Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Horacio Osorio | -- | 2337 | 2022-12-13 16:34:48 | | | |
| 2 | Peter Tang | Meta information modification | 2337 | 2022-12-14 07:11:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sánchez-Gloria, J.L.; Rada, K.M.; Juárez-Rojas, J.G.; Sánchez-Lozada, L.G.; Sánchez-Muñoz, F.; Osorio-Alonso, H. Sulfur Compounds in Garlic for Asthma Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/38708 (accessed on 04 March 2026).
Sánchez-Gloria JL, Rada KM, Juárez-Rojas JG, Sánchez-Lozada LG, Sánchez-Muñoz F, Osorio-Alonso H. Sulfur Compounds in Garlic for Asthma Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/38708. Accessed March 04, 2026.
Sánchez-Gloria, José L., Karla M. Rada, Juan G. Juárez-Rojas, Laura G. Sánchez-Lozada, Fausto Sánchez-Muñoz, Horacio Osorio-Alonso. "Sulfur Compounds in Garlic for Asthma Treatment" Encyclopedia, https://encyclopedia.pub/entry/38708 (accessed March 04, 2026).
Sánchez-Gloria, J.L., Rada, K.M., Juárez-Rojas, J.G., Sánchez-Lozada, L.G., Sánchez-Muñoz, F., & Osorio-Alonso, H. (2022, December 13). Sulfur Compounds in Garlic for Asthma Treatment. In Encyclopedia. https://encyclopedia.pub/entry/38708
Sánchez-Gloria, José L., et al. "Sulfur Compounds in Garlic for Asthma Treatment." Encyclopedia. Web. 13 December, 2022.
Copy Citation
Asthma is a chronic inflammatory disease in the airways with a multifactorial origin but with inflammation and oxidative stress as related pathogenic mechanisms. Garlic (Allium sativum) is a nutraceutical with different biological properties due to sulfur-containing natural compounds. Studies have shown that several compounds in garlic may have beneficial effects on cardiovascular diseases, including those related to the lungs. Therefore, it is possible to take advantage of the compounds from garlic as nutraceuticals for treating lung diseases.
asthma
inflammation
oxidative stress
garlic
sulfur compounds
1. Introduction
The lungs are exposed to the environment and therefore are vulnerable to potentially harmful factors (pathogens, chemicals, etc.) that result in pathological processes, such as neoplasms, infections, and inflammatory/allergic diseases [1]. According to the World Health Organization (WHO), chronic respiratory diseases (CRD) have a heavy economic burden on countries and an adverse impact on national economic development [2]. CRD includes asthma, respiratory allergies, chronic obstructive pulmonary disease (COPD), occupational lung diseases, sleep apnea syndrome, and pulmonary hypertension. It has been reported that more than 300 million people worldwide have asthma (according to the 2016 Global Burden of Disease Study) [3].
Asthma is among the most common CRD, affecting all age groups. Asthma has a multifactorial origin, but its pathophysiology involves the participation and presence of inflammatory mechanisms (an eosinophil-rich airway) and oxidative stress that induce airflow obstruction, mucus hypersecretion, and airway remodeling [4]. In asthma, chronic inflammation leads to an increase of reactive oxygen species (ROS), which translates into remodeling processes that damage the normal architecture of the airways, weakening lung function and, consequently, a worse clinical prognosis [4] (Figure 1). In addition, studies have reported that oxidative stress may be central to the development (cause) and progression (consequence) of airway inflammation. On the other hand, several investigations have shown that supplementation with nutraceuticals with antioxidant properties (e.g., curcumin, zinc, selenium, vitamin D) has a positive effect on the reduction of airway inflammation [5]. Furthermore, nutraceuticals with antioxidant and anti-inflammatory properties could be beneficial in the progression of asthma, as preventive, protective, or therapeutic alternatives.
