Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Javan Grisente dos Reis da Costa | -- | 3723 | 2022-12-12 14:52:06 | | | |
| 2 | Conner Chen | Meta information modification | 3723 | 2022-12-13 09:30:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Costa, J.G.D.R.D.; Costa, J.M.; Neto, A.F.D.A. Electrodeposition of Common Metals. Encyclopedia. Available online: https://encyclopedia.pub/entry/38605 (accessed on 08 February 2026).
Costa JGDRD, Costa JM, Neto AFDA. Electrodeposition of Common Metals. Encyclopedia. Available at: https://encyclopedia.pub/entry/38605. Accessed February 08, 2026.
Costa, Javan Grisente Dos Reis Da, Josiel Martins Costa, Ambrósio Florêncio De Almeida Neto. "Electrodeposition of Common Metals" Encyclopedia, https://encyclopedia.pub/entry/38605 (accessed February 08, 2026).
Costa, J.G.D.R.D., Costa, J.M., & Neto, A.F.D.A. (2022, December 12). Electrodeposition of Common Metals. In Encyclopedia. https://encyclopedia.pub/entry/38605
Costa, Javan Grisente Dos Reis Da, et al. "Electrodeposition of Common Metals." Encyclopedia. Web. 12 December, 2022.
Copy Citation
The electrodeposition process of metals and their alloys is widely used in the automotive, space, electronics, computing, jewelry, and other consumer items industries.
batteries
coating
corrosion
electroplating
1. Introduction
Among the main coating processes, electrodeposition stands out as a fast, low-cost method, in which the thickness of the deposit is easy to control. It is widely used in the automotive, aerospace, and oil industries [1]. In addition, electrodeposition has applications in the metallurgy and jewelry industries. This process is based on the mass transport of the solvated ion from the interior of the electrolyte solution toward the cathode. Adsorption of the coating metal occurs due to its complete desolvation and total charge loss (electron transfer) at a convenient location on the cathode surface. The atoms clump together until they form a growth center. This step is known as nucleation, and subsequent crystal growth occurs, controlled by interfacial phenomena. The interaction between these growth nuclei occurs through the superposition of crystals, giving rise to the growth of multilayers on the electrolytic surface, as shown in Figure 1 [2]. Although the process is known, the requirement to meet performance criteria has required ways to improve the electrolytic bath. Depending on application and service conditions, criteria include adhesion, ductility, and corrosion resistance [3][4].
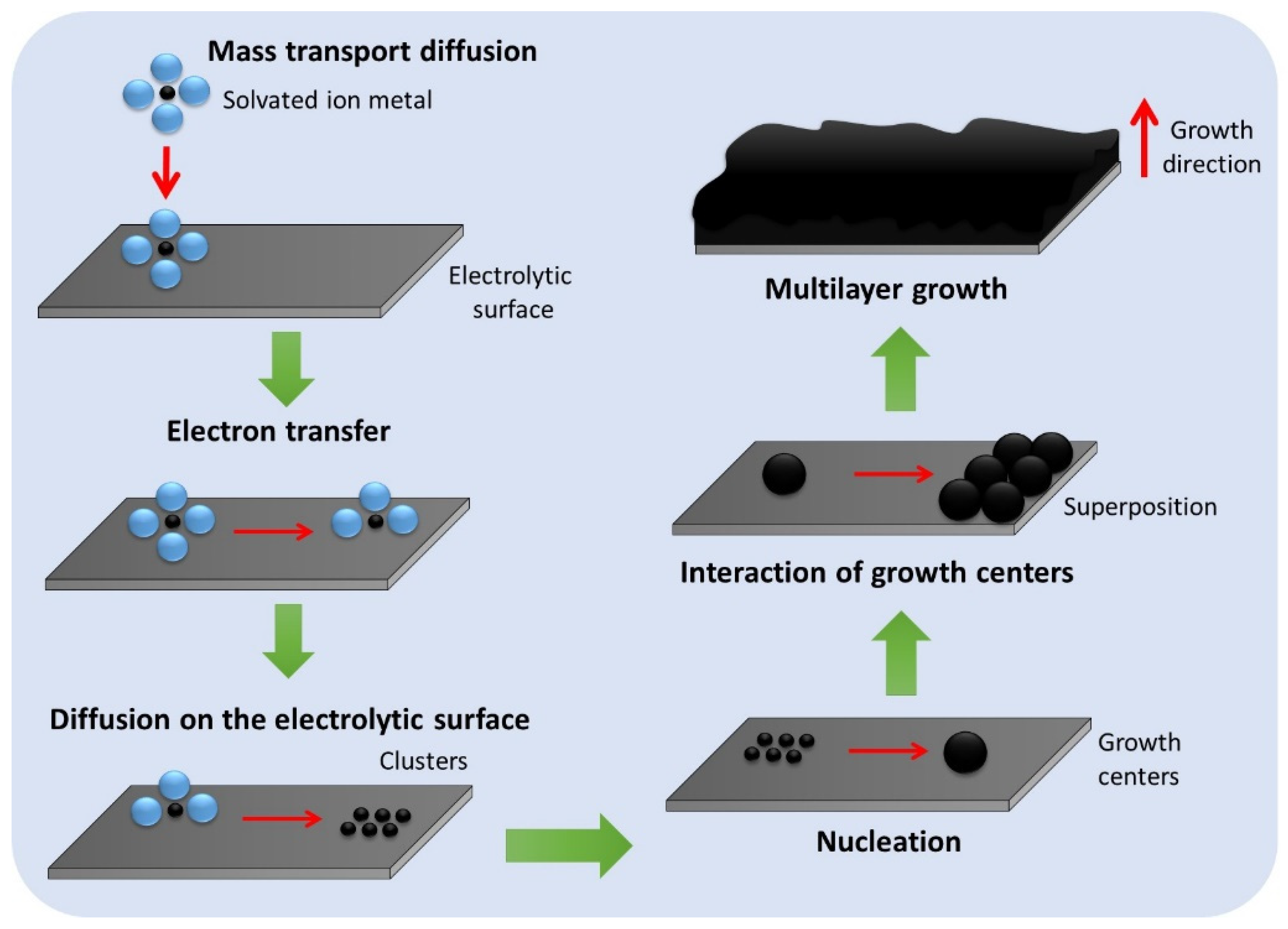
Figure 1. Deposit formation process and its involved phenomena.
Electrodeposition is categorized into the following three processes: (1) Underpotential deposition, (2) Electrophoretic deposition, and (3) Electroplating. Its main advantages and disadvantages are shown in Figure 2. Underpotential deposition is used in one- and two-step bimetallic particle syntheses. The metal is reduced on a surface at a potential less negative than the Nernst potential for the reduction of the metal on itself. Therefore, the reduction of the host metal is more favorable, forming monolayers or submonolayers of the secondary metal. On the other hand, electrophoretic electrodeposition generally uses two-electrode cells. The direct current electric field causes the charged particles to move towards the oppositely charged electrode, forming a compact and homogeneous film. Finally, electroplating employs an electric current to reduce dissolved metal cations in solution, forming a thin coating on the surface of the electrode.
