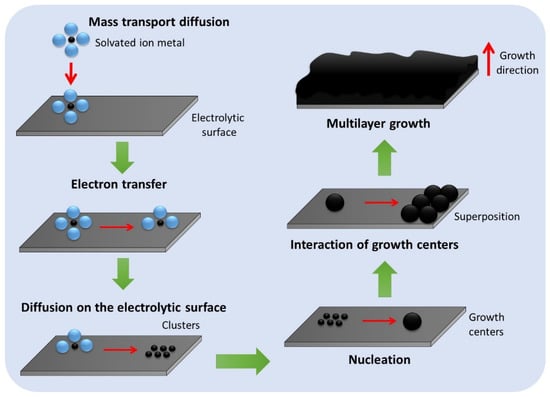
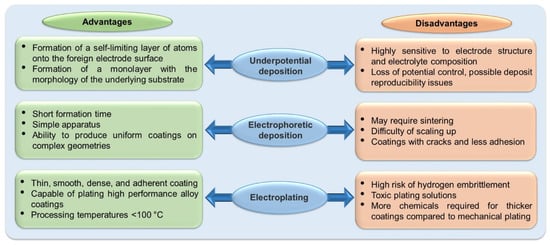
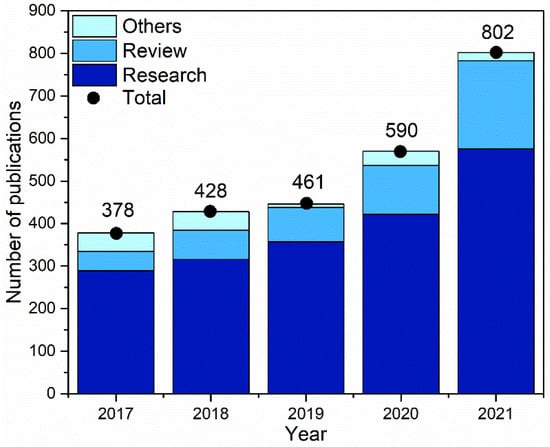
2. Electrodeposition of Common Metals
2.1. Aluminum
Aluminum plays an essential role as an excellent coating material due to its thermal properties, electrical conductivity, light weight, and corrosion resistance
[5]. In addition, Al coatings and alloys tend to develop a natural passive oxide layer on their surface during contact with air, making them resistant to corrosion in different environments
[25][55]. In recent years, the electrodeposition of Al from ILs has generated great interest among academic and industry researchers due to its industrial potential, benefiting many industries such as automotive, aeronautics, naval, construction, electronics, and others
[26][56]. The outstanding characteristics are the ability to operate at low deposition temperatures, ease of preparation, low cost, and high electrochemical stability. Furthermore, chloroaluminate ILs are the simplest compounds, containing inorganic halide anions and organic cations
[27][171], which, in solution, form the ions [(Al
2Cl
7)]
− and [(AlCl
4)]
− as electroactive species available for reduction.
Several authors change the AlCl
3/IL molar ratio with the ionic cation to improve conductivity and electrochemical potential window and favor electroplating on an industrial scale. The feasibility of the electrodeposition process depends on the AlCl
3/IL molar ratio and the characteristic of the studied IL. For [C
5H
14N
3+][CF
2ClCFClOCF
2CF
2SO
3−], the studied molar ratio ranged from 0.9 to 1.3. This composition range showed better conductivity values that allowed sufficient mobility of the electroactive species
[28][155]. At room temperature, [C
5H
14N
3+][CF
2ClCFClOCF
2CF
2SO
3−], due to its immiscibility in water, produced excellent deposits for an AlCl
3/IL molar ratio of 0.95:1
[29][154]. For AlCl
3/4-EP, the reduction current with a molar ratio of 1.3:1 was greater than that with a molar ratio of 1.1:1, suggesting that the reduction of [AlCl
2(4-EP)
2]
+ is thermodynamically favorable than [AlCl
2(4-EP)
2]
+ [30][124].
For BMIC, with a 1:2 molar ratio, the deposition of Al on a copper substrate was efficient at 90 °C
[31][57]. In contrast, Wang et al.
[32][58], inverting the proportion to 2:1, evaluated the temperature variation during the electrodeposition process of Al on Cu. They demonstrated that at 100 °C, there was a better diffusion and/or migration of Al
2Cl
7− ions, generating an increase in current density with a contribution to greater nucleation and efficiency of Al deposition. However, temperatures higher than room temperature may not favor electrodeposition on IL
[33][59] since these conditions also depend on the IL and the porosity of the substrate
[25][55].