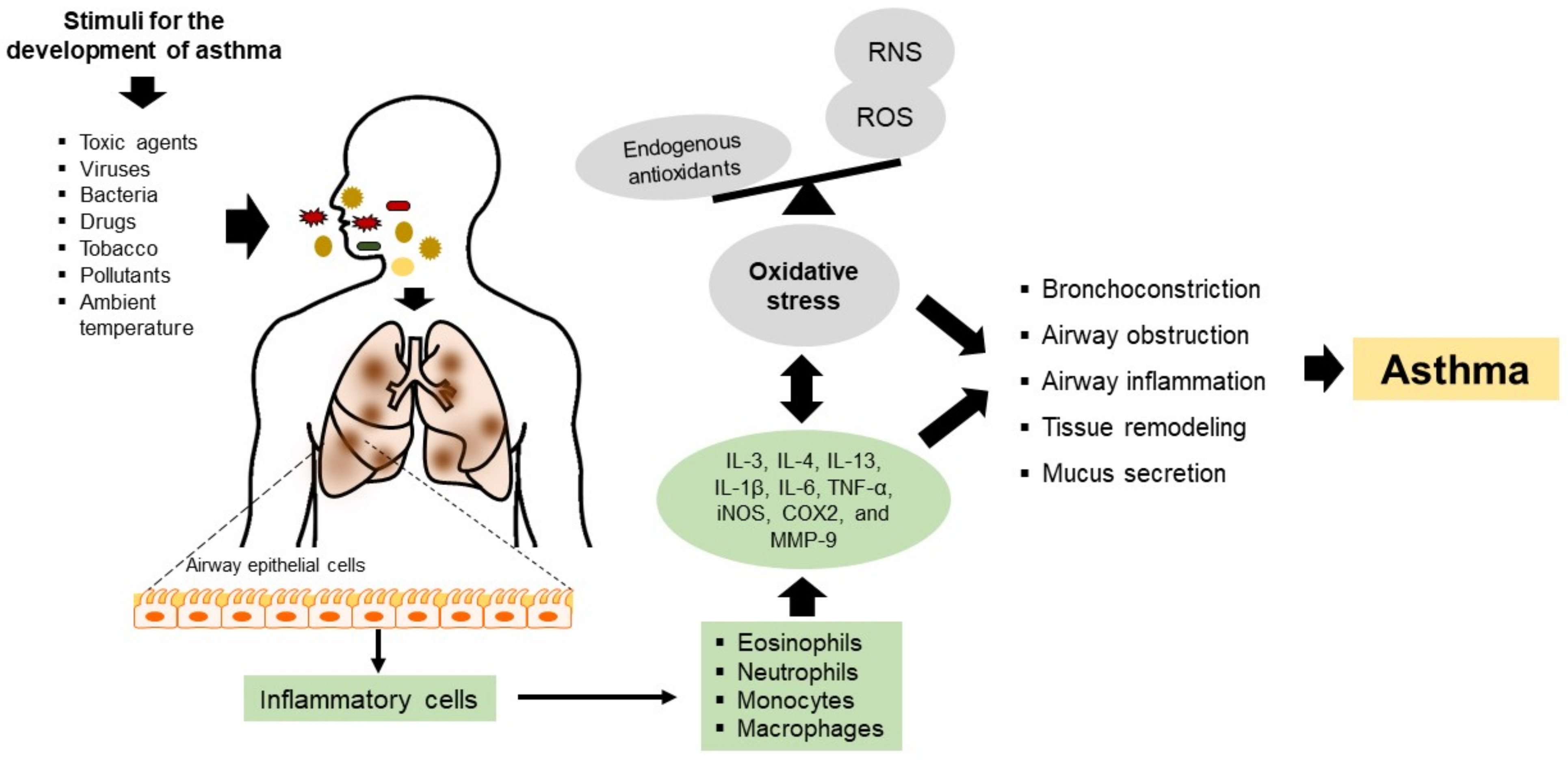
Figure 1. Pathophysiology mechanisms of asthma. Toxic agents, viruses, bacteria, drugs, tobacco, pollutants, and ambient temperature damage airway epithelial cells, inducing the recruitment of inflammatory cells such as eosinophils, neutrophils, monocytes, and macrophages. The inflammatory cells produce cytokines, chemokines, and substances that contribute to local and systemic inflammatory events that aggravate and cause inflammation and oxidative stress in the airways. Thus, chronic inflammation in asthma is accompanied by increased ROS production; however, this imbalance may also contribute to the induction of airway inflammation. Oxidative stress can cause or be a consequence of asthma and even exacerbate this condition. Th2: T helper cells type 2; IL: interleukin 1β, 3, 4, 6, and 13; TNF-α: tumor necrosis factor-alpha; ROS: reactive oxygen species; RNS: reactive nitrogen species; MMP-9: matrix metalloproteinase-9; iNOS: inducible nitric oxide synthase; and COX2: cyclooxygenase 2.