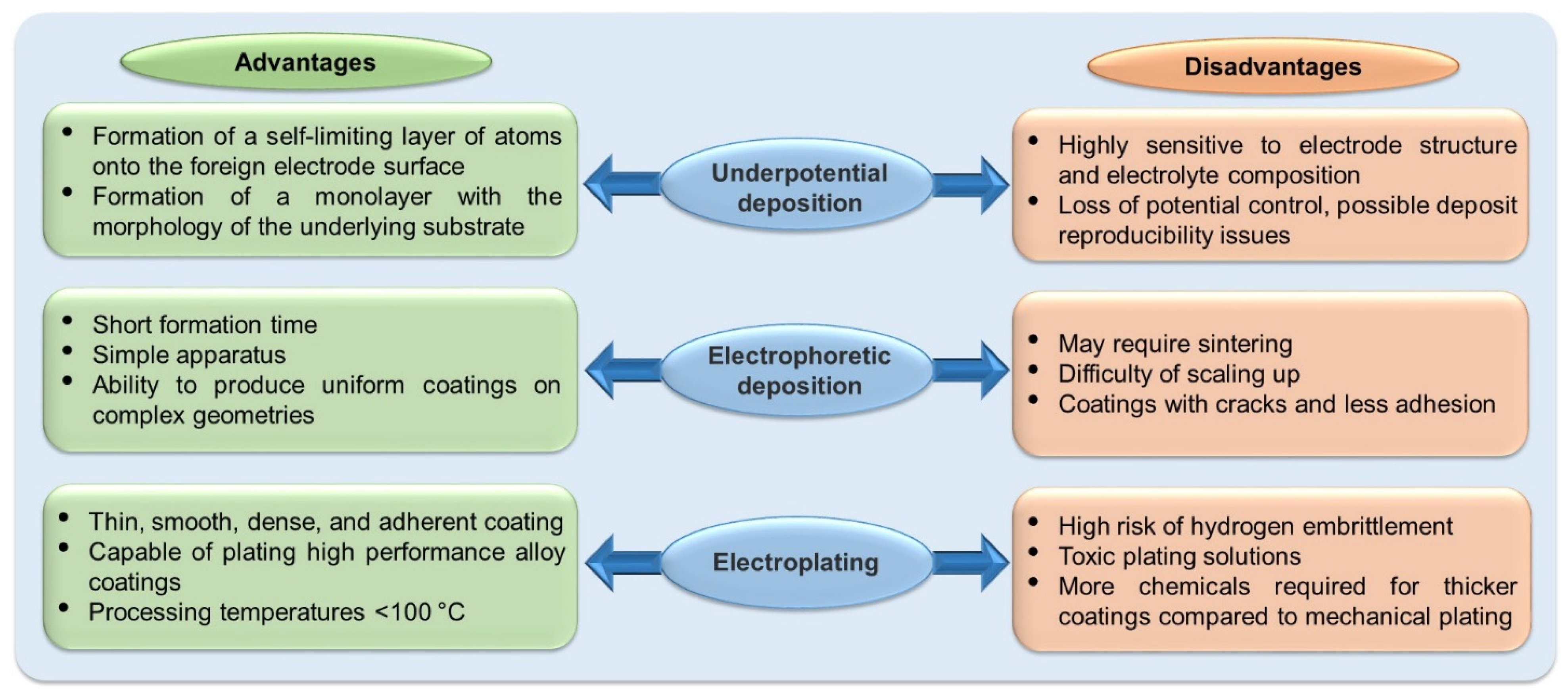
Figure 2. Main advantages and disadvantages of electrodeposition processes.
Al and its alloys are widely synthesized by electrodeposition. The studies use different aqueous baths, such as molten inorganic salts, organic solvents, and ionic liquids (ILs) [5]. ILs emerged as “green solvents” due to their easy incorporation into systems that were previously mostly dominated by volatile organic solvents. These salts are non-flammable, have a low melting temperature (<100 °C), have good thermal stability, and an almost insignificant vapor pressure [3][6]. In addition to being ecologically friendly, due to their low toxicity, they are easily recovered by extraction or distillation and recycled for subsequent use [7][8][9]. The composition of ILs (generally organic salts) comprises large cations derived from N-alkyl ammonium, phosphonium, sulfonium, guanidinium, imidazolium, pyrazolium, pyridinium, and others, and are linked to anions with a delocalized or shielded charge such as bis(triflyl)imide, hexafluorophosphate, tetrafluoroborate, trifluoromethylsulfonate, dicyanamide, bromide, chloride, tetrachloroferrate(III), and others [3].
One of the main features of ILs is the flexibility to adjust and adapt their thermophysical properties by varying the constituent ions [6][7]. This flexibility reflects the ability to chemically and structurally modify the ionic pairs, resulting in various forms of application such as solvents, electrolytes, solar cells, gas storage, organometallic and enzymatic catalysis, lubricants, hydraulic fluids, and others, making it a valuable product for many kinds of research [3].
As displayed in Figure 3, the increase in the number of studies related to electrodeposition using ILs has been growing over the years. They have a wide electrochemical window and good electrical conductivity. The higher speed of the process, with very negative redox potential and no hydrogen evolution, can result in a low risk of corrosion to the equipment, including increased mass transfer and better control of the nucleation process and growth of deposit grains [10]. Another important factor is that they can be aprotic, which can solve questions concerning hydrogen ions in protic solvents [6].
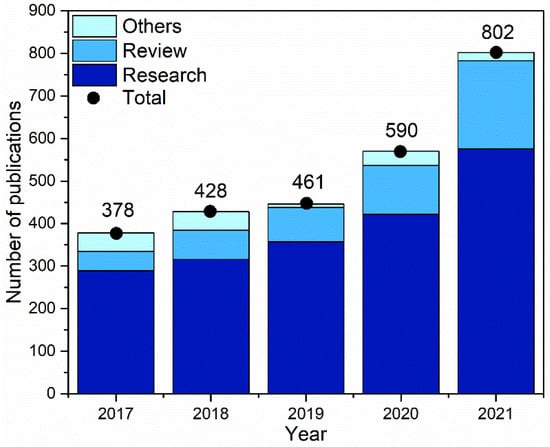
Figure 3. Number of articles per year containing the word ‘electroplating ionic liquid solution’ in the titles, keywords, and abstract section.
A combination of 79 ILs, 25 metals, and 40 alloys were found. Low-viscosity ILs are preferred in metal and alloy electrodeposition studies due to their ease of synthesis and stability under oxidative and reducing conditions [6][11]. However, some ILs have a high viscosity and can be a diminutive factor for mass transfer, making the process slower than aqueous electrolytes [10]. Therefore, they can also be presented from a mixture of deep eutectic solvents (DES). For example, choline chloride has a viscosity of 14.2 mm2/s. In some studies, proportions are made with urea and/or ethylene glycol [12][13][14][15] to reduce the viscosity and obtain a lower melting point than pure components, improving mass transfer [16][17][18].
Among the metals, Al [5][19], Co [20], Ni [21], and Zn [22] appear more frequently in electrodeposition using ILs, mainly due to their protection characteristics and rapid mass transfer during the process. In addition, they are favorites to serve as base metals, including for alloys such as Fe [23] and Cu [24], since they perform efficient codeposition and rapid nucleation. Another strong application for ILs is the ability to dissolve salts or oxides of rare earth and noble metals and then perform their electrodeposition. The process separates the metal of interest from a complex mixture, making the application of ILs fundamental.
2. Electrodeposition of Common Metals
2.1. Aluminum
Aluminum plays an essential role as an excellent coating material due to its thermal properties, electrical conductivity, light weight, and corrosion resistance [5]. In addition, Al coatings and alloys tend to develop a natural passive oxide layer on their surface during contact with air, making them resistant to corrosion in different environments [25]. In recent years, the electrodeposition of Al from ILs has generated great interest among academic and industry researchers due to its industrial potential, benefiting many industries such as automotive, aeronautics, naval, construction, electronics, and others [26]. The outstanding characteristics are the ability to operate at low deposition temperatures, ease of preparation, low cost, and high electrochemical stability. Furthermore, chloroaluminate ILs are the simplest compounds, containing inorganic halide anions and organic cations [27], which, in solution, form the ions [(Al2Cl7)]− and [(AlCl4)]− as electroactive species available for reduction.
