There are various physical, chemical, and biochemical modifications approaches for potato constituents. Physical modifications to alter the physicochemical properties of powders including starches and proteins are e.g., hydrothermal treatment, irradiation, ultrasonication and high-pressure treatment. Starches and proteins, which have been modified via physical methods do not have to be claimed as “modified”. Physical modification is also viewed as cost-efficient and environmentally friendly, because no hazardous substances (chemicals) are used. Chemical modifications refer to the substitution, cross-linking or degradation of a polymer via chemical reaction. Starch contains a large number of hydroxyl groups, and proteins contain a variety of different functional groups (hydroxyl-, carboxyl-, amine groups, etc.). These functional groups can be used as reactive sides for chemical modification reactions such as acylation, esterification, etherification, cross-linking, grafting, acid hydrolysis and oxidation. Biochemical modifications of starches and proteins including enzymatic substitution, cross-linking or hydrolysis are usually regarded as a clean or green alternative to chemical modification. Throughout the different biochemical modification methods, substrate specific enzymes can be used such as in enzymatic de-/branching modification, where the branched structure of potato starch can be altered to effect starch crystallinity and thus its properties.
- potato starch
- potato protein
- potato peel-based films
- biopolymer modification
1. Potato Tubers
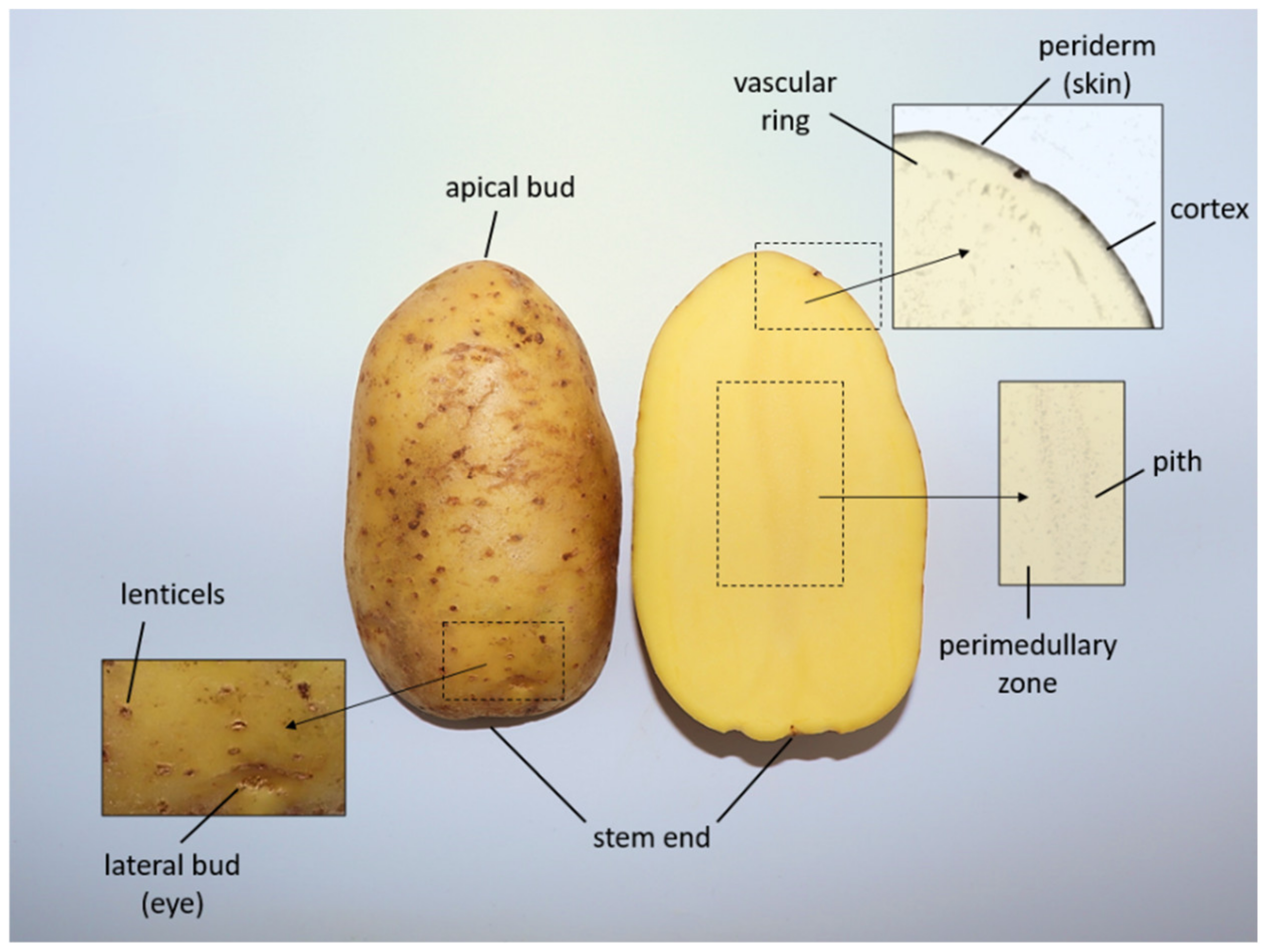
1.1. Potato Starch
With 70 to 80% w/w of dry matter content, starch is the major component of potato tubers. The structure of starch on molecular to macromolecular level is schematically illustrated in its main levels in Figure 2. The structure of potato starch typically consists of 20 to 30% (w/w) amylose, which is a linear to slightly branched (degree of polymerization > 60) polymer chain consisting of α-d-(1,4)-linked glucose units (Figure 2a), and 70 to 80% (w/w) of amylopectin, which is a highly branched polymer based on relatively short chains of α-d-(1,4)-linked glucose units with 4 to 6% (w/w) additional α-d-(1,6)-linkages (Figure 2b). Low molecular weight amylose (0.13–0.5 MDa) is assumed to form a helical structure (Figure 2c). Amylopectin is characterized by its high molecular weight (10–1000 MDa) and its branched structure, with branched chains being classified by their substitution into A chains (not substituted with other chains), and B chains (substituted with other chains) (Figure 2d). These branched chains derive from the main chain, which is called C chain (Figure 2d). The reducing end of the amylopectin polymer can be found at the end of the C chain (Figure 2d). Due to the tight packing of A and B chains, they can form double helices with six glucose units building one turn (Figure 2e). The arrangement of interlinked clusters results in lamellar structures, where alternating amorphous (branching points/interconnecting chains) and (semi-) crystalline (A and B chain clusters) parts are present (Figure 2f). On a macromolecular level, several of the alternating lamellar structures are organized in so called growth rings forming the starch granules (Figure 2g). The amorphous center of the granule (hilum) is formed mainly by amylose chains (Figure 2h) [13][14][15].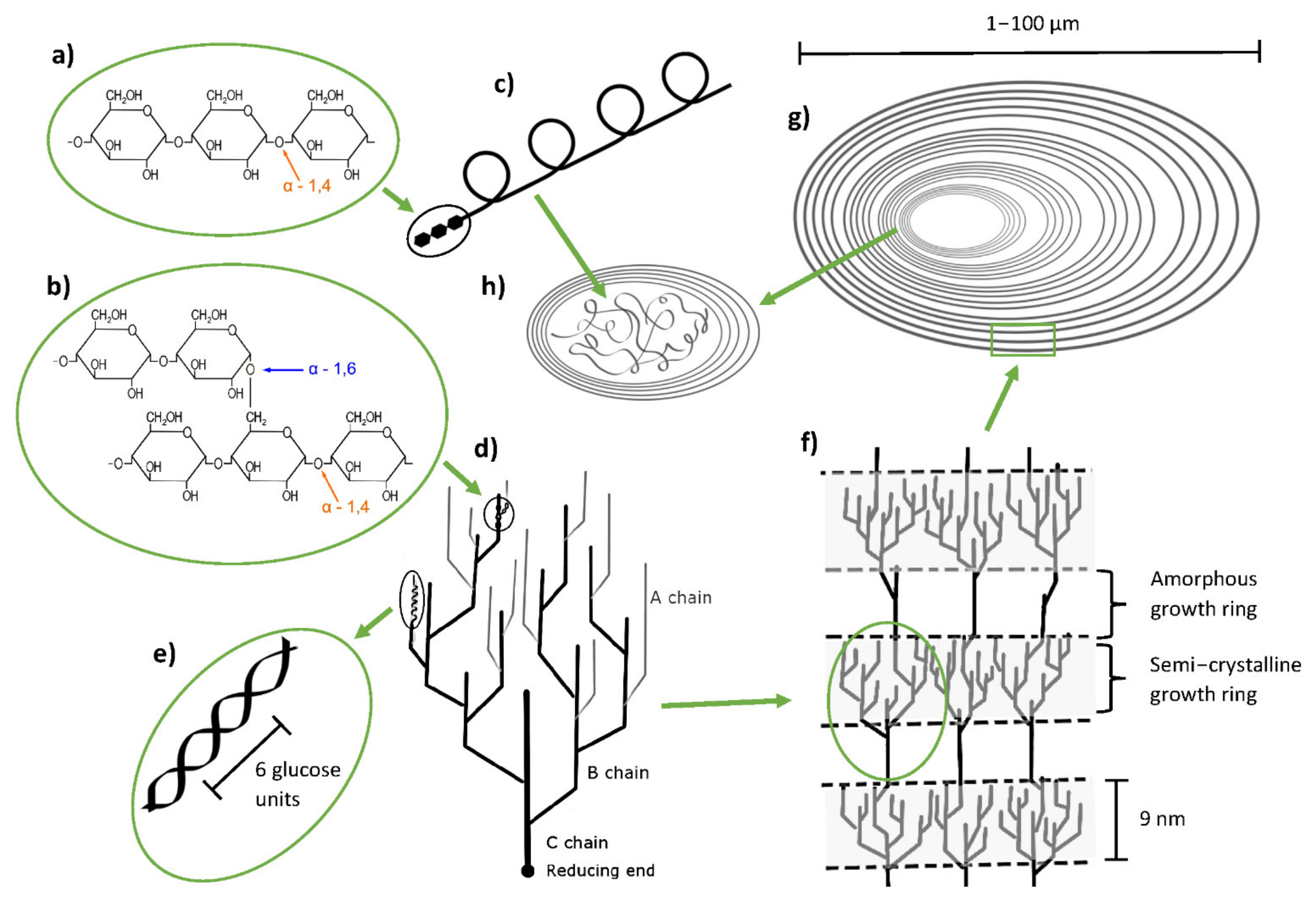
1.2. Potato Protein
2. Modifications
2.1. Physical Modification
2.1.1. Thermal Physical Modification
Heat moisture treatment (HMT) refers to the treatment of a product with heat in combination with a certain amount of moisture. Thus, the parameters for temperature, time, moisture and cycles/repetitions are crucial for this treatment method. In HMT the amount of water in the treated samples is limited to 10–35%, to prevent starch gelatinization [17]. Therefore, granular morphology of starch is preserved during HMT [18]. However, several scholars noted that HMT leads to some granular damage [18][19]. Overall, the thermal properties, such as gelatinization temperatures (onset (To), peak (Tp) and conclusion (Tc)) of potato starch significantly increased and melting enthalpy (ΔH) decreased by HMT. This increase in thermal properties was accelerated and/or intensified with increasing temperature [19], increasing holding time [20] and decreasing moisture content [21]. Shi et al. [19] linked the changes in gelatinization temperatures with increasing HMT temperatures to intramolecular interactions towards more amylose–amylose and amylose–lipid interactions at higher temperatures, which hindered the mobility of the amorphous region thus increasing the gelatinization temperature. Decreased mobility leads to decreased water uptake and swelling, which is necessary to facilitate melting of crystals and double helices. Therefore, stability of the crystalline regions of potato starch increased and higher temperatures for gelatinization were required after HMT. Accordingly, amylose content increased, degree of crystallinity decreased and type of crystallinity varied upon HMT of potato starch, using different temperatures and moisture contents [18][19][21][22]. It seems that the change in type of crystallinity of potato starch from B to C or A, which occurs at 120 °C, depends on the moisture content of the material: C-type occurred at 30% [18], but A-type at 10–27% [5][19][21] moisture content. At lower temperature (100 °C) a change from B to A-type crystallinity occurred at 24% moisture content [22].Annealing