Currently, asthma is treated with corticosteroids, and patients can also require pharmacological co-treatment. However, corticosteroids cause several adverse effects limiting their application, and patients frequently worsen despite treatments [6]. Therefore, finding more effective therapeutic alternatives with no side effects or toxic properties is necessary.
2. Association of Oxidative Stress and Inflammation on Asthma
The pathogenesis of asthma involves chronic airway inflammation triggered by allergen agents, ambient temperature, tobacco, bacteria, viruses, respiratory infections, or mechanical stimuli such as exercise. Cells mediating the inflammatory response, such as eosinophils, neutrophils, monocytes, and macrophages, generate ROS and reactive nitrogen species (RNS), contributing to oxidative stress and aggravating the detrimental effects of airway inflammation (Figure 1). On the other hand, oxidative stress in asthma may also be attributed to viral or bacterial infections and is even considered a factor that can exacerbate this pathology [7]. Such a situation was demonstrated in patients with asthma, where a considerable percentage of viral and bacterial co-infections were observed, and the predominant viruses were rhinoviruses, respiratory syncytial virus, influenza virus, and metapneumovirus. At the same time, the major species of bacteria present were Streptococcus pneumoniae and Haemophilus influenzae. In both cases, the patients had lower exhaled nitric oxide (NO) levels, lower immunoglobulin E (IgE) titers, and a higher incidence of comorbid sinusitis, COPD, or pneumonia [8]. These findings have also been related to an increased risk of hospital readmission. This phenomenon is possible because these allergens regulate the toll-like receptor (TLR) and intracellular adhesion molecule-1 (ICAM-1) pathways, causing an increase in neutrophil degranulation and cell lysis, thus exacerbating the clinical severity of the pathology [9].
Airway inflammation is an essential feature of this pathology where the nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) regulates several genes involved in the immune and inflammatory responses. Here, NF-κB is translocated to the nucleus due to the degradation of inhibitory kappa B (IκB), where NF-κB binds to the promoter region of proinflammatory mediators, including inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2) and matrix metallopeptidase-9 (MMP-9) [10]. Thus, the over-expression of iNOS and COX2 increases NO synthesis, which aggravates the inflammatory response. Chronic inflammation and oxidative stress results in cell proliferation, apoptosis of respiratory epithelium, bronchoconstriction, an increase in mucus secretion and airway remodeling, and, finally, irreversible airflow limitations (Figure 1) [11].
In allergic asthma, exposure to allergens causes an imbalance between the T helper type 1 (Th1) and Th2 cells. The activation of Th2 cells is related to the inflammatory response, leading to tracheal hypersensitivity owing to cytokine release, including interleukin 13 (IL-13), IL-5, and IL-4 [12].
