
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuan Zhang | -- | 1841 | 2022-12-13 10:19:36 | | | |
| 2 | Peter Tang | Meta information modification | 1841 | 2022-12-14 04:35:15 | | |
Video Upload Options
The pathophysiological process of intracerebral hemorrhage (ICH) is very complex, involving various mechanisms such as apoptosis, oxidative stress and inflammation. As one of the key factors, the inflammatory response is responsible for the pathological process of acute brain injury and is associated with the prognosis of patients. Abnormal or dysregulated inflammatory responses after ICH can aggravate cell damage in the injured brain tissue. The NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is a multiprotein complex distributed in the cytosol, which can be triggered by multiple signals. The NLRP3 inflammasome is activated after ICH, thus promoting neuroinflammation and aggravating brain edema.
1. Introduction
2. Functions of the NLRP3 Inflammasome in ICH
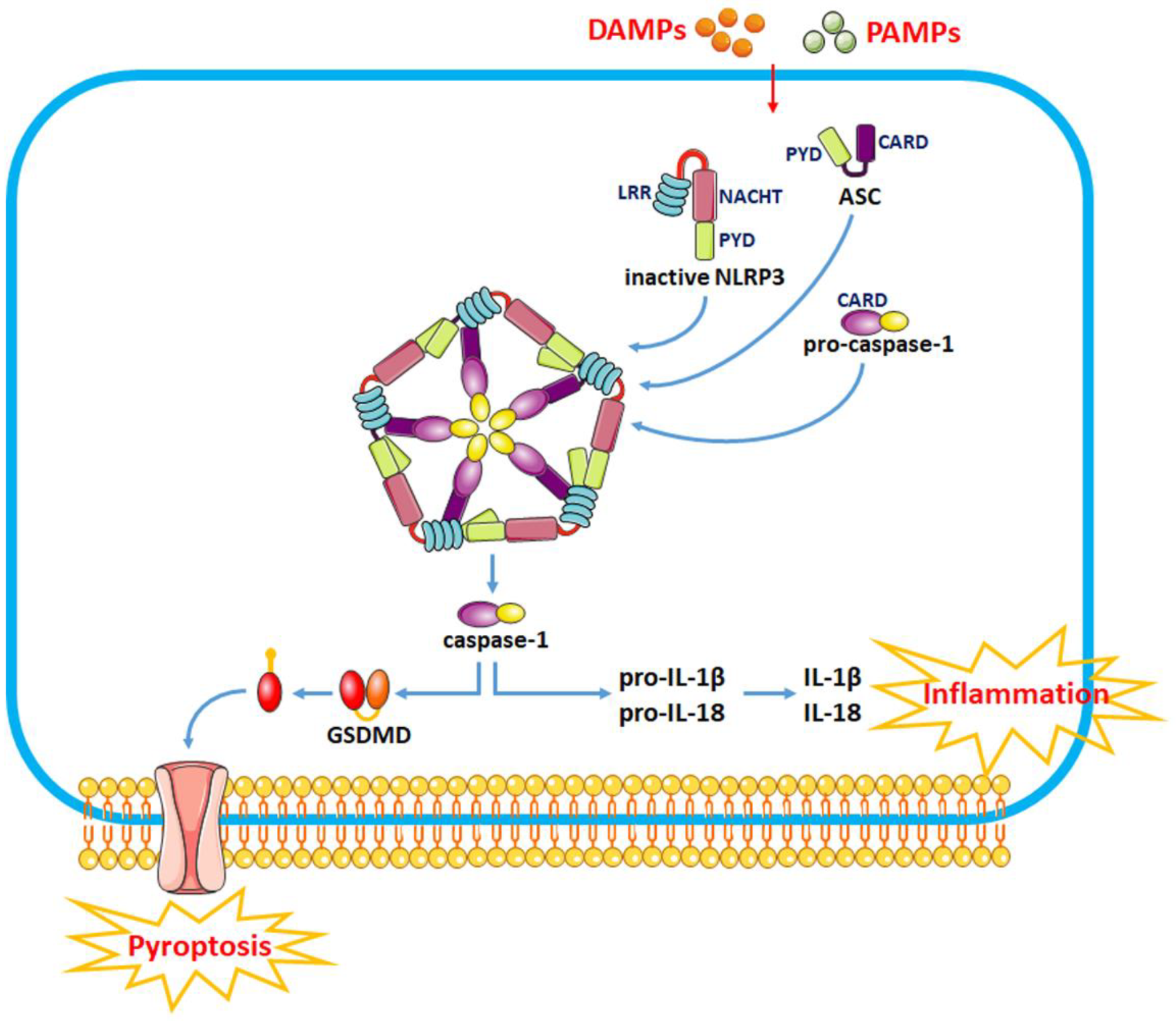
2.1. Activation of the NLRP3 Inflammasome
2.2. Modulation of NLRP3 Inflammasome Activity as a Therapeutic Strategy for SBI after ICH
|
Drugs |
Models |
Efficacy |
References |
|---|---|---|---|
|
Pioglitazone |
blood-induced mouse ICH model |
brain edema↓, lactate↑ |
[18] |
|
Edaravone |
autologous blood-induced rat ICH model |
IL-1β↓, caspase-1↓, NF-κB↓, brain edema↓, neurological deficits↓ |
[19] |
|
Adiponectin |
autologous blood-induced rat ICH model |
IL-1β↓, IL-18↓, brain edema↓, neurological deficits↓ |
[8] |
|
Glibenclamide |
autologous blood-induced mouse ICH model |
IL-1β↓, IL-18↓, IL-6↓, TNF-α↓, brain edema↓, disrupted BBB↓, neurological deficits↓ |
[20] |
|
Memantine |
collagenase-induced rat ICH model |
IL-1β↓, disrupted BBB↓, neurological deficits↓ |
[21] |
|
Atorvastatin |
collagenase-induced mouse ICH model |
IL-1β↓, IL-6↓, TNF-α↓, brain edema↓, neurological deficits↓ |
[22] |
|
Isoliquiritigenin |
collagenase IV-induced rat ICH model |