Annealing (ANN) is another method to physically modify starch, without destroying its granular morphology. In contrast to HMT, moisture content of the samples is not limited (usually being > 60%, w/w) and temperature used during ANN is below the sample To (onset gelatinization temperature). Common processing conditions during ANN of potato starch are 30 to 55 °C for 12 h to 96 h. Here, iterations of ANN treatment cycles can be applied [23][24][25]. Overall, ANN treatment of potato starch increased gelatinization temperatures (To, Tp, Tc), had no effect on type of crystallinity and granule surface, but increased pasting properties (pasting temperature and final viscosity) [23][24][25], which were favored by higher temperatures [24] and longer treatment times/iterations [25] used for ANN treatment. Furthermore, increased hot water solubility and hot water swelling power of ANN treated potato starch granules were reported [25]. Through processing of starch in water having temperatures between 50 and 70 °C, solubility and swelling power of ANN potato starch was lower than for native potato starch [25].Microwave Treatment
Microwave (MW) treatment is a thermal heating method using microwaves (300 MHz to 300 GHz), that can be absorbed by polar materials, such as potato starch. Upon MW heating, molecular vibrations and frictions occur due to their alignment to the vibrating electromagnetic field, resulting in the generation of heat within the product. Therefore, MW heating requires less treatment time and can be more efficient and less energy consuming than conventional heating [26][27]. The ability of a product to be heated via MW treatment is dependent on moisture content, frequency, as well as compaction density and temperature of the sample [28][29][30]. Compared to native potato starch granules, MW treated potato starch granules showed damaged structure or severe cracking, which correlated to an increased water absorption capacity [31]. For potato starch with low moisture content (16.5%) decreasing gelatinization temperatures and decreasing final viscosities have been reported after MW treatment [32]. Whereas increasing To, decreasing relative crystallinity and increasing pasting properties (pasting temperature and final viscosity) have been observed for MW treated potato starch with higher moisture contents (21% and 30%) [31][33].Radio Frequency Treatment
Similar to MW heating, radio frequency (RF) are electromagnetic waves in the frequency range of 1 to 300 MHz. In comparison to MW, RF has been reported to penetrate products even more deeply and distribute heat more uniformly.Others
Other thermal modification methods including freezing and thawing (FT), flash drying, autoclaving or steam heating can be used to alter potato starch, flour and protein properties. In general, there are methods that can be used in the presence or absence of water to generate/transfer heat. FT can be applied to induce structural and functional changes in potato constituents. For instance, Zhang et al. [34] investigated the effect of FT treatment on potato starch showing disruption and aggregation of granule fragments after FT treatment. Furthermore, pasting temperature slightly increased and final viscosity of potato starch slightly decreased. Similarly, an increase in gelatinization properties (To, Tp, Tc), final viscosity, and a reduction in granule damage and gelatinization were achieved in potato flour upon flash drying (130–135 °C, 1–2 min) as compared to commercial potato flakes and granules [35]. The influence of superheated steam (100–160 °C) on potato starch properties was investigated by Hu et al. [36], showing minor effects on granule surface, compared to native starch granules. The type of crystalline structure was not affected by the superheated steam treatment, but relative crystallinity decreased progressively with increasing temperature. Furthermore, thermal properties (To, Tp, Tc and ΔH), solubility and swelling power decreased, and pasting properties increased upon superheated steam treatment [36].2.1.2. Non-Thermal Physical Modification
High Pressure Treatment
High pressure (HP) treatment (30–1000 MPa) is a non-thermal modification method, which can cause starch gelatinization and protein denaturation/gelation [37][38]. Thereby, HP can be applied in different ways, which are commonly referred to as high hydrostatic pressure/high isostatic pressure, high pressure homogenization and dynamic high pressure microfluidization. During high hydrostatic pressure/high isostatic pressure modification of starch and protein, pressure is transmitted uniformly through a pressure-transmitting medium (e.g., water) to the sample (sample-water suspensions), which is usually packed in vacuum bags and placed inside the high pressure vessel [39][40]. The high hydrostatic pressure treatment of starch in its granular form has been previously reported [41]. During high pressure homogenization a liquid sample is pressed through a small orifice causing shear forces. In dynamic high pressure microfluidization the pressure is transferred in an interaction chamber [42][43]. Similar to other modification methods, the effect of HP treatment can vary for starch from different plant sources as summarized elsewhere [44][45].Ultrasonication
During ultrasound (US) treatment, acoustic waves with a frequency > 16 kHz are transmitted through solid, liquid or gaseous systems. US can be classified into different types based on frequency and intensity [46][47]. As for the modification of potato constituents, US treatment is usually carried out in a liquid medium (usually in a water bath), with starch- or protein-based samples treated in their granular form or in the form of suspensions before or after the gelatinization/gelation process. US can be conducted at room or elevated temperatures. During US treatment cavitation occurs, which is the formation and implosion of gas bubbles that can break polymer chains by mechanical shear forces, which occur when these bubbles collapse. As a side effect, local temperature increases contributing to the modification effect [48]. For different carbohydrate and protein-based films or coatings US treatment has shown to be able to improve gelling properties and tensile properties, increase solubility and surface hydrophobicity, and reduce water vapor permeability [49][50].Ionizing Irradiation