Several authors change the AlCl3/IL molar ratio with the ionic cation to improve conductivity and electrochemical potential window and favor electroplating on an industrial scale. The feasibility of the electrodeposition process depends on the AlCl3/IL molar ratio and the characteristic of the studied IL. For [C5H14N3+][CF2ClCFClOCF2CF2SO3−], the studied molar ratio ranged from 0.9 to 1.3. This composition range showed better conductivity values that allowed sufficient mobility of the electroactive species [28]. At room temperature, [C5H14N3+][CF2ClCFClOCF2CF2SO3−], due to its immiscibility in water, produced excellent deposits for an AlCl3/IL molar ratio of 0.95:1 [29]. For AlCl3/4-EP, the reduction current with a molar ratio of 1.3:1 was greater than that with a molar ratio of 1.1:1, suggesting that the reduction of [AlCl2(4-EP)2]+ is thermodynamically favorable than [AlCl2(4-EP)2]+ [30].
For BMIC, with a 1:2 molar ratio, the deposition of Al on a copper substrate was efficient at 90 °C [31]. In contrast, Wang et al. [32], inverting the proportion to 2:1, evaluated the temperature variation during the electrodeposition process of Al on Cu. They demonstrated that at 100 °C, there was a better diffusion and/or migration of Al2Cl7− ions, generating an increase in current density with a contribution to greater nucleation and efficiency of Al deposition. However, temperatures higher than room temperature may not favor electrodeposition on IL [33] since these conditions also depend on the IL and the porosity of the substrate [25].
The Al deposit studies have a rich variation of the AlCl3/IL ratio. However, [EMIm]Cl stands out as the main IL for the electrodeposition of Al and its alloys [34][35]. The most evaluated AlCl3/[EMIm]Cl ratio was 2:1 [11][19][36]. However, ratios between 1:1 and 2:1 [37][38] and 1:2 [35] also obtained excellent deposition results. [EMIm]Cl confers a less negative applied potential and a higher current density to Al deposition in addition to a uniform coating and instantaneous nucleation of the coating, promoting lower energy consumption during the deposition.
The lower energy consumption and uniform coating have sparked interest in the Li-ion battery field. For example, Peng et al. [39] studied [EMIm]Cl as an electrolyte for the deposition of Al on Ni. As a result, they obtained a film free of dendrites, improving the corrosion resistance of Ni, and making it suitable for use as anodes of Li-ion batteries. The smooth and uniform deposition was also obtained at a current density of 20 mA/cm2 for the uranium substrate, significantly increasing the corrosion resistance [40]. Another recent advance was the magnetohydrodynamic (MHD) effect of the AlCl3–[EMIm]Cl composition in reducing the activation energy required to promote electrochemical reduction [41]. In addition, it increased the capacitance of the electrical double layer and promoted greater mass transport of Al [42].
The AlCl3/IL ratio inspires researchers to develop Al alloys from electrolytes containing ILs. Several alloys were synthesized in different temperature ranges, such as Al–Li [43] and Al–Ga [44] at 25 °C, Al–Mg [45] at 50 °C, and Al-Cu [46] at 70 °C. However, the two most cited Al alloys in the literature refer to Al–Ti and Al–W. The 2:1 AlCl3–[EMIm]Cl electrolyte containing TiCl4 (0.1 M) resulted in an Al–Ti alloy deposited on a copper electrode after a multi-step reduction [47]. The reduction to TiCl42− contributed to the codeposition of Ti on Al, with a Ti content of up to 7.37%. In contrast, Xu et al. [48], using [Bmim][Cl] as an electrolyte, obtained an Al–Ti alloy with a Ti content of up to 11.4 at.%.
Another study co-deposited Al on Ti (Ti–Al alloy) using [Bmim][Cl] [49]. The authors maintained the highest proportion of Ti of up to 40%, demonstrating that increasing the IL chain showed a greater ability to control the composition and morphology of Al–Ti coatings. For Al–W, as well as Al deposits, [EMIM][Cl] is the most used cation [50], with W contents in the range of 9–12 at.% using W6Cl12 as the W source for AlC3/EMImCl ratios 2:1 [51][52].
Unlike the studies cited, Higashino et al. [51] obtained a W content of 20 at.%, with a 1:1.5 ratio of AlCl3/EMImCl, demonstrating that the increase in the cation made the electrolyte more polarizable. Furthermore, the increase in current density favored the codeposition of W over Al. Bright and adherent deposits of ternary alloys with Al have also been studied. However, the proportions of the other metals added together were at most 10%, demonstrating the need for a careful view of the proportions to favor the codeposition [53][54].
In addition, by evaluating parameters such as component proportions, temperature, and current density, additives have been highlighted in the electrolyte composition combined with IL for Al and its alloys. For [BMIM][Cl], additives such as dichloromethane (DCM) and toluene (C7H8) were evaluated as improving agents for Al electrodeposition processes [55]. The morphology of the deposits improved with DCM due to its interaction with the [BMIM][Cl] cations, which can be adsorbed on the protruding part of the electrode surface during the electrochemical process. On the other hand, as the interaction of C7H8 with the cation was low, the characteristics of the deposit were inferior to those of DCM.
For IL, 3-butyl-1-ethylimidazolium, light aromatic naphtha (LAN) [56], as well as DCM for [BMIM][Cl], also improved the morphology of the deposits due to attraction with the 3-butyl-1-ethylimidazolium cation, demonstrating a brighter appearance to the metal. Although the electrodeposition of Al and its alloys with the ILs in the electrolytic environment is already present in many studies, research on ternary alloys and additives that improve the electrodeposition process can be further explored.
2.2. Iron
Due to the ease of oxidation, Fe is one of the few metals not studied individually in electrodeposition processes. All studies used Fe as a base metal and inducer of the codeposition of other metals to obtain stable and anticorrosive alloys [57] or as an element codeposited by a base metal [58]. Fe–Cu [59] and Fe–Al [60] alloys were obtained by electrodeposition with [Py1,4]TfO, both on a copper substrate, aimed to “damp” these alloys. For both studies, there was a thin deposition of the alloys on the substrate, in which the interaction phenomenon was charge transfer. The difference between the depositions was the locations of the damping peaks. For Fe–Cu, a damping peak occurred around 680 K, leading to the appearance of Fe particles at the interface between Cu and Fe (Cu + α-Fe phase). The driving force was the diffusion of Cu atoms around the Cu + α-Fe phase particles. For Fe–Al, the peak appeared around 800 K. However, the phenomenon depended on the dissolution of small precipitates or agglomerates of defects during heating. During cooling, the phenomenon depended on the reprecipitation/reagglomeration of the particles. Therefore, [Py1,4]TfO favored the electrodeposition of Fe alloys, improving the damping of the process.
Although Fe is preferentially studied as an alloy, alloys with Ni were the most found considering Fe codeposited [58][61][62]. One of these studies aimed to improve the anticorrosion properties of the Watts bath of Ni–Fe alloy with boric acid [63]. [BMIM]HSO4 improved the nucleation and three-dimensional growth of Ni–Fe alloy grains due to the increase in charge transfer involved in the process [61]. For the authors, the anticorrosive improvement was evident.
2.3. Nickel
Most studies involving Ni use the NiCl2 salt as the metal source since the Cl ions, together with the ILs, show the viscosity and electrical conductivity of the medium can be improved, in addition to improving the Ni nucleation mechanism [12][21]. Baths containing choline chloride favor electrodeposition, generating instantaneous three-dimensional nucleation at negative potentials. In addition to urea, ethylene glycol can also form an IL with choline chloride for Ni deposition. Diffusion controls this process. In this way, the formation of nanostructured Ni films improves the surface area of the electrode, promoting an increase in the catalytic activity in the hydrogen evolution reaction [18].