NF-κB↓, IL-1β↓, brain edema↓, disrupted BBB↓, neurological deficits↓ |
[26] |
|
Silymarin |
collagenase II-induced mouse ICH model |
NF-κB↓, caspase-1↓, IL-1β↓ |
[24] |
|
Baicalein |
collagenase VII-induced rat ICH model |
ROS↓, SOD↑, GSH-Px↑, ASC↓, caspase-1↓ |
[25] |
|
Cordycepin |
autologous blood-induced mouse ICH model |
IL-1β↓, IL-18↓, brain edema↓, neurological deficits↓ |
[27] |
References
- Askenase, M.H.; Sansing, L.H. Stages of the Inflammatory Response in Pathology and Tissue Repair after Intracerebral Hemorrhage. Semin. Neurol. 2016, 36, 288–297.
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46.
- Seoane, P.I.; Lee, B.; Hoyle, C.; Yu, S.; Lopez-Castejon, G.; Lowe, M.; Brough, D. The NLRP3-inflammasome as a sensor of organelle dysfunction. J. Cell Biol. 2020, 219, e202006194.
- Liu, Y.; Li, C.; Yin, H.; Zhang, X.; Li, Y. NLRP3 Inflammasome: A Potential Alternative Therapy Target for Atherosclerosis. Evid.-Based Complement. Altern. Med. Ecam 2020, 2020, 1561342.
- Wani, K.; AlHarthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public Health 2021, 18, 511.
- Wu, X.; Zhang, Y.; Zhang, Y.; Xia, L.; Yang, Y.; Wang, P.; Xu, Y.; Ren, Z.; Liu, H. MST4 attenuates NLRP3 inflammasome-mediated neuroinflammation and affects the prognosis after intracerebral hemorrhage in mice. Brain Res. Bull. 2021, 177, 31–38.
- Wang, S.; Yao, Q.; Wan, Y.; Wang, J.; Huang, C.; Li, D.; Yang, B. Adiponectin reduces brain injury after intracerebral hemorrhage by reducing NLRP3 inflammasome expression. Int. J. Neurosci. 2020, 130, 301–308.
- Ma, Q.; Chen, S.; Hu, Q.; Feng, H.; Zhang, J.H.; Tang, J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann. Neurol. 2014, 75, 209–219.
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 2022, 118, 785–797.
- Chen, Z.; Chopp, M.; Zacharek, A.; Li, W.; Venkat, P.; Wang, F.; Landschoot-Ward, J.; Chen, J. Brain-Derived Microparticles (BDMPs) Contribute to Neuroinflammation and Lactadherin Reduces BDMP Induced Neuroinflammation and Improves Outcome After Stroke. Front. Immunol. 2019, 10, 2747.