3. Garlic
Nowadays, it is indisputable that dietary interventions play a key role in the survival and maintenance of health, as well as in managing diseases, including cardiovascular disease, diabetes mellitus, metabolic syndrome, and cancer [13]. Several studies have reported that nutraceuticals (foods or parts of them) have antioxidant and anti-inflammatory properties that can protect, prevent, or improve chronic diseases such as the lung, including asthma [14]. Garlic is a nutraceutical with health benefits due mainly to the organic sulfur compounds derived from cysteine contained in it [15]. Intact garlic contains non-volatile (non-odiferous and stable) γ-glutamyl-S-alk(en)yl-l-cysteines, namely, γ-glutamyl-S-allyl-l-cysteine, γ-glutamyl-S-trans-1-propenyl-l-cysteine, and S-alk(en)yl-l-cysteine sulfoxides such as S-allyl-l-cysteine sulfoxide (alliin), S-(trans-1-propenyl)-l-cysteine sulfoxide (isoalliin), and S-methyl-l-cysteine sulfoxide (methiin) with a small amount of S-allyl cysteine (SAC). When garlic cloves are crushed or chopped, the enzyme alliinase stored in the vacuoles is released, which encounters cytosolic alliin to convert it into a series of thiosulfinates, most notably allicin (diallyl thiosulfinate). The highly reactive, unstable, and volatile allicin breaks down to produce a large number of sulfides, which are oil-soluble compounds responsible for garlic’s characteristic odor and taste. These sulfides correspond to diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), methyl allyl disulfide (MADS), methyl allyl sulfide (MAS), ajoene, and vinyl dithiins (2-vinyl-1,3-dithiin, 3-vinyl-1,2-dithiin). The water-soluble garlic compounds are SAC, S-allylmercapto-l-cysteine (SAMC), and S-methyl cysteine [16].
4. Effect of Garlic Compounds on Asthma
A study investigated the effects of intraperitoneal injection of aged garlic extract (AGE) on established allergic airway inflammation in a murine model (BALB/c mice) [17]. The injection of AGE caused a decrease in the allergic airway inflammation, including eosinophil percentage in bronchoalveolar lavage fluid (BALF), immunoglobulin G1 (IgG1) levels in BALF and serum, the proportion of mucous-producing goblet cells, and peribronchial and perivascular inflammation. It also increased BALF’s interferon-gamma (IFN-γ) levels. The results suggested that AGE could attenuate inflammatory features of allergic airway inflammation.
Shin et al. investigated the effects of DADS on airway inflammation using a mouse model of ovalbumin-induced asthma [18]. In this study, DADS suppressed the expression of iNOS, COX2, and MMP-9, which decreased NF-κB activation, thus inhibiting the production of inflammatory markers (IL-1β and IL-6) in experiments in vitro and in vivo. The suppressed expression of MMP-9 by DADS treatment caused a reduction in IL-4, IL-5, IL-13, and IgE in the lung tissue of rats with asthma. In addition, ovalbumin-induced asthma decreased the expression of IFN-γ levels, while DADS treatment significantly increased the expression of IFN-γ and the expression of antioxidant proteins, such as nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and hemeoxygenase-1 (HO-1) in experiments in vivo and in vitro, leading to reduced ROS production [18]. Thus, the results showed that DADS decreases the inflammatory response by enhancing the antioxidant status induced by Nrf2 activation.
Other studies investigated the effects of DAS orally administered on ovalbumin-induced pulmonary inflammation of asthma mice [19]. In this study, DAS decreased airway inflammation, mucus secretion, and oxidative damage in the lung of asthma mice. In asthmatic responses, Nrf2 disruption causes an increase in the levels of Th2 cytokines IL-4 and IL-13. DAS administration elevated the Nrf2 translocation from the cytosol to the nucleus in the lung cells and consequently decreased the inflammatory state. Specifically, DAS reduced the number of eosinophils in BALF, preventing inflammatory cell infiltration and the generation of Th2 cytokines (IL-4 and IL-10) via Nrf2. Furthermore, DAS decreased the expression of 8-Hydroxy-2’-deoxyguanosine (8-OHdG) and 8-isoprostane, two biomarkers of oxidative damage, suggesting that DAS reduced ROS generation and prevented oxidant-induced damage in the lung [19]. In addition, DAS treatment downregulated the expression of microRNAs (miRNAs) such as miR-144, miR-34a, and miR-34b/c, which play a role in oxidant and inflammatory activities [20][21].