Upon interaction with ionizing irradiation, cleavage of water molecules (in the product) into free radicals and high energy electrons occur, which can facilitate cross-linking and hydrolysis reaction of the polymer chains. The affinity of a polymer towards crosslinking and chain scission is described by the G(x) and G(s) value (quantification of the chemical yield obtained from ionizing irradiation treatment), respectively, which can highly differ among polymers. Thereby, the ratio between G(s):G(x) indicates the overall prevailing reaction (cross-linking < 1, hydrolysis > 1) which, however, depends on the irradiation dose and temperature. Accordingly, the molecular weight is either increased (cross-linking is prevailing), decreased (chain scission is prevailing) or not significantly changed (cross-linking and chain scission occur equally). For starches (including potato starch) mostly decreasing average molecular weights upon irradiation have been reported in several studies which were summarized by Bashir and Aggarwal [51], indicating the prevalence of hydrolysis [51].Others
In addition to HP, US treatment and ionizing irradiation, there are other non-thermal modification methods to change the structural and functional properties of potato constituents, such as milling and electric field treatment. Milling is commonly applied in the food industry to reduce the particle size and produce flour/powder. Ball-milling of potato starch does not only reduce the particle size of the samples but can also highly damage granule morphology, induce partial gelatinization and destroy B-type crystallinity, which is accelerated with increasing milling time [52]. Electric field treatment is the generic term for a number of different applications, including high voltage electric field (HVEF), or induced electric field (IEF). HVEF application is regarded as a method to physically modify starch properties in the absence of thermal influence, as no significant changes in product temperature occur during this treatment. In HVEF application, the sample is exposed to an electric field in liquid or gaseous medium. High voltage electric field can be classified into high electrostatic field (uniform electric field with no currents or varying voltages) and into high voltage electrical discharge (current flow causing ionizing/plasma) [53].2.2. Chemical Modification
2.2.1. Chemical Substitution
Acetylation
Acetylation refers to the chemical reaction of potato starch or potato protein with acetic anhydride [54][55][56]. Acetylation of potato starch resulted in an increase in moisture and amylose content as well as increased relative crystallinity, solubility and paste clarity. However, type of crystallinity and gelatinization temperatures of potato starch were not significantly influenced by acetylation [54].Phosphorylation
Phosphorylation of starch can be performed using different agents including phosphoryl chloride, sodium trimetaphosphate, sodium tripolyphosphate and sodium or potassium orthophosphate to obtain mono- and/or di-starch phosphates. In di-starch phosphates, orthophosphate groups can act as intramolecular or intermolecular bridges between the C2, C3 and C6 atoms of glucose units from the same or different (cross-linking) chain [57]. Phosphorylation of potato starch resulted in slightly decreased moisture content, no change in amylose content and a slight change in color, which was however not visible to the human eye. Compared to native starch, gelatinization temperatures were slightly decreased when phosphorylation was performed at 15 °C, but slightly increased when phosphorylation was performed at 45 °C. The scholars interpreted these effects as loosening and strengthening of the potato starch structure at 15 °C and 45 °C, respectively. Accordingly, an increase in viscosity was observed in potato starch pastes, which were phosphorylated at 45 °C [57].Fatty Acid Esterification
Another approach to modify potato starch by chemical substitution is the esterification of hydroxyl groups by fatty acid chains. Vanmarcke et al. [58] investigated the effect of fatty acid chain length (C8, C12 and C16) on potato starch fatty acid ester cast films. Results showed that chain length did not influence esterification reactivity and FT-IR spectra confirmed the almost complete substitution of hydroxyl groups by disappearance of the 3300 cm−1 band. However, with increasing chain length, weight of the samples increased and the X-ray diffraction peak in the low angle region shifted towards lower angles, indicating an increase in nanometer scale ordered structure based on the esterification of longer fatty acid chains. Thermographs showed that the thermal stability of potato starch increased due to fatty acid esterification, as hydrophobicity increased, which was indicated by the fact that the mass did not decrease due to water evaporation and the starch degradation shifted to higher temperatures. However, no significant differences between different chain lengths were observed. Fatty acid chain length highly influenced tensile properties of the modified starch films. With increasing chain length from C8, C12 to C16, elongation at break decreased by 150%, 133% and 13%, respectively. Tensile strength and elongation modulus showed no linear trend but highly depended on the fatty acid chain length. For C8, C12 and C16 fatty acid esterified potato starch films, the tensile strength was measured to be 3.8, 2.5 and 6.5 MPa and for the elongation modulus 68, 40 and 122 MPa was determined, respectively [58].Octenyl Succinylation
