Polym. Anal. Charact. 2021, 26, 97–110.
- Sun, B.; Tian, Y.; Wei, B.; Chen, L.; Bi, Y.; Jin, Z. Effect of reaction solvents on the multi-scale structure of potato starch during acid treatment. Int. J. Biol. Macromol. 2017, 97, 67–75.
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559.
- Hutla, P.; Kolaříková, M.; Hájek, D.; Doležal, P.; Hausvater, E.; Petráčková, B. Ozone treatment of stored potato tubers. Agron. Res. 2020, 18, 100–112.
- Boruczkowska, H.; Boruczkowski, T.; Gubica, T.; Anioł, M.; Tomaszewska-Ciosk, E. Analysis of the chemical structure of insoluble products of enzymatic esterification of starch and transesterification of acetylated starch with oleic acid by solid-state CP/MAS 13 C NMR. Starch-Stärke 2016, 68, 1180–1186.
- Li, M.; Karboune, S.; Light, K.; Kermasha, S. Oxidative cross-linking of potato proteins by fungal laccases: Reaction kinetics and effects on the structural and functional properties. Innov. Food Sci. Emerg. Technol. 2021, 71, 102723.
- Isaschar-Ovdat, S.; Fishman, A. Crosslinking of food proteins mediated by oxidative enzymes—A review. Trends Food Sci. Technol. 2018, 72, 134–143.
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A.; Kukavica, B. Partial characterization of bean and maize root peroxidases and their ability to crosslink potato protein. Arch. Biol. Sci. 2019, 71, 293–303.
- Gui, Y.; Li, J.; Zhu, Y.; Guo, L. Roles of four enzyme crosslinks on structural, thermal and gel properties of potato proteins. LWT 2020, 123, 109116.
- Li, M.; Blecker, C.; Karboune, S. Molecular and air-water interfacial properties of potato protein upon modification via laccase-catalyzed cross-linking and conjugation with sugar beet pectin. Food Hydrocoll. 2021, 112, 106236.
- Zhu, Y.; Tao, H.; Janaswamy, S.; Zou, F.; Cui, B.; Guo, L. The functionality of laccase- or peroxidase-treated potato flour: Role of interactions between protein and protein/starch. Food Chem. 2021, 341, 128082.
- Glusac, J.; Isaschar-Ovdat, S.; Kukavica, B.; Fishman, A. Oil-in-water emulsions stabilized by tyrosinase-crosslinked potato protein. Food Res. Int. 2017, 100, 407–415.
- Wang, R.; Liu, P.; Cui, B.; Kang, X.; Yu, B.; Qiu, L.; Sun, C. Effects of pullulanase debranching on the properties of potato starch-lauric acid complex and potato starch-based film. Int. J. Biol. Macromol. 2020, 156, 1330–1336.
- Guo, L.; Deng, Y.; Lu, L.; Zou, F.; Cui, B. Synergistic effects of branching enzyme and transglucosidase on the modification of potato starch granules. Int. J. Biol. Macromol. 2019, 130, 499–507.
- Ulbrich, M.; Asiri, S.A.; Bussert, R.; Flöter, E. Enzymatic modification of granular potato starch using isoamylase—Investigation of morphological, physicochemical, molecular, and techno-functional properties. Starch-Stärke 2021, 73, 2000080.
- Asiri, S.A.; Ulbrich, M.; Flöter, E. Partial hydrolysis of granular potato starch using α-amylase—Effect on physicochemical, molecular, and functional properties. Starch-Stärke 2019, 71, 1800253.
- Guo, L.; Li, J.; Li, H.; Zhu, Y.; Cui, B. The structure property and adsorption capacity of new enzyme-treated potato and sweet potato starches. Int. J. Biol. Macromol. 2020, 144, 863–873.
- Vafina, A.; Proskurina, V.; Vorobiev, V.; Evtugin, V.G.; Egkova, G.; Nikitina, E. Physicochemical and morphological characterization of potato starch modified by bacterial amylases for food industry applications. J. Chem. 2018, 2018, 1627540.