Since Ni is an excellent inductor for the co-deposition of other metals, unusual metals have been co-deposited with Ni. Choline chloride with ethylene glycol was also crucial for depositing Ni–Sn [64] and Ni–Sn–P [65] alloys from ILs. Rosoiu et al. [64] deposited Ni–Sn by a pulsed and direct current. However, using pulsed current, the hardness of the Ni–Sn alloy showed better results. In another study, although the authors did not assess the hardness of Ni-Sn-P alloy synthesized from choline chloride-ethylene glycol, this alloy exhibited superior corrosion resistance to Ni-P coatings [65]. Furthermore, its corrosion resistance improved with increasing Sn content. According to Yang et al. [66], EMIC–EG (1:2 molar ratio) improved the induction of Ni to deposit Ni–La alloy with instantaneous nucleation and three-dimensional growth. In addition to the use of IL in the electrolyte solution, the control of current density and temperature resulted in the CFC or amorphous structure of the alloy.
The evaluation of catalytic activity during the electrodeposition of Ni and its alloys using ILs has been growing since ILs have significant effects on parameters such as current density, pH, and deposition time [67][68]. According to Gao et al. [69], the catalytic activity in BMP–DCA medium was verified due to low overpotentials (−279 mV) even operating at 100 mA cm−2. This demonstrates that Ni–La or Ni–rare earth alloys can be important in the evaluation of hydrogen evolution processes. In addition to this process, Jesmani et al. [70] evaluated the electrocatalytic properties of Ni–Mo alloys for methanol oxidation in a modified Watts bath (EMIM [Br]). The increase in current density (from 1 to 6 A/dm2) decreased the Mo content, reducing the catalytic activity of the process. The optimal current density was 3.5 A/dm2, showing better catalytic performance. Likewise, another study with EMIM[Br] also showed excellent catalytic activity for the methanol oxidation process [71]. However, changes in pH directly interfered with current density. The best catalytic activity occurred at pH 7 with a current density of 15 mA/cm2.
2.4. Cobalt
Cobalt and cobalt-based alloys are essential materials in many industrial and technological fields due to their magnetic properties, hardness, thermal stability, and corrosion resistance [72]. As a ferromagnetic, its applicability includes microelectronics, high-density magnetic storage media, spintronics, drug and gene delivery, and immunological magnetic separation [73][74]. However, most Co depositions show high overpotentials due to the formation of negatively charged metal complexes due to the coordination of anions at room temperature [75]. Therefore, studies seek external and internal modifications to improve the deposition of Co. Ries et al. [76], through a rigorous diffusional control, obtained a deposit of Co at 50 °C from a solution containing [BMI][BF4], producing a diffusion coefficient of 8.21 × 10−10 cm2 s−1. In addition, the Co films were compact, smooth, shiny, and adherent to the substrate, resulting in crack-free metallic Co layers.
Alloys containing Co as the base metal has been little discussed recently in electrodeposition with ILs in the electrolyte solution composition [77][78][79]. However, as with Fe, studies report Co as a metal co-deposited by Ni since the ferromagnetic properties of these elements favor their affinity [13][15]. The peak reduction of the Ni–Co alloy can be observed between −0.6 and −1.2 V using choline chloride as the IL. The highest reduction peak occurred at −1.0 V, which favored the diffusional process deposited with 20 wt.% of Co [15]. However, by adding ethylene glycol to form a eutectic solvent, the diffusion process reduced the Co content to about 12 wt.%. This reduction indicated a worsening in corrosion protection.
[MOFIM]I is considered to be a prominent additive for Watts baths of Ni–Mo alloys [80]. In contrast, [FPIM]Br was an effective bleach for smooth and uniform Ni, Co, and Ni–Co deposits due to the reduced grain sizes imposed by IL [81]. Likewise, [FPIM]Br, compared to [MOFIM]I, resulted in deposits with less improved morphology, obtaining an atomic radius of 51.1 nm, almost twice the atomic radius using [MOFIM]I (28 nm) [82]. In addition, there was a better inhibition efficiency, obtaining a Co content of up to 30 wt.%.
2.5. Copper
Due to its high electrical conductivity, copper is the most applicable metal in the electronics industry. In galvanizing processes, copper-based films have a wide application as an undercoat for other metallic finishes as they cover minor imperfections in the substrate [83][84]. Due to these properties, Cu electrodeposition is one of the preferred methods for introducing ILs into electrolyte solutions, second only to Al [85][86]. As with Al, the major Cu source for electrodeposition using ILs is metal chloride [87][88].
As with most studies with other metals, choline chloride-based ILs also promote a homogeneous, smooth, shiny, and corrosion-resistant deposit due to their ability to generate hydrophobicity on the metal surface [89][90]. However, the addition of nicotinic acid (NA) in the electrolyte can inhibit the electrochemical processes due to the formation of transient species near the electrode surface, influencing the nucleation kinetics and the faster growth of Cu deposits [91]. In contrast, sodium bromide modified the morphology of the deposits, not only of Cu, but also of Al, Sn, Ag, and Ni [92]. However, the electrochemical results did not indicate significant differences compared to the deposits formed by ChCl–EG without additives.
Recent studies highlight the use of Cu2O as a source of Cu for its deposition under suitable dissolution conditions [85][93]. According to He et al. [93], Cu2O dissolved in an electrolyte containing [EMIM]F–urea–H2O at 353 K. Likewise, in another study, theu se of urea–EMIC as an electrolyte at 363 K increased the diffusion coefficient of the reaction to 1.02 × 10−10 m2/s. In addition, urea–EMIC efficiently dissolved Cu2O, improving the diffusion reaction, nuclei formation capacity, and Cu grain growth.
In contrast to pure copper deposition studies, for Cu–Pb alloys, the use of [Hbet][TFSA] promoted a complete dissolution of a mixture of water and CuO/PbO. The alloy morphology was altered by the nitrate reduction capacity [94] and the glucose oxidation [95]. Therefore, the system showed greater flexibility for electrocatalytic reduction or oxidation activities. The Cu–Sn alloy, popularly known as bronze, has good anti-corrosion properties. However, studies seek to improve the ratio of Cu:Sn using IL based on imidazolium cation, such as [Bmim]Cl [96][97], [EMIm]DCA [98], [EMIM]Cl [99], and [EMIm][TFSA] [100], with an Sn content of 13.7 at.% [96][97], 15 at.% [99], and 25 at.%, respectively [100].
In addition to improving anticorrosive properties, Jie et al. [96] reported that [BMIM]Cl in the Cu–Sn deposition system resulted in a crystalline plane (111). Due to the IL, the coloration became darker, making it interesting for industries that produce products with decorative aspects. The cyclic voltammetry peaks of Cu with [BMIM]Cl occur in two steps, 0.45~0.55 V for Cu2+ → Cu+ and 1.25~−1.5 V for Cu+ → Cu0, which could change the current density of the system during the process [96][97]. However, the reduction of the Cu–Sn alloy covered an intermediate reduction peak between −0.5 and −1.0 V, without interference in the alloy synthesis, with the reduction process occurring spontaneously and irreversibly. On the other hand, a system with [EMIm]DCA, polycarbonate membranes, and polystyrene models did not obtain a Cu–Sn alloy in the plane (111) [98]. The authors reported an oriented Cu6Sn5 alloy with nanofibers in the structure (221), being interesting for application in Li-ion batteries as anodic hosts.