The hydrophobic group of alkenyl succinic anhydrides can be esterified with starch, to weaken internal bonding and disorder the internal structure to obtain more fluid and clear pastes. Due to its food approval at low substitution levels, the influence of octenyl succinic anhydride on starch properties is widely studied [59][60][61]. Investigating the effect of octenyl succinic anhydride modification on potato starch structure, Won et al. [61] observed an increase in surface roughness, porosity, granule size and the occurrence of cavities and slight deformation after octenyl succinic anhydride modification. However, no differences between different degrees of substitutions were observed and crystallinity of potato starch was not affected by octenyl succinic anhydride modification [61].Citric Acid Esterification
Based on its three carboxyl groups and acidity, it has been reported that citric acid can induce esterification, cross-linking and hydrolysis, when reacting under elevated temperatures with potato starch [62][63]. SEM images of citric acid esterified potato starch revealed the occurrence of some surface damage due to citric acid treatment, which was, however, not intensified using a higher citric acid concentration (up to 10%). Furthermore, amylose content of potato starch slightly in-/or decreased and moisture content and relative crystallinity decreased upon citric acid modification [62][64]. A change in type of crystallinity was not reported [62]. In addition to structural changes, citric acid esterification of potato starch also influenced different functional/physicochemical properties. Upon citric acid esterification, the solubility of potato starch decreased, whereas swelling power, water binding capacity and gelatinization temperatures increased. The reported effects of citric acid modification on potato starch pasting properties were slightly different, although pasting temperature did not appear to be significantly affected and final viscosity appeared to increase with citric acid modification compared to native potato starch [62][64].2.2.2. Chemical Cross-Linking
Cross-linking of potato starch describes the formation of intermolecular covalent bonds (between different chains) via esterification or etherification reaction with a cross-linking agent. Thereby, the cross-linking agents possess bi- or multi- functional groups, which enable the reaction on different sides with multiple chains. In the case of starch, it has been reported that cross-linking only occurs between two amylopectin molecules or between amylopectin and amylose, but not between two amylose molecules [65]. The reduction in amylose content by cross-linking reaction, as cross-linked molecules can be considered as amylopectin molecules, can (among others) be used to determine the degree of cross-linking by the starch–iodine method [65]. Cross-linking of potato starch can be performed using cross-linking agents such as acetylmalic acid chloroanhydride [66], sodium trimetaphosphate (STMP)/sodium tripolyphosphate (STPP) [67] and deep eutectic solvents [68]. According to Shulga et al. [66] cross-linking of potato starch resulted in destruction of its granular form and reduced relative crystallinity as well as reduced thermal stability. Furthermore, Heo et al. [67] reported that the increased formation of covalent bridges between starch molecules due to cross-linking hindered granular swelling and thermally induced gelatinization. In addition, pasting temperature and (final) viscosity of potato starch pastes increased upon cross-linking. For potato starch-based films, the formation of new covalent bonds through cross-linking generally strengthened the starch gel network, resulting in an increase in rheological, thermal and tensile (elongation modulus, tensile strength) properties. Solubility of potato starch-based films was not affected and water sorption degree increased upon deep eutectic solvent addition [68].2.2.3. Degradation
Acid Hydrolysis
Acid hydrolysis describes the process of chemical bond cleavage via a nucleophilic substitution reaction with water under thermal conditions and in an acidic environment. Common acids that are used in acid hydrolysis include hydrochloric acid (HCl), acetic acid, citric acid and sulfuric acid (H2SO4). Generally, hydrolysis of potato starch in water results in a decrease in molecular weight and some granular damage. Furthermore, an increase in thermal properties (gelatinization temperatures and stability), gel strength and viscosity of hydrolyzed potato starch was observed [64][69][70].Oxidation
Oxidation of potato starch can be carried out using oxidizing agents under controlled pH to oxidize hydroxyl groups into carbonyl and carboxyl groups. Common oxidizing agents used, include sodium hypochlorite, hydrogen peroxide and ozone [71]. Degree of oxidation is usually expressed as the sum of carbonyl and carboxyl content. In contrast to the use of chemical oxidizing agents, ozone can be decomposed after the oxidation reaction into oxygen by an ozone destructor. Thus, ozone oxidation is often described as a safe and environmental friendly method [71], which can also be applied to fresh potato tubers to reduce the spread of potato diseases during storage [72].2.3. Biochemical Modification
2.3.1. Enzymatic Substitution
One example of enzymatic substitution is the esterification/transesterification of fatty acids by lipases. Based on fatty acid solubility, organic solvents or ionic liquids need to be used. Dependent on the lipase used, degree of esterification and esterification site can vary [73].