Inhalant allergens such as dust or house mites are considered the most important source of allergens worldwide. Dust mites, particularly Dermatophagoides pteronyssinus (Der p), constitute one of the most critical risk factors for allergic respiratory diseases in patients with a genetic predisposition. In this context, the oral administration of the water-soluble fraction of garlic (collected in Taichung City, Taiwan) on Der p-induced allergic airway inflammation in mice was evaluated [22]. The total inflammatory cells determined in the lung of asthmatic mice were increased by Der p; however, garlic treatment inhibited the total cell counts and inflammatory cell infiltration (eosinophils and lymphocytes) around perivascular space. These results are supported by the reduction of Th2 cytokines by garlic treatment, an important cytokine that regulates the secretion of IgE. In contrast, the garlic extract fraction was found to increase IFN-γ levels. These results indicate that garlic reduces airway inflammation by decreasing Th2 cytokines and increasing Th1 cytokines. In addition, IgE is related to the NF-κB activation [23]. The reduction of IgE by the garlic fraction treatment also inhibited the phosphorylation of NF-κB and the decrement in the expression of IL-13 and IL-4. Garlic extract administration modulated the anti-inflammatory response by inhibiting the IL-6/PI3K/Akt/NF-κB pathway.
Hsieh et al. also reported that garlic extract (collected in Taichung City, Taiwan) corrected the imbalance of Th1 and Th2 cells in BALB/c mice with Der p-induced asthma. Again, Th2 cytokines (IL-4, IL-5, and IL-13) decreased with garlic administration, causing a reduction in the stimulation of mucus secretion from epithelial cells in the airways, decreasing the expression of vascular endothelial cell adhesion molecules (VCAM) and inhibiting IgE production. On the other hand, it increased IFN-γ and IL-12 and restored the expression of IL-10 in BALF [22]. SAC is another compound in garlic that has been reported to have beneficial effects on the ovalbumin-induced asthma model [15]. In this study, SAC attenuated airway hyperresponsiveness and inflammatory cell infiltration by significantly reducing inflammatory cell counts. In asthma, goblet cells show an increase in mucin 5AC (MUC5AC), a major component of airway mucus, caused by an increase in Th2 cytokines. Mucus production from goblet cells is triggered by an increase in Th2 cytokines and increased activity of molecules in the inflammatory signaling pathway, such as NF-κB [24]. In addition, the oral administration of SAC decreased Th2 cytokines and IgE levels in BALF and serum. Finally, SAC administration inhibited NF-κB translocation to the nucleus and, thus, the transcription of inflammatory proteins, resulting in reduced airway hyperresponsiveness and MUC5AC.