2.6. Zinc
Zn is a low-cost material widely used in electroplating for application in the automotive and aerospace industries [101]. In addition, it has applications for energy storage due to the reversible capacity of its deposits [102][103]. However, the control of Zn alloys can be improved in terms of morphology [104], resulting in nanotubes [105]. In this context, IL-based electrolytes, instead of conventional aqueous-based electrolytes, have been promising in reducing harmful morphologies and forming a uniform metallic film.
The main dissolution of Zn for electrodeposition in ILs uses ZnCl2 [17][106]. The IL control variables, such as viscosity, density, and conductivity for Zn electrodeposition, are analyzed with ZnCl2 as the Zn source [107]. This control of the properties of an electrolyte containing ChCl–EG in a 1:2 ratio can result in Zn–Co alloys with corrosion resistivity up to 30 times higher than uncoated Cu [79]. ChCl–EG also improved charge transfer by agglomerating Zn–Ce alloy particles [108]. The Ce deposit occurred in cathodic and higher pH areas, demonstrating a tendency to improve the self-repair capacity of the alloy due to the Ce content.
Corrosion resistance was also evaluated for the ternary Zn–Mn–Sn alloy synthesized from an IL [109]. The control of the Sn content occurred due to the increase of the concentration and the potential. In addition, the ease of dissolution in Sn2+ and ChCl–EG improves the conductivity of the electrolyte solution. In this sense, both studies demonstrated the affinity of Zn and its alloys with ChCl–EG, encouraging the synthesis of new materials.
References
- Djokic, S.S. Electrodeposition—Theory and Practice, 1st ed.; Springer: New York, NY, USA, 2010; p. XVII-295.
- Gamburg, Y.D.; Zangari, G. Theory and Practice of Metal Electrodeposition, 1st ed.; Springer: New York, NY, USA, 2011; p. XVII-378.
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556.
- Lebedeva, O.; Kultin, D.; Zakharov, A.; Kustov, L. Advances in application of ionic liquids: Fabrication of surface nanoscale oxide structures by anodization of metals and alloys. Surf. Interfaces 2022, 34, 102345.
- Maniam, K.K.; Paul, S. A Review on the Electrodeposition of Aluminum and Aluminum Alloys in Ionic Liquids. Coatings 2021, 11, 80.
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812.
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706.
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864.
- Dołżonek, J.; Kowalska, D.; Maculewicz, J.; Stepnowski, P. Regeneration, Recovery, and Removal of Ionic Liquids. In Encyclopedia of Ionic Liquids; Zhang, S., Ed.; Springer: Singapore, 2020; pp. 1–9.
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289.
- Zhang, M.; Peng, D.; Peng, F.; Huang, A.; Song, K.; He, Q.; Yin, C.; Rao, J.; Zhang, Y.; Chen, H.; et al. Effects of Additives Containing Cyanopyridine on Electrodeposition of Bright Al Coatings from AlCl3-EMIC Ionic Liquids. Coatings 2021, 11, 1396.
- Yavuz, A.; Ozdemir, N.; Yilmaz Erdogan, P.; Zengin, H.; Zengin, G.; Bedir, M. Effect of electrodeposition potential and time for nickel film generation from ionic liquid electrolytes for asymmetric supercapacitor production. Thin Solid Film. 2020, 711, 138309.
- Li, W.; Hao, J.; Liu, W.; Mu, S. Electrodeposition of nano Ni–Co alloy with (220) preferred orientation from choline chloride-urea: Electrochemical behavior and nucleation mechanism. J. Alloy. Compd. 2021, 853, 157158.
- Sides, W.D.; Huang, Q. Electrodeposition of manganese thin films on a rotating disk electrode from choline chloride/urea based ionic liquids. Electrochim. Acta 2018, 266, 185–192.
- Li, W.; Hao, J.; Mu, S.; Liu, W. Electrochemical behavior and electrodeposition of Ni-Co alloy from choline chloride-ethylene glycol deep eutectic solvent. Appl. Surf. Sci. 2020, 507, 144889.
- Alesary, H.F.; Cihangir, S.; Ballantyne, A.D.; Harris, R.C.; Weston, D.P.; Abbott, A.P.; Ryder, K.S. Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim. Acta 2019, 304, 118–130.
- Bakkar, A.; Neubert, V. Recycling of cupola furnace dust: Extraction and electrodeposition of zinc in deep eutectic solvents. J. Alloys Compd. 2019, 771, 424–432.
- Wang, S.; Zou, X.; Lu, Y.; Rao, S.; Xie, X.; Pang, Z.; Lu, X.; Xu, Q.; Zhou, Z. Electrodeposition of nano-nickel in deep eutectic solvents for hydrogen evolution reaction in alkaline solution. Int. J. Hydrog. Energy 2018, 43, 15673–15686.
- Yamagami, M.; Higashino, S.; Yamamoto, T.; Ikenoue, T.; Miyake, M.; Hirato, T. Aluminum Electrodeposition in Dry Air Atmosphere: Comparative Study of an Acetamide–AlCl3 Deep Eutectic Solvent and a 1-Ethyl-3-Methylimidazolium Chloride–AlCl3 Ionic Liquid. J. Electrochem. Soc. 2022, 169, 062502.
- Nishi, N.; Ezawa, K.; Sakka, T. In Situ Surface Roughness Analysis of Electrodeposited Co Films in an Ionic Liquid Using Electrochemical Surface Plasmon Resonance: Effect of Leveling Additives. J. Electrochem. Soc. 2021, 168, 072505.
- Du, C.; Yang, H.; Chen, X.-B.; Wang, L.; Dong, H.; Ning, Y.; Lai, Y.; Jia, J.; Zhao, B. Effect of coordinated water of hexahydrate on nickel platings from choline–urea ionic liquid. J. Mater. Sci. 2018, 53, 10758–10771.
- Wang, Y.-S.; Chen, P.-Y. Electrochemical Study and Electrodeposition of Zn-Ni Alloys in an Imide-Type Hydrophobic Room-Temperature Ionic Liquid: Feasibility of Using Metal Chlorides as the Metal Sources. J. Electrochem. Soc. 2018, 165, D76.
- Yavuz, A.; Yilmaz, N.F.; Artan, M. Fe-Cu Alloy-Based Flexible Electrodes from Ethaline Ionic Liquid. J. Electron. Mater. 2021, 50, 3478–3487.
- Zhang, J.; Ma, X.; Zhang, J.; Yang, P.; An, M.; Li, Q. Electrodeposition of Cu-Zn alloy from EMImTfO ionic liquid/ethanol mixtures for replacing the cyanide zincate layer on Al alloy. J. Alloy. Compd. 2019, 806, 79–88.
- Zhang, D.; Mu, Y.; Li, H.; Yang, Z.; Yang, Y. A new method for electrodeposition of Al coatings from ionic liquids on AZ91D Mg alloy in air. RSC Adv. 2018, 8, 39170–39176.
- Caporali, S.; Martinuzzi, S.M.; Von Czarnecki, P.; Schubert, T.J.S.; Bardi, U. Effects of Metal Ions on the Aluminum Electrodeposition from Ionic Liquids. J. Mater. Eng. Perform. 2017, 26, 685–691.
- Li, M.; Li, Y. Aluminum Electrodeposition using AlCl3/urea Ionic Liquid. Int. J. Electrochem. Sci. 2020, 15, 8498–8505.
- Oriani, A.V.; Cojocaru, P.; Monzani, C.; Vallés, E.; Gómez, E. Aluminium electrodeposition from a novel hydrophobic ionic liquid tetramethyl guanidinium-perfluoro-3-oxa-4,5 dichloro-pentan-sulphonate. J. Electroanal. Chem. 2017, 793, 85–92.