2.3.2. Enzymatic Cross-Linking
Enzymatic cross-linking of potato protein can be performed in a variety of different ways including acyl-transfer reaction, oxidation/radical formation and 1,4-addition reaction. For potato protein, enzymatic cross-linking using transglutaminase, laccase, tyrosinase and peroxidase has been reported [74][75][76][77]. Of these four enzymes, transglutaminase and peroxidase showed higher quantitative impacts on potato protein properties than tyrosinase and laccase [77]. For instance, thermal stability of potato protein was increased to a higher extent using transglutaminase and peroxidase than using laccase or tyrosinase as the cross-linking enzyme. Similarly, rheological properties increased using transglutaminase and peroxidase but decreased using laccase or tyrosinase as the cross-linking enzyme [77][78][79]. The extent of enzymatic cross-linking using laccase could be enhanced using a mediator such as ferulic acid [78]. Furthermore, Glusac et al. [80] demonstrated that tyrosinase-cross-linking could improve emulsion properties of potato protein.2.3.3. Enzymatic De-/Branching
During enzymatic branching of potato starch, α-1,4-glycosidic linkages are cleaved and new α-1,6-glycosidic bonds are formed via a transglycosylation reaction using enzymes such as branching enzymes and/or transglucosidase. Therefore, polymer chain length distributions shift from longer chains towards shorter chains, indicating the occurrence of enzymatic hydrolysis. This means that starch polymers become more branched, resulting in a decrease in amylose and increase in amylopectin content [81][82]. Overall, branching of potato starch induced granular damaging, affected type of crystallinity (B → B + C or C), decreased relative crystallinity, gelatinization temperatures and shear viscosity and increased solubility [82]. The opposite happens during enzymatic debranching of potato starch, where debranching enzymes such as pullulanase [81] or isoamylase [83] can be used. Here α-1,6-glycosidic bonds are cleaved and new α-1,4-glycosidic bonds are formed. As a result, starch polymers become less branched, resulting in a decrease in amylopectin and increase in amylose content [83]. Accordingly, average molecular weight of the samples gradually decreased with increasing isoamylase concentration and granular damaging occurred, which intensified with increasing enzyme concentration. However, B-type crystallinity, thermal properties and solubility were not significantly affected upon isoamylase treatment, but gel strength and viscosity of debranched potato starch pastes decreased [83].2.3.4. Enzymatic Hydrolysis
Enzymatic hydrolysis of potato starch and potato protein refers to the enzymatic cleavage of glycosidic and peptide bonds, respectively, to decrease chain length and molecular weight and therefore, alter product properties. Different enzymes including α-amylase [84][85][86], glucoamylase [85], glycosyltransferase [85], or mixtures of different enzymes [86] can be used to hydrolyze potato starch. In general, the enzymatic hydrolysis of potato starch results in a decrease in average molecular weight, average chain length and therefore amylose content [84][85][86]. In starch granules, this was expressed by surface roughness and cracking, which increased with increasing enzyme concentration used, which in turn increased the degree of hydrolysis [84].2.4. Dual Modification
2.4.1. Physical–Physical
As demonstrated by Wang et al. [87] and Cao and Gao [88], the order in which the two different physical treatment methods are performed can also influence the resulting qualitative and quantitative properties of the dual-modified samples, compared to native samples. For instance, ANN treatment prior to HP treatment resulted in a decrease in potato starch relative crystallinity, whereas an increase was reported when physical treatments were applied vice versa [87]. In potato starch, solubility and swelling power decreased, and gel hardness increased, when treated with US prior to electric field treatment, compared to untreated native potato starch. Treating potato starch first with an electric field and then with US resulted in reverse effects meaning increase in solubility and swelling power. Interestingly, the simultaneous treatment of electric field and US resulted in similar changes as the electric field prior to US treatment, except for a decrease in swelling power and increase in gel adhesiveness [88]. Some additional general information on dual-modification of starch can be found elsewhere [89].
2.4.2. Physical-Chemical
To enhance the effect of chemical modification on product properties, several studies were performed in the last years, combining different physical treatment methods with chemical modification methods. For instance, the assistance of acetylation or octenyl succinylation of potato starch by high voltage electric field, ultrasonication, pulsed electric field or microwave treatment was reported to increase the degree of substitution compared to a single chemical modification [90][91][92][93]. Moreover, some structural, pasting, rheological, thermal and other functional properties were affected by US treatment assisted acid hydrolysis [94], dry heat and CaCl2 dual-treated [95] and annealing treated acetylated [96] potato starch samples.2.4.3. Physical–Biochemical
Among biochemical modifications, enzymatic hydrolysis is often reported to be amplified by a physical pre-treatment. For instance, enzymatic hydrolysis of potato protein was increased by US treatment [97], and the enzymatic hydrolysis of potato starch by HP [98] and HMT [99]. This can be mostly attributed to an increase in susceptibility due to increasing structural damaging and/or surface cracking. With increasing degree of hydrolysis, different sample properties can be further in-/decreased, as demonstrated by Mu et al. [98] with increasing enzyme concentration and pressure.2.4.4. Chemical–Chemical
For potato starch different dual modifications, including etherification + esterification, etherification + acid hydrolysis, and CaCl2 treatment + succinylation, have been reported [100][101][102]. One commonly applied combination is acetylation + cross-linking. In potato starch granules, this dual modification resulted in a decrease in granule size and relative crystallinity and in an increase in thermal and pasting properties. Regarding potato starch-based films, acetylation prior to cross-linking caused lower water vapor permeability, solubility, moisture sorption and relative crystallinity, while flexibility of the films was increased [103].References
- Singh, J.; Kaur, L. Chemistry, processing, and nutritional attributes of potatoes–An introduction. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 23–26. ISBN 9780128000021.
- Leonel, M.; do Carmo, E.L.; Fernandes, A.M.; Soratto, R.P.; Ebúrneo, J.A.M.; Garcia, É.L.; Dos Santos, T.P.R. Chemical composition of potato tubers: The effect of cultivars and growth conditions. J. Food Sci. Technol. 2017, 54, 2372–2378.
- Lieberei, R.; Reisdorff, C. Nutzpflanzen, 8th ed.; Thieme: Stuttgart, Germany, 2012; ISBN 9783131516381.
- Karki, D.B.; KC, Y.; Khanal, H.; Bhattarai, P.; Koirala, B.; Khatri, S.B. Analysis of biodegradable films of starch from potato waste. Asian Food Sci. J. 2020, 14, 28–40.
- Subroto, E.; Indiarto, R.; Marta, H.; Shalihah, S. Effect of heat-moisture treatment on functional and pasting properties of potato (Solanum tuberosum L. var. Granola) starch. Food Res. 2018, 3, 469–476.