The Al deposit studies have a rich variation of the AlCl
3/IL ratio. However, [EMIm]Cl stands out as the main IL for the electrodeposition of Al and its alloys
[34][35][82,83]. The most evaluated AlCl
3/[EMIm]Cl ratio was 2:1
[11][19][36][11,84,85]. However, ratios between 1:1 and 2:1
[37][38][54,86] and 1:2
[35][83] also obtained excellent deposition results. [EMIm]Cl confers a less negative applied potential and a higher current density to Al deposition in addition to a uniform coating and instantaneous nucleation of the coating, promoting lower energy consumption during the deposition.
The lower energy consumption and uniform coating have sparked interest in the Li-ion battery field. For example, Peng et al.
[39][87] studied [EMIm]Cl as an electrolyte for the deposition of Al on Ni. As a result, they obtained a film free of dendrites, improving the corrosion resistance of Ni, and making it suitable for use as anodes of Li-ion batteries. The smooth and uniform deposition was also obtained at a current density of 20 mA/cm
2 for the uranium substrate, significantly increasing the corrosion resistance
[40][88]. Another recent advance was the magnetohydrodynamic (MHD) effect of the AlCl
3–[EMIm]Cl composition in reducing the activation energy required to promote electrochemical reduction
[41][89]. In addition, it increased the capacitance of the electrical double layer and promoted greater mass transport of Al
[42][90].
The AlCl
3/IL ratio inspires researchers to develop Al alloys from electrolytes containing ILs. Several alloys were synthesized in different temperature ranges, such as Al–Li
[43][91] and Al–Ga
[44][92] at 25 °C, Al–Mg
[45][23] at 50 °C, and Al-Cu
[46][35] at 70 °C. However, the two most cited Al alloys in the literature refer to Al–Ti and Al–W. The 2:1 AlCl
3–[EMIm]Cl electrolyte containing TiCl4 (0.1 M) resulted in an Al–Ti alloy deposited on a copper electrode after a multi-step reduction
[47][94]. The reduction to TiCl
42− contributed to the codeposition of Ti on Al, with a Ti content of up to 7.37%. In contrast, Xu et al.
[48][62], using [Bmim][Cl] as an electrolyte, obtained an Al–Ti alloy with a Ti content of up to 11.4 at.%.
Another study co-deposited Al on Ti (Ti–Al alloy) using [Bmim][Cl]
[49][63]. The authors maintained the highest proportion of Ti of up to 40%, demonstrating that increasing the IL chain showed a greater ability to control the composition and morphology of Al–Ti coatings. For Al–W, as well as Al deposits, [EMIM][Cl] is the most used cation
[50][95], with W contents in the range of 9–12 at.% using W
6Cl
12 as the W source for AlC
3/EMImCl ratios 2:1
[51][52][96,97].
Unlike the studies cited, Higashino et al.
[51][96] obtained a W content of 20 at.%, with a 1:1.5 ratio of AlCl
3/EMImCl, demonstrating that the increase in the cation made the electrolyte more polarizable. Furthermore, the increase in current density favored the codeposition of W over Al. Bright and adherent deposits of ternary alloys with Al have also been studied. However, the proportions of the other metals added together were at most 10%, demonstrating the need for a careful view of the proportions to favor the codeposition
[53][54][93,125].
In addition, by evaluating parameters such as component proportions, temperature, and current density, additives have been highlighted in the electrolyte composition combined with IL for Al and its alloys. For [BMIM][Cl], additives such as dichloromethane (DCM) and toluene (C
7H
8) were evaluated as improving agents for Al electrodeposition processes
[55][61]. The morphology of the deposits improved with DCM due to its interaction with the [BMIM][Cl] cations, which can be adsorbed on the protruding part of the electrode surface during the electrochemical process. On the other hand, as the interaction of C
7H
8 with the cation was low, the characteristics of the deposit were inferior to those of DCM.
For IL, 3-butyl-1-ethylimidazolium, light aromatic naphtha (LAN)
[56][123], as well as DCM for [BMIM][Cl], also improved the morphology of the deposits due to attraction with the 3-butyl-1-ethylimidazolium cation, demonstrating a brighter appearance to the metal. Although the electrodeposition of Al and its alloys with the ILs in the electrolytic environment is already present in many studies, research on ternary alloys and additives that improve the electrodeposition process can be further explored.
2.2. Iron
Due to the ease of oxidation, Fe is one of the few metals not studied individually in electrodeposition processes. All studies used Fe as a base metal and inducer of the codeposition of other metals to obtain stable and anticorrosive alloys
[57][132] or as an element codeposited by a base metal
[58][133]. Fe–Cu
[59][172] and Fe–Al
[60][47] alloys were obtained by electrodeposition with [Py
1,4]TfO, both on a copper substrate, aimed to “damp” these alloys. For both studies, there was a thin deposition of the alloys on the substrate, in which the interaction phenomenon was charge transfer. The difference between the depositions was the locations of the damping peaks. For Fe–Cu, a damping peak occurred around 680 K, leading to the appearance of Fe particles at the interface between Cu and Fe (Cu + α-Fe phase). The driving force was the diffusion of Cu atoms around the Cu + α-Fe phase particles. For Fe–Al, the peak appeared around 800 K. However, the phenomenon depended on the dissolution of small precipitates or agglomerates of defects during heating. During cooling, the phenomenon depended on the reprecipitation/reagglomeration of the particles. Therefore, [Py
1,4]TfO favored the electrodeposition of Fe alloys, improving the damping of the process.