- Wang, S.; Guo, P.; Xiang, F.; Wang, J.; Yu, J.; Wang, S. Effect of dual modification by annealing and ultrahigh pressure on properties of starches with different polymorphs. Carbohyd. Polym. 2017, 174, 549–557.
- Cao, M.; Gao, Q. Effect of dual modification with ultrasonic and electric field on potato starch. Int. J. Biol. Macromol. 2020, 150, 637–643.
- Ashogbon, A.O. Dual modification of various starches: Synthesis, properties and applications. Food Chem. 2021, 342, 128325.
- Cao, M.; Gao, Q. Internal structure of high degree substitution acetylated potato starch by chemical surface gelatinization. Int. J. Biol. Macromol. 2020, 145, 133–140.
- Chen, B.-R.; Wen, Q.-H.; Zeng, X.-A.; Abdul, R.; Roobab, U.; Xu, F.-Y. Pulsed electric field assisted modification of octenyl succinylated potato starch and its influence on pasting properties. Carbohyd. Polym. 2021, 254, 117294.
- Hong, J.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Effect of pulsed electric fields treatment on the nanostructure of esterified potato starch and their potential glycemic digestibility. Innov. Food Sci. Emerg. Technol. 2018, 45, 438–446.
- Zhao, K.; Li, B.; Xu, M.; Jing, L.; Gou, M.; Yu, Z.; Zheng, J.; Li, W. Microwave pretreated esterification improved the substitution degree, structural and physicochemical properties of potato starch esters. LWT 2018, 90, 116–123.
- Ulbrich, M.; Bai, Y.; Flöter, E. The supporting effect of ultrasound on the acid hydrolysis of granular potato starch. Carbohyd. Polym. 2020, 230, 115633.
- Zhang, H.; He, F.; Wang, T.; Chen, G. Thermal, pasting, and rheological properties of potato starch dual-treated with CaCl2 and dry heat. LWT 2021, 146, 111467.
- Zięba, T.; Wilczak, A.; Kobryń, J.; Musiał, W.; Kapelko-Żeberska, M.; Gryszkin, A.; Meisel, M. The annealing of acetylated potato starch with various substitution degrees. Molecules 2021, 26, 2096.
- Cheng, Y.; Liu, Y.; Wu, J.; Ofori Donkor, P.; Li, T.; Ma, H. Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: Thermodynamics and kinetics. Ultrason. Sonochem. 2017, 37, 351–359.
- Mu, T.-H.; Zhang, M.; Raad, L.; Sun, H.-N.; Wang, C. Effect of α-amylase degradation on physicochemical properties of pre-high hydrostatic pressure-treated potato starch. PLoS ONE 2015, 10, e0143620.
- Qi, X.; Tester, R.F. Heat and moisture modification of native starch granules on susceptibility to amylase hydrolysis. Starch-Stärke 2016, 68, 816–820.
- Wang, J.; Ren, F.; Huang, H.; Wang, Y.; Copeland, L.; Wang, S.; Wang, S. Effect of CaCl2 pre-treatment on the succinylation of potato starch. Food Chem. 2019, 288, 291–296.
- Li, L.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Synergistic effect of sodium dodecyl sulfate and salts on the gelation properties of acid-hydrolyzed-hydroxypropylated potato starch. LWT 2018, 93, 556–562.
- He, X.; Gong, X.; Li, W.; Cao, W.; Yan, J.; Guo, R.; Niu, B.; Jia, L. Preparation and characterization of amphiphilic composites made with double-modified (etherified and esterified) potato starches. Starch-Stärke 2019, 71, 1900089.
- González-Soto, R.A.; Núñez-Santiago, M.C.; Bello-Pérez, L.A. Preparation and partial characterization of films made with dual-modified (acetylation and crosslinking) potato starch. J. Sci. Food Agric. 2019, 99, 3134–3141.