- Awokoya, K.N.; Odeleye, I.E.; Muhammed, Y.A.; Ndukwe, N.A.; Ibikunle, A.A. Impact of microwave irradiation energy levels on molecular rotation, structural, physicochemical, proximate and functional properties of potato (Ipomoea batatas) starch. Ghana J. Sci. 2021, 61, 57–72.
- Van Koningsveld, G.A.; Gruppen, H.; Jongh, H.H.; de Wijngaards, G.; van Boekel, M.A.; Walstra, P.; Voragen, A.G. The solubility of potato proteins from industrial potato fruit juice as influenced by pH and various additives. J. Sci. Food Agric. 2002, 82, 134–142.
- Løkra, S.; Strætkvern, K.O. Industrial proteins from potato juice. A review. Food 2009, 3, 88–95.
- Lee, C.H. A simple outline of methods for protein isolation and purification. Endocrinol. Metab. 2017, 32, 18–22.
- Li, H.; Zeng, X.; Shi, W.; Zhang, H.; Huang, S.; Zhou, R.; Qin, X. Recovery and purification of potato proteins from potato starch wastewater by hollow fiber separation membrane integrated process. Innov. Food Sci. Emerg. Technol. 2020, 63, 102380.
- Knorr, D.; Kohler, G.O.; Betschart, A.A. Potato protein concentrates: The influence of various methods of recovery upon yield, compositional and functional characteristics. J. Food Process. Preserv. 1977, 1, 235–247.
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382.
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA J. Food 2018, 16, 1003–1017.
- Bertoft, E.; Blennow, A. Structure of Potato Starch. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–73. ISBN 9780128000021.
- Raigond, P.; Singh, B.; Dutt, S.; Chakrabarti, S.K. Potato; Springer: Singapore, 2020; ISBN 978-981-15-7661-4.
- Waglay, A.; Karboune, S. Potato proteins: Functional food ingredients. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 75–104. ISBN 9780128000021.
- Fonseca, L.M.; Halal, S.L.M.E.; Dias, A.R.G.; Zavareze, E.D.R. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohyd. Polym. 2021, 274, 118665.
- Zhang, B.; Saleh, A.S.M.; Su, C.; Gong, B.; Zhao, K.; Zhang, G.; Li, W.; Yan, W. The molecular structure, morphology, and physicochemical property and digestibility of potato starch after repeated and continuous heat-moisture treatment. J. Food Sci. 2020, 85, 4215–4224.
- Shi, M.; Gao, Q.; Liu, Y. Corn, potato, and wrinkled pea starches with heat-moisture treatment: Structure and digestibility. Cereal Chem. 2018, 95, 603–614.
- Lin, C.-L.; Lin, J.-H.; Lin, J.-J.; Chang, Y.-H. Progressive alterations in crystalline structure of starches during heat-moisture treatment with varying iterations and holding times. Int. J. Biol. Macromol. 2019, 135, 472–480.
- Oliveira, C.S.; de Bet, C.D.; Bisinella, R.Z.B.; Waiga, L.H.; Colman, T.A.D.; Schnitzler, E. Heat-moisture treatment (HMT) on blends from potato starch (PS) and sweet potato starch (SPS). J. Therm. Anal. Calorim. 2018, 133, 1491–1498.
- Bartz, J.; da Rosa Zavareze, E.; Dias, A.R.G. Study of heat-moisture treatment of potato starch granules by chemical surface gelatinization. J. Sci. Food Agric. 2017, 97, 3114–3123.
- Chen, X.; Luo, J.; Liang, Z.; Zhu, J.; Li, L.; Wang, Q. Structural and physicochemical/digestion characteristics of potato starch-amino acid complexes prepared under hydrothermal conditions. Int. J. Biol. Macromol. 2020, 145, 1091–1098.
- Wang, S.; Wang, J.; Wang, S.; Wang, S. Annealing improves paste viscosity and stability of starch. Food Hydrocoll. 2017, 62, 203–211.
- Xu, M.; Saleh, A.S.M.; Gong, B.; Li, B.; Jing, L.; Gou, M.; Jiang, H.; Li, W. The effect of repeated versus continuous annealing on structural, physicochemical, and digestive properties of potato starch. Food Res. Int. 2018, 111, 324–333.
- Zhu, Z.; Guo, W. Frequency, moisture content, and temperature dependent dielectric properties of potato starch related to drying with radio-frequency/microwave energy. Sci. Rep. 2017, 7, 9311.
- Shen, G.; Zhang, L.; Hu, T.; Li, Z.; Chen, A.; Zhang, Z.; Wu, H.; Li, S.; Hou, X. Preparation of potato flour by freeze-thaw pretreatment: Effect of different thawing methods on hot-air drying process and physicochemical properties. LWT 2020, 133, 110157.
- Ozturk, S.; Kong, F.; Trabelsi, S.; Singh, R.K. Dielectric properties of dried vegetable powders and their temperature profile during radio frequency heating. J. Food Eng. 2016, 169, 91–100.
- Fan, D.; Wang, L.; Shen, H.; Huang, L.; Zhao, J.; Zhang, H. Ultrastructure of potato starch granules as affected by microwave treatment. Int. J. Food Prop. 2017, 20, S3189–S3194.
- Zhu, H.; Li, D.; Li, S.; Wang, S. A novel method to improve heating uniformity in mid-high moisture potato starch with radio frequency assisted treatment. J. Food Eng. 2017, 206, 23–36.
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of microwave treatment (low power and varying time) on potato starch: Microstructure, thermo-functional, pasting and rheological properties. Int. J. Biol. Macromol. 2020, 155, 27–35.
- Przetaczek-Rożnowska, I.; Fortuna, T.; Wodniak, M.; Łabanowska, M.; Pająk, P.; Królikowska, K. Properties of potato starch treated with microwave radiation and enriched with mineral additives. Int. J. Biol. Macromol. 2019, 124, 229–234.
- Xu, X.; Chen, Y.; Luo, Z.; Lu, X. Different variations in structures of A- and B-type starches subjected to microwave treatment and their relationships with digestibility. LWT 2019, 99, 179–187.
- Zhang, C.; Lim, S.-T.; Chung, H.-J. Physical modification of potato starch using mild heating and freezing with minor addition of gums. Food Hydrocoll. 2019, 94, 294–303.
- Li, J.; Shen, C.; Ge, B.; Wang, L.; Wang, R.; Luo, X.; Chen, Z. Preparation and application of potato flour with low gelatinization degree using flash drying. Dry. Technol. 2018, 36, 374–382.