Other studies have reported that oral administration of SAC has beneficial effects in neonatal asthmatic rats (in an ovalbumin-induced asthmatic animal model) [25]. SAC administration decreased the inflammation and the infiltration of eosinophils, lymphocytes, mast cells, and monocytes, as well as the number of goblet cells in the airway. In addition, SAC decreased smooth muscle mass, mucous gland hypertrophy, and vascular congestion in the asthmatic model. In this study, the administration of SAC decreased the expression of fibrinogen, prothrombin, and thrombin time; these parameters are related to the degree of inflammation in asthma. Moreover, SAC administration decreased the expression of TNF-α, IL-1β, IL-6, IL-13, and IL-17. In contrast, the expression levels of IL-10 were increased with the treatment [25]. Studies have reported that the production of several proinflammatory cytokines is inhibited in the presence of prostaglandin E2 (PGE2). PGE2 suppresses the production of the Th1 cytokine secretion [26]. However, PGE2 can act on uncommitted B lymphocytes to promote isotype switching to IgE or IgG1 [27]. IL-6 is a proinflammatory mediator involved in synthesizing PGE2 and the infiltration of eosinophils in the airway. In asthmatic animals, high expression of COX2 correlates with IL-6, which regulates immune cells to generate PGE2. SAC treatment decreased IL-6, PGE2, and COX2. In addition, other compounds derived from arachidonic acid, such as leukotriene, cysteinyl leukotrienes, and leukotrienes B4 in eosinophils, act as potent bronchoconstrictor and cause airway smooth muscle constriction and increase mucus secretion. These effects were decreased by SAC treatment [25]. IL-13 also plays a role in this pathology by regulating the inflammation and remodeling of the lung tissues by Th2 cytokines. This mechanism is accompanied by a coordinated response of the various chemokines, such as eotaxin, which, upon activation, regulated normal T cell expressed and secreted macrophage inflammatory protein-1 beta (MIP1-β) and monocyte chemoattractant protein-1 (MCP-1), resulting in the Th2 inflammatory response in the lungs. This phenomenon increases the traffic of eosinophils from the bloodstream to the airways, increasing adhesion molecules to join the epithelial cells of the airways. In this study, IL-13 inhibition through SAC administration could ameliorate asthma-related inflammatory events by downregulating Th2 cytokines. Therefore, SAC may be an important nutraceutical for inhibiting airway inflammation by decreasing the expression of inflammatory cytokines in asthma patients. The garlic compounds could also be used as a coadjutant or therapeutic option in treating pathogen-infected asthma exacerbation patients, mainly bacterial and viral infections. Based on this knowledge, the dietary intake of these nutraceuticals as coadjuvant therapy could reduce the adverse effects of antiviral drugs, including preventing bacterial or viral infections that exacerbate asthma.
References
- Fonseca, C.; Abraham, D.; Black, C.M. Lung fibrosis. Springer Semin. Immunopathol. 1999, 21, 453–474.
- Bousquet, J.; Kaltaev, N. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach; World Health Organization: Geneva, Switzerland, 2007; p. 146.
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182.
- Jesenak, M.; Zelieskova, M.; Babusikova, E. Oxidative Stress and Bronchial Asthma in Children-Causes or Consequences? Front. Pediatr. 2017, 5, 162.
- Thomson, N.C.; Spears, M. Inhaled corticosteroids for asthma: On-demand or continuous use. Expert Rev. Respir. Med. 2013, 7, 687–699.
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; De Bont, L.G.M.; Bossios, A.; Bousquet, J.; et al. Viruses and bacteria in acute asthma exacerbation—A GA2 LEN-DARE systematic review. Allergy 2011, 66, 458–468.
- Iikura, M.; Hojo, M.; Koketsu, R.; Watanabe, S.; Sat, A.; Chino, H.; Ro, S.; Masaki, H.; Hirashima, J.; Ishii, S.; et al. The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS ONE 2015, 10, e0123584.
- Wark, P.A.; Johnston, S.L.; Moric, I.; Simpson, J.L.; Hensley, M.J.; Gibson, P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002, 19, 68–75.
- Lee, M.Y.; Yuk, J.E.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahn, K.S. Zuonin B Inhibits Lipopolysaccharide-Induced Inflammation via Downregulation of the ERK1/2 and JNK Pathways in RAW264.7 Macrophages. Evid. -Based Complement. Altern. Med. 2012, 2012, 728196.
- Silveira, J.S.; Antunes, G.L.; Kaiber, D.B.; da Costa, M.S.; Marques, E.P.; Ferreira, F.S.; Gassen, R.B.; Breda, R.V.; Wyse, A.; Pitrez, P.; et al. Reactive oxygen species are involved in eosinophil extracellular traps release and in airway inflammation in asthma. J. Cell. Physiol. 2019, 234, 23633–23646.