- Li, C.; Chen, M.; He, X.; Ouyang, D. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation. Acta Biochim. Biophys. Sin. 2021, 53, 131–139.
- Wang, L.; Zheng, S.; Zhang, L.; Xiao, H.; Gan, H.; Chen, H.; Zhai, X.; Liang, P.; Zhao, J.; Li, Y. Histone Deacetylation 10 Alleviates Inflammation After Intracerebral Hemorrhage via the PTPN22/NLRP3 Pathway in Rats. Neuroscience 2020, 432, 247–259.
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225.
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2021, 17, 2975–2990.
- Cheng, Y.; Liu, M.; Tang, H.; Chen, B.; Yang, G.; Zhao, W.; Cai, Y.; Shang, H. iTRAQ-Based Quantitative Proteomics Indicated Nrf2/OPTN-Mediated Mitophagy Inhibits NLRP3 Inflammasome Activation after Intracerebral Hemorrhage. Oxidative Med. Cell Longev. 2021, 2021, 6630281.
- Zheng, S.; Jian, D.; Gan, H.; Wang, L.; Zhao, J.; Zhai, X. FUNDC1 inhibits NLRP3-mediated inflammation after intracerebral hemorrhage by promoting mitophagy in mice. Neurosci. Lett. 2021, 756, 135967.
- Kim, H.; Lee, J.E.; Yoo, H.J.; Sung, J.H.; Yang, S.H. Effect of Pioglitazone on Perihematomal Edema in Intracerebral Hemorrhage Mouse Model by Regulating NLRP3 Expression and Energy Metabolism. J. Korean Neurosurg. Soc. 2020, 63, 689–697.
- Miao, H.; Jiang, Y.; Geng, J.; Zhang, B.; Zhu, G.; Tang, J. Edaravone Administration Confers Neuroprotection after Experimental Intracerebral Hemorrhage in Rats via NLRP3 Suppression. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 104468.
- Xu, F.; Shen, G.; Su, Z.; He, Z.; Yuan, L. Glibenclamide ameliorates the disrupted blood-brain barrier in experimental intracerebral hemorrhage by inhibiting the activation of NLRP3 inflammasome. Brain Behav. 2019, 9, e01254.
- Chen, X.; Xiang, X.; Xie, T.; Chen, Z.; Mou, Y.; Gao, Z.; Xie, X.; Song, M.; Huang, H.; Gao, Z.; et al. Memantine protects blood-brain barrier integrity and attenuates neurological deficits through inhibiting nitric oxide synthase ser1412 phosphorylation in intracerebral hemorrhage rats: Involvement of peroxynitrite-related matrix metalloproteinase-9/NLRP3 inflammasome activation. Neuroreport 2021, 32, 228–237.
- Chen, D.; Sui, L.; Chen, C.; Liu, S.; Sun, X.; Guan, J. Atorvastatin suppresses NLRP3 inflammasome activation in intracerebral hemorrhage via TLR4- and MyD88-dependent pathways. Aging 2022, 14, 462–476.
- Ikeda, H. Effect of Ni2+ on Ca2+-stimulated adenosine triphosphatase in the microsomal fraction of the rat parotid gland in vitro. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1985, 81, 7–9.
- Yuan, R.; Fan, H.; Cheng, S.; Gao, W.; Xu, X.; Lv, S.; Ye, M.; Wu, M.; Zhu, X.; Zhang, Y. Silymarin prevents NLRP3 inflammasome activation and protects against intracerebral hemorrhage. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 93, 308–315.
- Chen, X.; Zhou, Y.; Wang, S.; Wang, W. Mechanism of Baicalein in Brain Injury After Intracerebral Hemorrhage by Inhibiting the ROS/NLRP3 Inflammasome Pathway. Inflammation 2022, 45, 590–602.
- Zeng, J.; Chen, Y.; Ding, R.; Feng, L.; Fu, Z.; Yang, S.; Deng, X.; Xie, Z.; Zheng, S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-kappaB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J. Neuroinflammation 2017, 14, 119.
- Cheng, Y.; Wei, Y.; Yang, W.; Song, Y.; Shang, H.; Cai, Y.; Wu, Z.; Zhao, W. Cordycepin confers neuroprotection in mice models of intracerebral hemorrhage via suppressing NLRP3 inflammasome activation. Metab. Brain Dis. 2017, 32, 1133–1145.
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179.