- Galindo, M.; Sebastián, P.; Cojocaru, P.; Gómez, E. Electrodeposition of aluminium from hydrophobic perfluoro-3-oxa-4,5 dichloro-pentan-sulphonate based ionic liquids. J. Electroanal. Chem. 2018, 820, 41–50.
- Chen, J.-A.; Chen, P.-Y.; Sun, I.W. An Assessment of Aluminum Electrodeposition from Aluminum Chloride/4-ethylpyridine Ionic Liquid at Ambient Temperature. J. Electrochem. Soc. 2022, 169, 052505.
- Peng, Y.; Shinde, P.; Reddy, R.G. Nucleation Study on Deposition of Aluminum from 1-Butyl-3-Methylimidazolium Chloride and Aluminum Chloride Ionic Liquid Electrolyte. ECS Trans. 2020, 98, 199.
- Wang, Y.; Reddy, R.G.; Wang, R. Dendrite-free Al recycling via electrodeposition using ionic liquid electrolytes: The effects of deposition temperature and cathode surface roughness. J. Clean. Prod. 2021, 287, 125043.
- Lang, H.; Wang, Q.; Tu, X.; Chen, S. Template-free preparation of spherical Al particles in aluminum chloride and 1-butyl-3-methylimidazolium chloride ionic liquid. Ionics 2018, 24, 1781–1788.
- Xu, D.; Li, J.; Liang, C.; Liu, J.; Wang, H.; Ling, G. Increasing anhydrous chromium chloride concentration in AlCl3–EMIC ionic liquid: A step towards non-hydrogen-embrittlement chromium electroplating. RSC Adv. 2022, 12, 1855–1861.
- Farisi, M.S.A.; Hertel, S.; Wiemer, M.; Otto, T. Investigation of aluminum patterned electrodeposition process from AlCl3-Cl ionic liquid for microsystems application. In Proceedings of the 2018 International Conference on Electronics Packaging and iMAPS All Asia Conference (ICEP-IAAC), Mie, Japan, 17–21 April 2018; pp. 415–418.
- Rodríguez González, P.; Ling, G. Anodic behavior in AlCl3-EMIC ionic liquid of Ti6Al4V alloy and its application for enhancing the adhesion strength of aluminum coatings. Surf. Coat. Technol. 2017, 331, 57–65.
- Peng, Y.; Shinde, P.S.; Reddy, R.G. Diffusion coefficient and nucleation density studies on electrochemical deposition of aluminum from chloroaluminate ionic liquid electrolytes. J. Electroanal. Chem. 2021, 895, 115363.
- Elterman, V.A.; Shevelin, P.Y.; Yolshina, L.A.; Borozdin, A.V. Electrodeposition of aluminium from the chloroaluminate ionic liquid 1-ethyl-3-methylimidazolium chloride. Electrochim. Acta 2021, 389, 138715.
- Yang, Y.; Liu, S.; Chi, C.; Hao, J.; Zhao, J.; Xu, Y.; Li, Y. Electrodeposition of a continuous, dendrite-free aluminum film from an ionic liquid and its electrochemical properties. J. Mater. Sci. Mater. Electron. 2020, 31, 9937–9945.
- Jiang, Y.; Ding, J.; Luo, L.; Shi, P.; Wang, X. Electrodepositing aluminum coating on uranium from aluminum chloride-1-ethyl-3-methylimidazolium chloride ionic liquid. Surf. Coat. Technol. 2017, 309, 980–985.
- Wang, J.; Liu, X.; Xie, H.; Yin, H.; Song, Q.; Ning, Z. Effect of a Magnetic Field on the Electrode Process of Al Electrodeposition in a Cl-AlCl3 Ionic Liquid. J. Phys. Chem. B 2021, 125, 13744–13751.
- Takahashi, H.; Matsushima, H.; Ueda, M. Al Film Electrodeposition from the AlCl3-EMIC Electrolyte under a Magnetic Field. J. Electrochem. Soc. 2017, 164, H5165.
- Zhang, B.; Shi, Z.; Shen, L.; Liu, A.; Xu, J.; Hu, X. Electrodeposition of Al, Al-Li Alloy, and Li from an Al-Containing Solvate Ionic Liquid under Ambient Conditions. J. Electrochem. Soc. 2018, 165, D321.
- Yang, Y.; Hao, J.; Xue, J.; Liu, S.; Chi, C.; Zhao, J.; Xu, Y.; Li, Y. Co-electrodeposited Al-Ga composite electrode from ionic liquid with volume expansion adaptability in energy storage. Mater. Lett. 2021, 303, 130484.
- Zhang, X.; Liu, A.; Liu, F.; Shi, Z.; Zhang, B.; Wang, X. Electrodeposition of aluminum–magnesium alloys from an aluminum-containing solvate ionic liquid at room temperature. Electrochem. Commun. 2021, 133, 107160.
- Ismail, A.S. Electrodeposition of aluminium–copper alloy from 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl) imide ionic liquid. Egypt. J. Pet. 2017, 26, 61–65.
- Hibino, Y.; Azumi, K. Enhancement of Al-Ti Alloy Electrodeposition from AlCl3-EMIC Ionic Liquid Based Bath by Mg Additive. J. Electrochem. Soc. 2019, 166, D776.
- Xu, C.; Hua, Y.; Zhang, Q.; Li, J.; Lei, Z.; Lu, D. Electrodeposition of Al-Ti alloy on mild steel from AlCl3-BMIC ionic liquid. J. Solid State Electrochem. 2017, 21, 1349–1356.
- Shinde, P.S.; Peng, Y.; Reddy, R.G. Potentiostatic Electrodeposition of Ti–Al Alloy with 40% Titanium from the Lewis Acidic 1-Butyl-3-Methylimidazolium Chloride-Aluminum Chloride Ionic Liquid Electrolyte. In TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2022; pp. 74–86.
- Higashino, S.; Takeuchi, Y.; Miyake, M.; Ikenoue, T.; Tane, M.; Hirato, T. Tungsten(II) chloride hydrates with high solubility in chloroaluminate ionic liquids for the electrodeposition of Al–W alloy films. J. Electroanal. Chem. 2022, 912, 116238.
- Higashino, S.; Miyake, M.; Takahashi, A.; Matamura, Y.; Fujii, H.; Kasada, R.; Hirato, T. Evaluation of the hardness and Young’s modulus of electrodeposited Al–W alloy films by nano-indentation. Surf. Coat. Technol. 2017, 325, 346–351.
- Höhlich, D.; Wachner, D.; Müller, M.; Scharf, I.; Lampke, T. Electrodeposition and characterisation of Al-W alloy films from ionic liquid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 373, 012007.
- Yang, J.; Chang, L.; Jiang, L.; Wang, K.; Huang, L.; He, Z.; Shao, H.; Wang, J.; Cao, C.-N. Electrodeposition of Al-Mn-Zr ternary alloy films from the Lewis acidic aluminum chloride-1-ethyl-3-methylimidazolium chloride ionic liquid and their corrosion properties. Surf. Coat. Technol. 2017, 321, 45–51.
- Suneesh, P.V.; Ramachandran, T.; Satheesh Babu, T.G. Electrodeposition of Al-Zr-Cu Ternary Alloy from AlCl3-Et3NHCl Ionic Liquid containing Acetylacetonates of Copper and Zirconium. Mater. Today Proc. 2018, 5, 16640–16645.
- Tian, G.-C.; Yuan, Q.-X. Effect of dichloromethane and toluene on the structure, property, and Al electrodeposition in 1-butyl-3-methylimidazolium chloroaluminate ionic liquid. Chin. J. Eng. 2021, 43, 1037.