- Hu, X.; Guo, B.; Liu, C.; Yan, X.; Chen, J.; Luo, S.; Liu, Y.; Wang, H.; Yang, R.; Zhong, Y.; et al. Modification of potato starch by using superheated steam. Carbohyd. Polym. 2018, 198, 375–384.
- Liu, Y.; Chao, C.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. New insights into starch gelatinization by high pressure: Comparison with heat-gelatinization. Food Chem. 2020, 318, 126493.
- Kunugi, S.; Tanaka, N. Cold denaturation of proteins under high pressure. Biochim. Biophys. Acta 2002, 1595, 329–344.
- Wang, J.; Zhu, H.; Li, S.; Wang, S.; Wang, S.; Copeland, L. Insights into structure and function of high pressure-modified starches with different crystalline polymorphs. Int. J. Biol. Macromol. 2017, 102, 414–424.
- Baier, A.K.; Knorr, D. Influence of high isostatic pressure on structural and functional characteristics of potato protein. Food Res. Int. 2015, 77, 753–761.
- Słomińska, L.; Zielonka, R.; Jarosławski, L.; Krupska, A.; Szlaferek, A.; Kowalski, W.; Tomaszewska-Gras, J.; Nowicki, M. High pressure impact on changes in potato starch granules. Pol. J. Chem. Technol. 2015, 17, 65–73.
- Dos Santos Aguilar, J.G.; Cristianini, M.; Sato, H.H. Modification of enzymes by use of high-pressure homogenization. Food Res. Int. 2018, 109, 120–125.
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234.
- Castro, L.M.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Impact of high pressure on starch properties: A review. Food Hydrocoll. 2020, 106, 105877.
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Impact of pressure on physicochemical properties of starch dispersions. Food Hydrocoll. 2017, 68, 164–177.
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154.
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207.
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505.
- Wang, X.; Majzoobi, M.; Farahnaky, A. Ultrasound-assisted modification of functional properties and biological activity of biopolymers: A review. Ultrason. Sonochem. 2020, 65, 105057.
- Brodnjak, U.V. Influence of ultrasonic treatment on properties of bio-based coated paper. Prog. Org. Coat. 2017, 103, 93–100.
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523.
- Fu, Z.; Wu, M.; Zhang, H.; Wang, J. Retrogradation of partially gelatinised potato starch prepared by ball milling. Int. J. Food Sci. Technol. 2018, 53, 1065–1071.
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakis, E. The principles of high voltage electric field and its application in food processing: A review. Food Res. Int. 2016, 89, 48–62.
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Influence of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J. Food Eng. 2012, 108, 320–326.
- Zięba, T.; Kapelko, M.; Szumny, A. Effect of preparation method on the properties of potato starch acetates with an equal degree of substitution. Carbohyd. Polym. 2013, 94, 193–198.
- Miedzianka, J.; Pęksa, A.; Aniołowska, M. Properties of acetylated potato protein preparations. Food Chem. 2012, 133, 1283–1291.
- Rożnowski, J.; Juszczak, L.; Szwaja, B.; Przetaczek-Rożnowska, I. Effect of esterification conditions on the physicochemical properties of phosphorylated potato starch. Polymers 2021, 13, 2548.
- Vanmarcke, A.; Leroy, L.; Stoclet, G.; Duchatel-Crépy, L.; Lefebvre, J.-M.; Joly, N.; Gaucher, V. Influence of fatty chain length and starch composition on structure and properties of fully substituted fatty acid starch esters. Carbohyd. Polym. 2017, 164, 249–257.
- Wang, C.; Tang, C.-H.; Fu, X.; Huang, Q.; Zhang, B. Granular size of potato starch affects structural properties, octenylsuccinic anhydride modification and flowability. Food Chem. 2016, 212, 453–459.
- Won, C.; Jin, Y.I.; Chang, D.-C.; Kim, M.; Lee, Y.; Gansean, P.; Lee, Y.-K.; Chang, Y.H. Rheological, pasting, thermal and retrogradation properties of octenyl succinic anhydride modified potato starch. Food Sci. Technol. 2017, 37, 321–327.
- Won, C.; Jin, Y.; Kim, M.; Lee, Y.; Chang, Y.H. Structural and rheological properties of potato starch affected by degree of substitution by octenyl succinic anhydride. Int. J. Food Prop. 2017, 20, 3076–3089.
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohyd. Polym. 2018, 202, 29–38.
- Van Hung, P.; Vien, N.L.; Lan Phi, N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73.
- Martins, P.C.; Gutkoski, L.C.; Martins, V.G. Impact of acid hydrolysis and esterification process in rice and potato starch properties. Int. J. Biol. Macromol. 2018, 120, 959–965.
- Kou, T.; Gao, Q. New insight in crosslinking degree determination for crosslinked starch. Carbohyd. Res. 2018, 458–459, 13–18.
- Shulga, O.; Simurova, N.; Shulga, S.; Smirnova, J. Modification of potato starch by acetylmalic acid chloroanhydride and physicochemical research of the new product. Int. J. Polym. Sci. 2018, 2018, 1–7.
- Heo, H.; Lee, Y.-K.; Chang, Y.H. Rheaological, pasting, and structural properties of potato starch by cross-linking. Int. J. Food Prop. 2017, 2, 1–13.
- Zdanowicz, M.; Johansson, C. Mechanical and barrier properties of starch-based films plasticized with two- or three component deep eutectic solvents. Carbohyd. Polym. 2016, 151, 103–112.
- Celikci, N.; Dolaz, M.; Colakoglu, A.S. An environmentally friendly carton adhesive from acidic hydrolysis of waste potato starch. Int. J. Polym. Anal. Charact. 2021, 26, 97–110.
- Sun, B.; Tian, Y.; Wei, B.; Chen, L.; Bi, Y.; Jin, Z. Effect of reaction solvents on the multi-scale structure of potato starch during acid treatment. Int. J. Biol. Macromol. 2017, 97, 67–75.
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559.
- Hutla, P.; Kolaříková, M.; Hájek, D.; Doležal, P.; Hausvater, E.; Petráčková, B. Ozone treatment of stored potato tubers. Agron. Res. 2020, 18, 100–112.
- Boruczkowska, H.; Boruczkowski, T.; Gubica, T.; Anioł, M.; Tomaszewska-Ciosk, E. Analysis of the chemical structure of insoluble products of enzymatic esterification of starch and transesterification of acetylated starch with oleic acid by solid-state CP/MAS 13 C NMR. Starch-Stärke 2016, 68, 1180–1186.
- Li, M.; Karboune, S.; Light, K.; Kermasha, S. Oxidative cross-linking of potato proteins by fungal laccases: Reaction kinetics and effects on the structural and functional properties. Innov. Food Sci. Emerg. Technol. 2021, 71, 102723.