- Wang, J.; Jin, R.G.; Xiao, L.; Wang, Q.J.; Yan, T.H. Anti-asthma effects of synthetic salidroside through regulation of Th1/Th2 balance. Chin. J. Nat. Med. 2014, 12, 500–504.
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 2005, 98, 3–30.
- Wyness, L. Nutrition in early life and the risk of asthma and allergic disease. Br. J. Community Nurs. 2014, 19, S28–S32.
- Shin, N.-R.; Kwon, H.-J.; Ko, J.-W.; Kim, J.-S.; Lee, I.-C.; Kim, J.-C.; Kim, S.-H.; Shin, I.-S. S-Allyl cysteine reduces eosinophilic airway inflammation and mucus overproduction on ovalbumin-induced allergic asthma model. Int. Immunopharmacol. 2019, 68, 124–130.
- Sánchez-Gloria, J.L.; Arellano-Buendía, A.S.; Juárez-Rojas, J.G.; García-Arroyo, F.E.; Argüello-García, R.; Sánchez-Muñoz, F.; Sánchez-Lozada, L.G.; Osorio-Alonso, H. Cellular Mechanisms Underlying the Cardioprotective Role of Allicin on Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 9082.
- Zare, A.; Farzaneh, P.; Pourpak, Z.; Zahedi, F.; Moin, M.; Shahabi, S.; Hassan, Z.M. Purified aged garlic extract modulates allergic airway inflammation in BALB/c mice. Iran. J. Allergy Asthma Immunol. 2008, 7, 133–141.
- Shin, I.S.; Hong, J.; Jeon, C.M.; Shin, N.R.; Kwon, O.K.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 62, 506–513.
- Ho, C.Y.; Lu, C.C.; Weng, C.J.; Yen, G.C. Protective Effects of Diallyl Sulfide on Ovalbumin-Induced Pulmonary Inflammation of Allergic Asthma Mice by MicroRNA-144, -34a, and -34b/c-Modulated Nrf2 Activation. J. Agric. Food Chem. 2016, 64, 151–160.
- Kim, J.S.; Kim, E.J.; Lee, S.; Tan, X.; Liu, X.; Park, S.; Kang, K.; Yoon, J.-S.; Yoon, H.K.; Kurie, J.M.; et al. MiR-34a and miR-34b/c have distinct effects on the suppression of lung adenocarcinomas. Exp. Mol. Med. 2019, 51, 1–10.
- Sangokoya, C.; Telen, M.J.; Chi, J.-T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010, 116, 4338–4348.
- Hsieh, C.C.; Peng, W.H.; Tseng, H.H.; Liang, S.Y.; Chen, L.J.; Tsai, J.C. The Protective Role of Garlic on Allergen-Induced Airway Inflammation in Mice. Am. J. Chin. Med. 2019, 47, 1099–1112.
- Wang, J. Casticin alleviates lipopolysaccharide-induced inflammatory responses and expression of mucus and extracellular matrix in human airway epithelial cells through Nrf2/Keap1 and NF-κB pathways. Phytother. Res. 2018, 32, 1346–1353.
- Williams, O.W.; Sharafkhaneh, A.; Kim, V.; Dickey, B.F.; Evans, C.M. Airway mucus: From production to secretion. Am. J. Respir. Cell Mol. Biol. 2006, 34, 527–536.
- Jiang, L.; Li, Y.; Wang, F.; Zhang, X.; Zhao, R. Protective Effect of S-Allyl Cysteine Against Neonatal Asthmatic Rats. Dose-Response 2020, 18, 1559325820982189.
- Kunkel, S.L.; Spengler, M.; May, M.A.; Spengler, R.; Larrick, J.; Remick, D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J. Biol. Chem. 1988, 263, 5380–5384.
- Roper, R.L.; Brown, D.M.; Phipps, R.P. Prostaglandin E2 promotes B lymphocyte Ig isotype switching to IgE. J. Immunol. 1995, 154, 162–170.
More
Information
Subjects:
Allergy; Health Policy & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
14 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