- Guinea, E.; Salicio-Paz, A.; Iriarte, A.; Grande, H.-J.; Medina, E.; García-Lecina, E. Robust Aluminum Electrodeposition from Ionic Liquid Electrolytes Containing Light Aromatic Naphta as Additive. ChemistryOpen 2019, 8, 1094–1099.
- Wang, Z.; Wu, T.; Geng, X.; Ru, J.; Hua, Y.; Bu, J.; Xue, Y.; Wang, D. The role of electrolyte ratio in electrodeposition of nanoscale FeCr alloy from choline chloride-ethylene glycol ionic liquid: A suitable layer for corrosion resistance. J. Mol. Liq. 2022, 346, 117059.
- Danilov, F.I.; Bogdanov, D.A.; Smyrnova, O.V.; Korniy, S.A.; Protsenko, V.S. Electrodeposition of Ni–Fe alloy from a choline chloride-containing ionic liquid. J. Solid State Electrochem. 2022, 26, 939–957.
- Lambri, O.A.; Weidenfeller, B.; Bonifacich, F.G.; Mohr-Weidenfeller, L.; Lambri, F.D.; Xu, J.; Zelada, G.I.; Endres, F. Study of the damping behaviour in samples consisting of iron electro-deposited on copper in an ionic liquid. J. Alloy. Compd. 2022, 918, 165462.
- Lambri, O.A.; Weidenfeller, B.; Bonifacich, F.G.; Pulletikurthi, G.; Xu, J.; Weidenfeller, L.; Endres, F. Damping of Fe-Al alloy electrodeposited in an ionic liquid. Mat. Res. 2018, 21, e20170805.
- He, X.; Zhang, C.; Zhu, Q.; Lu, H.; Cai, Y.; Wu, L. Electrodeposition of Nanocrystalline Ni-Fe Alloy Coatings Based on 1-Butyl-3-Methylimidazolium-Hydrogen Sulfate Ionic Liquid. J. Nanosci. Nanotechnol. 2017, 17, 1108–1115.
- Mardani, R.; Shahmirzaee, H.; Ershadifar, H.; Vahdani, M.R. Electrodeposition of Ni32Fe48Mo20 and Ni52Fe33W15 alloy film on Cu microwire from ionic liquid containing plating bath. Surf. Coat. Technol. 2017, 324, 281–287.
- Maizi, R.; Meddour, A.; Rousse, C. Structural and electrochemical properties of thin layers of binary Ni-Fe alloys electrodeposited by two different baths: Acid and ionic liquid. Surf. Rev. Lett. 2017, 25, 1950025.
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Comparative Study of Ni-Sn Alloys Electrodeposited from Choline Chloride-Based Ionic Liquids in Direct and Pulsed Current. Coatings 2019, 9, 801.
- Fashu, S.; Mudzingwa, L.; Khan, R.; Tozvireva, M. Electrodeposition of high corrosion resistant Ni–Sn–P alloy coatings from an ionic liquid based on choline chloride. Trans. IMF 2018, 96, 20–26.
- Yang, Y.; Xu, C.; Hua, Y.; Wang, M.; Su, Z. Electrochemical preparation of Ni-La alloys from the EMIC-EG eutectic-based ionic liquid. Ionics 2017, 23, 1703–1710.
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Baskevich, A.S.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 24604–24616.
- Protsenko, V.S.; Butyrina, T.E.; Bobrova, L.S.; Korniy, S.A.; Danilov, F.I. Electrochemical Corrosion Behavior of Ni–TiO2 Composite Coatings Electrodeposited from a Deep Eutectic Solvent-Based Electrolyte. Coatings 2022, 12, 800.
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Hua, Y.X.; Xu, C.Y.; Li, Y. Electrochemical Preparation of Ni-La Alloy Films from N-butyl-N-Methyl Pyrrolidinium Dicyanamide Ionic Liquid as Electrocatalysts for Hydrogen Evolution Reaction. J. Electrochem. Soc. 2017, 164, D778.
- Jesmani, S.M.; Amini, R.; Abdollah-Pour, H.; Mohammadian-Semnani, H. Effect of current density on Ni-Mo electrodeposition using EMIM . Surf. Eng. 2019, 35, 1088–1096.
- Jesmani, S.M.; Mohammadian-Semnani, H.; Abdollah-Pour, H.; Amini, R. The effect of pH on electrocatalytic properties of electrodeposited Ni–Mo/Ni coating using 1-ethyl-3-methylimidazolium bromide. Mater. Res. Express 2019, 6, 1065e1062.
- Panzeri, G.; Pedrazzetti, L.; Rinaldi, C.; Nobili, L.; Magagnin, L. Electrodeposition of Nanostructured Cobalt Films from Choline Chloride-Ethylene Glycol Deep Eutectic Solvent. J. Electrochem. Soc. 2018, 165, D580.
- Manjum, M.; Serizawa, N.; Katayama, Y. Electrodeposition of Co in an Amide-Type Ionic Liquid under an External Magnetic Field. J. Electrochem. Soc. 2021, 168, 042504.
- Pereira, N.M.; Brincoveanu, O.; Pantazi, A.G.; Pereira, C.M.; Araújo, J.P.; Fernando Silva, A.; Enachescu, M.; Anicai, L. Electrodeposition of Co and Co composites with carbon nanotubes using choline chloride-based ionic liquids. Surf. Coat. Technol. 2017, 324, 451–462.
- Motobayashi, K.; Shibamura, Y.; Ikeda, K. Origin of a High Overpotential of Co Electrodeposition in a Room-Temperature Ionic Liquid. J. Phys. Chem. Lett. 2020, 11, 8697–8702.
- Ries, L.A.d.S.; de Brito, H.A.; Gasparin, F.P.; Muller, I.L. Additive-free electrodeposition of cobalt on silicon from 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. J. Mol. Liq. 2021, 325, 114787.
- Chang, C.-C.; Sun, I.W. Template-Free Fabrication of Diameter-Modulated Co-Zn/Oxide Wires from a Chlorozincate Ionic Liquid by Using Pulse Potential Electrodeposition. J. Electrochem. Soc. 2017, 164, D425.
- Fong, J.-D.; Chen, P.-Y.; Sun, I.W. Template-Free Electrodeposition of Net-Like Co-Al/Oxide Structures from a Lewis Acidic Chloroaluminate Room Temperature Ionic Liquid Using a Potential Step Method. J. Electrochem. Soc. 2018, 165, D716.
- Yavuz, A.; Yilmaz Erdogan, P.; Zengin, H.; Zengin, G. Electrodeposition and Characterisation of Zn-Co Alloys from Ionic Liquids on Copper. J. Electron. Mater. 2022, 51, 5253–5261.
- Omar, I.M.A.; Al-Fakih, A.M.; Aziz, M.; Emran, K.M. Part II: Impact of ionic liquids as anticorrosives and additives on Ni-Co alloy electrodeposition: Experimental and DFT study. Arab. J. Chem. 2021, 14, 102909.
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Impact of ionic liquid Br on the electrodeposition of Ni and Co from an aqueous sulfate bath. J. Mater. Res. Technol. 2021, 12, 170–185.
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Part I: Ni-Co alloy foils electrodeposited using ionic liquids. Arab. J. Chem. 2020, 13, 7707–7719.
- Sun, J.; Ming, T.-Y.; Qian, H.-X.; Li, Q.-S. Preparation of copper–silver alloy with different morphologies by a electrodeposition method in 1-butyl-3-methylimidazolium chloride ionic liquid. Bull. Mater. Sci. 2019, 42, 227.