- Isaschar-Ovdat, S.; Fishman, A. Crosslinking of food proteins mediated by oxidative enzymes—A review. Trends Food Sci. Technol. 2018, 72, 134–143.
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A.; Kukavica, B. Partial characterization of bean and maize root peroxidases and their ability to crosslink potato protein. Arch. Biol. Sci. 2019, 71, 293–303.
- Gui, Y.; Li, J.; Zhu, Y.; Guo, L. Roles of four enzyme crosslinks on structural, thermal and gel properties of potato proteins. LWT 2020, 123, 109116.
- Li, M.; Blecker, C.; Karboune, S. Molecular and air-water interfacial properties of potato protein upon modification via laccase-catalyzed cross-linking and conjugation with sugar beet pectin. Food Hydrocoll. 2021, 112, 106236.
- Zhu, Y.; Tao, H.; Janaswamy, S.; Zou, F.; Cui, B.; Guo, L. The functionality of laccase- or peroxidase-treated potato flour: Role of interactions between protein and protein/starch. Food Chem. 2021, 341, 128082.
- Glusac, J.; Isaschar-Ovdat, S.; Kukavica, B.; Fishman, A. Oil-in-water emulsions stabilized by tyrosinase-crosslinked potato protein. Food Res. Int. 2017, 100, 407–415.
- Wang, R.; Liu, P.; Cui, B.; Kang, X.; Yu, B.; Qiu, L.; Sun, C. Effects of pullulanase debranching on the properties of potato starch-lauric acid complex and potato starch-based film. Int. J. Biol. Macromol. 2020, 156, 1330–1336.
- Guo, L.; Deng, Y.; Lu, L.; Zou, F.; Cui, B. Synergistic effects of branching enzyme and transglucosidase on the modification of potato starch granules. Int. J. Biol. Macromol. 2019, 130, 499–507.
- Ulbrich, M.; Asiri, S.A.; Bussert, R.; Flöter, E. Enzymatic modification of granular potato starch using isoamylase—Investigation of morphological, physicochemical, molecular, and techno-functional properties. Starch-Stärke 2021, 73, 2000080.
- Asiri, S.A.; Ulbrich, M.; Flöter, E. Partial hydrolysis of granular potato starch using α-amylase—Effect on physicochemical, molecular, and functional properties. Starch-Stärke 2019, 71, 1800253.
- Guo, L.; Li, J.; Li, H.; Zhu, Y.; Cui, B. The structure property and adsorption capacity of new enzyme-treated potato and sweet potato starches. Int. J. Biol. Macromol. 2020, 144, 863–873.
- Vafina, A.; Proskurina, V.; Vorobiev, V.; Evtugin, V.G.; Egkova, G.; Nikitina, E. Physicochemical and morphological characterization of potato starch modified by bacterial amylases for food industry applications. J. Chem. 2018, 2018, 1627540.
- Wang, S.; Guo, P.; Xiang, F.; Wang, J.; Yu, J.; Wang, S. Effect of dual modification by annealing and ultrahigh pressure on properties of starches with different polymorphs. Carbohyd. Polym. 2017, 174, 549–557.
- Cao, M.; Gao, Q. Effect of dual modification with ultrasonic and electric field on potato starch. Int. J. Biol. Macromol. 2020, 150, 637–643.
- Ashogbon, A.O. Dual modification of various starches: Synthesis, properties and applications. Food Chem. 2021, 342, 128325.
- Cao, M.; Gao, Q. Internal structure of high degree substitution acetylated potato starch by chemical surface gelatinization. Int. J. Biol. Macromol. 2020, 145, 133–140.
- Chen, B.-R.; Wen, Q.-H.; Zeng, X.-A.; Abdul, R.; Roobab, U.; Xu, F.-Y. Pulsed electric field assisted modification of octenyl succinylated potato starch and its influence on pasting properties. Carbohyd. Polym. 2021, 254, 117294.
- Hong, J.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Effect of pulsed electric fields treatment on the nanostructure of esterified potato starch and their potential glycemic digestibility. Innov. Food Sci. Emerg. Technol. 2018, 45, 438–446.
- Zhao, K.; Li, B.; Xu, M.; Jing, L.; Gou, M.; Yu, Z.; Zheng, J.; Li, W. Microwave pretreated esterification improved the substitution degree, structural and physicochemical properties of potato starch esters. LWT 2018, 90, 116–123.
- Ulbrich, M.; Bai, Y.; Flöter, E. The supporting effect of ultrasound on the acid hydrolysis of granular potato starch. Carbohyd. Polym. 2020, 230, 115633.
- Zhang, H.; He, F.; Wang, T.; Chen, G. Thermal, pasting, and rheological properties of potato starch dual-treated with CaCl2 and dry heat. LWT 2021, 146, 111467.
- Zięba, T.; Wilczak, A.; Kobryń, J.; Musiał, W.; Kapelko-Żeberska, M.; Gryszkin, A.; Meisel, M. The annealing of acetylated potato starch with various substitution degrees. Molecules 2021, 26, 2096.
- Cheng, Y.; Liu, Y.; Wu, J.; Ofori Donkor, P.; Li, T.; Ma, H. Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: Thermodynamics and kinetics. Ultrason. Sonochem. 2017, 37, 351–359.
- Mu, T.-H.; Zhang, M.; Raad, L.; Sun, H.-N.; Wang, C. Effect of α-amylase degradation on physicochemical properties of pre-high hydrostatic pressure-treated potato starch. PLoS ONE 2015, 10, e0143620.
- Qi, X.; Tester, R.F. Heat and moisture modification of native starch granules on susceptibility to amylase hydrolysis. Starch-Stärke 2016, 68, 816–820.
- Wang, J.; Ren, F.; Huang, H.; Wang, Y.; Copeland, L.; Wang, S.; Wang, S. Effect of CaCl2 pre-treatment on the succinylation of potato starch. Food Chem. 2019, 288, 291–296.
- Li, L.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Synergistic effect of sodium dodecyl sulfate and salts on the gelation properties of acid-hydrolyzed-hydroxypropylated potato starch. LWT 2018, 93, 556–562.
- He, X.; Gong, X.; Li, W.; Cao, W.; Yan, J.; Guo, R.; Niu, B.; Jia, L. Preparation and characterization of amphiphilic composites made with double-modified (etherified and esterified) potato starches. Starch-Stärke 2019, 71, 1900089.
- González-Soto, R.A.; Núñez-Santiago, M.C.; Bello-Pérez, L.A. Preparation and partial characterization of films made with dual-modified (acetylation and crosslinking) potato starch. J. Sci. Food Agric. 2019, 99, 3134–3141.