- Ramírez, C.; Bozzini, B.; Calderón, J.A. Electrodeposition of copper from triethanolamine as a complexing agent in alkaline solution. Electrochim. Acta 2022, 425, 140654.
- He, W.; Shi, Z.; Liu, A.; Guan, J.; Yang, S. Electro-reduction of Cu2O to Cu in urea/1-ethyl-3-methylimidazolium chloride. J. Appl. Electrochem. 2021, 51, 1145–1156.
- Pham, T.A.; Horwood, C.; Maiti, A.; Peters, V.; Bunn, T.; Stadermann, M. Solvation Properties of Silver and Copper Ions in a Room Temperature Ionic Liquid: A First-Principles Study. J. Phys. Chem. B 2018, 122, 12139–12146.
- Ezawa, K.; Nishi, N.; Sakka, T. In-situ electrochemical SPR study of gold surface smoothing by repetitive cathodic deposition and anodic dissolution of copper in an ionic liquid. J. Electroanal. Chem. 2020, 877, 114611.
- Bhujbal, A.V.; Rout, A.; Venkatesan, K.A.; Bhanage, B.M. Electrochemical Fabrication of Copper and Tin Micro-Crystals from a Protic Ionic Liquid Medium. ChemistrySelect 2020, 5, 3694–3699.
- Sato, Y.; Maruyama, S.; Matsumoto, Y. Electrodeposition of metallic Cu from CuCl gas source transported into ionic liquid in a vacuum. J. Vac. Sci. Technol. A 2018, 36, 031516.
- Kuang, Y.; Jiang, F.; Zhu, T.; Wu, H.; Yang, X.; Li, S.; Hu, C. One-step electrodeposition of superhydrophobic copper coating from ionic liquid. Mater. Lett. 2021, 303, 130579.
- Alesary, H.F.; Ismail, H.K.; Hameid Odda, A.; Watkins, M.J.; Arkan Majhool, A.; Ballantyne, A.D.; Ryder, K.S. Influence of different concentrations of nicotinic acid on the electrochemical fabrication of copper film from an ionic liquid based on the complexation of choline chloride-ethylene glycol. J. Electroanal. Chem. 2021, 897, 115581.
- Alesary, H.F.; Khudhair, A.F.; Rfaish, S.Y.; Ismail, H.K. Effect of Sodium Bromide on the Electrodeposition of Sn, Cu, Ag and Ni from a Deep Eutectic Solvent-Based Ionic Liquid. Int. J. Electrochem. Sci. 2019, 14, 7116–7132.
- He, W.; Shi, Z.; Liu, F.; Yang, S. Electrodeposition of Copper Metal from the 1-Ethyl-3-methylimidazolium Fluoride (F)-urea-H2O System Containing Cu2O. Electrochemistry 2020, 88, 253–255.
- Shao, Y.-A.; Chen, Y.-T.; Chen, P.-Y. Cu and CuPb electrodes prepared via potentiostatic electrodeposition from metal oxides in hydrophobic protic amide-type ionic liquid/water mixture under ambient air for nonenzymatic nitrate reduction. Electrochim. Acta 2019, 313, 488–496.
- Shao, Y.-A.; Chen, Y.-T.; Chen, P.-Y. Cu and CuPb Electrodes Electrodeposited from Metal Oxides in Hydrophobic Protic Amide-Type Ionic Liquid/Water Mixture for Nonenzymatic Glucose Oxidation. J. Electrochem. Soc. 2019, 166, D221.
- Sun, J.; Ming, T.-Y.; Qian, H.-X.; Li, Q.-S. Preparation of black Cu-Sn alloy with single phase composition by electrodeposition method in 1-butyl-3-methylimidazolium chloride ionic liquids. Mater. Chem. Phys. 2018, 219, 421–424.
- Sun, J.; Ming, T.Y.; Qian, H.X.; Li, Q.S. Electrochemical behaviors and electrodeposition of single-phase Cu-Sn alloy coating in Cl. Electrochim. Acta 2019, 297, 87–93.
- Elbasiony, A.M.; Prowald, A.; Abedin, S.Z.E.; Endres, F. Electrochemical synthesis of nanowires and macroporous CuSn alloy from ionic liquids. J. Solid State Electrochem. 2022, 26, 783–789.
- Lehmann, L.; Höhlich, D.; Mehner, T.; Lampke, T. Irregular Electrodeposition of Cu-Sn Alloy Coatings in Cl Outside the Glove Box with Large Layer Thickness. Coatings 2021, 11, 310.
- Soulmi, N.; Porras-Gutierrez, A.-G.; Mordvinova, N.E.; Lebedev, O.I.; Rizzi, C.; Sirieix-Plénet, J.; Groult, H.; Dambournet, D.; Gaillon, L. Sn(TFSI)2 as a suitable salt for the electrodeposition of nanostructured Cu6Sn5–Sn composites obtained on a Cu electrode in an ionic liquid. Inorg. Chem. Front. 2019, 6, 248–256.
- Maniam, K.K.; Paul, S. Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids. Appl. Sci. 2020, 10, 5321.
- Shimizu, M.; Sugiyama, Y.; Horita, M.; Yoshii, K.; Arai, S. Cation-Structure Effects on Zinc Electrodeposition and Crystallographic Orientation in Ionic Liquids. ChemElectroChem 2022, 9, e202200016.
- Schuett, F.M.; Heubach, M.-K.; Mayer, J.; Ceblin, M.U.; Kibler, L.A.; Jacob, T. Electrodeposition of Zinc onto Au(111) and Au(100) from the Ionic Liquid . Angew. Chem. Int. Ed. 2021, 60, 20461–20468.
- Keist, J.S.; Hammons, J.A.; Wright, P.K.; Evans, J.W.; Orme, C.A. Coupling in situ atomic force microscopy (AFM) and ultra-small-angle X-ray scattering (USAXS) to study the evolution of zinc morphology during electrodeposition within an imidazolium based ionic liquid electrolyte. Electrochim. Acta 2020, 342, 136073.
- Chen, Y.-T.; Chen, P.-Y.; Ju, S.-P. Preparation of Ni nanotube-modified electrodes via galvanic displacement on sacrificial Zn templates: Solvent effects and attempts for non-enzymatic electrochemical detection of urea. Microchem. J. 2020, 158, 105172.
- Yang, J.-M.; Hsieh, Y.-T.; Chu-Tien, T.-T.; Sun, I.W. Electrodeposition of Distinct One-Dimensional Zn Biaxial Microbelt from the Zinc Chloride-1-Ethyl-3-methylidazolium Chloride Ionic Liquid. J. Electrochem. Soc. 2011, 158, D235.
- Liu, A.-M.; Guo, M.-X.; Shi, Z.-N.; Liu, Y.-B.; Liu, F.-G.; Hu, X.-W.; Yang, Y.-J.; Tao, W.-J.; Wang, Z.-W. Physicochemical properties of 1,3-dimethyl-2-imidazolinone−ZnCl2 solvated ionic liquid and its application in zinc electrodeposition. Trans. Nonferrous Met. Soc. China 2021, 31, 832–841.
- Marín-Sánchez, M.; Gracia-Escosa, E.; Conde, A.; Palacio, C.; García, I. Deposition of Zinc–Cerium Coatings from Deep Eutectic Ionic Liquids. Materials 2018, 11, 2035.
- Fashu, S.; Khan, R.; Zulfiqar, S. Ternary Zn–Mn–Sn alloy electrodeposition from an ionic liquid based on choline chloride. Trans. IMF 2017, 95, 217–225.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.6K
Revisions:
2 times
(View History)
Update Date:
15 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