Although Fe is preferentially studied as an alloy, alloys with Ni were the most found considering Fe codeposited
[58][61][62][49,77,133]. One of these studies aimed to improve the anticorrosion properties of the Watts bath of Ni–Fe alloy with boric acid
[63][52]. [BMIM]HSO
4 improved the nucleation and three-dimensional growth of Ni–Fe alloy grains due to the increase in charge transfer involved in the process
[61][49]. For the authors, the anticorrosive improvement was evident.
2.3. Nickel
Most studies involving Ni use the NiCl
2 salt as the metal source since the Cl ions, together with the ILs, show the viscosity and electrical conductivity of the medium can be improved, in addition to improving the Ni nucleation mechanism
[12][21][12,145]. Baths containing choline chloride favor electrodeposition, generating instantaneous three-dimensional nucleation at negative potentials. In addition to urea, ethylene glycol can also form an IL with choline chloride for Ni deposition. Diffusion controls this process. In this way, the formation of nanostructured Ni films improves the surface area of the electrode, promoting an increase in the catalytic activity in the hydrogen evolution reaction
[18].
Since Ni is an excellent inductor for the co-deposition of other metals, unusual metals have been co-deposited with Ni. Choline chloride with ethylene glycol was also crucial for depositing Ni–Sn
[64][134] and Ni–Sn–P
[65][137] alloys from ILs. Rosoiu et al.
[64][134] deposited Ni–Sn by a pulsed and direct current. However, using pulsed current, the hardness of the Ni–Sn alloy showed better results. In another study, although the authors did not assess the hardness of Ni-Sn-P alloy synthesized from choline chloride-ethylene glycol, this alloy exhibited superior corrosion resistance to Ni-P coatings
[65][137]. Furthermore, its corrosion resistance improved with increasing Sn content. According to Yang et al.
[66][105], EMIC–EG (1:2 molar ratio) improved the induction of Ni to deposit Ni–La alloy with instantaneous nucleation and three-dimensional growth. In addition to the use of IL in the electrolyte solution, the control of current density and temperature resulted in the CFC or amorphous structure of the alloy.
The evaluation of catalytic activity during the electrodeposition of Ni and its alloys using ILs has been growing since ILs have significant effects on parameters such as current density, pH, and deposition time
[67][68][135,173]. According to Gao et al.
[69][164], the catalytic activity in BMP–DCA medium was verified due to low overpotentials (−279 mV) even operating at 100 mA cm
−2. This demonstrates that Ni–La or Ni–rare earth alloys can be important in the evaluation of hydrogen evolution processes. In addition to this process, Jesmani et al.
[70][78] evaluated the electrocatalytic properties of Ni–Mo alloys for methanol oxidation in a modified Watts bath (EMIM [Br]). The increase in current density (from 1 to 6 A/dm
2) decreased the Mo content, reducing the catalytic activity of the process. The optimal current density was 3.5 A/dm
2, showing better catalytic performance. Likewise, another study with EMIM[Br] also showed excellent catalytic activity for the methanol oxidation process
[71][79]. However, changes in pH directly interfered with current density. The best catalytic activity occurred at pH 7 with a current density of 15 mA/cm
2.
2.4. Cobalt
Cobalt and cobalt-based alloys are essential materials in many industrial and technological fields due to their magnetic properties, hardness, thermal stability, and corrosion resistance
[72][138]. As a ferromagnetic, its applicability includes microelectronics, high-density magnetic storage media, spintronics, drug and gene delivery, and immunological magnetic separation
[73][74][33,139]. However, most Co depositions show high overpotentials due to the formation of negatively charged metal complexes due to the coordination of anions at room temperature
[75][120]. Therefore, studies seek external and internal modifications to improve the deposition of Co. Ries et al.
[76][72], through a rigorous diffusional control, obtained a deposit of Co at 50 °C from a solution containing [BMI][BF
4], producing a diffusion coefficient of 8.21 × 10
−10 cm
2 s
−1. In addition, the Co films were compact, smooth, shiny, and adherent to the substrate, resulting in crack-free metallic Co layers.
Alloys containing Co as the base metal has been little discussed recently in electrodeposition with ILs in the electrolyte solution composition
[77][78][79][99,126,141]. However, as with Fe, studies report Co as a metal co-deposited by Ni since the ferromagnetic properties of these elements favor their affinity
[13][15][13,15]. The peak reduction of the Ni–Co alloy can be observed between −0.6 and −1.2 V using choline chloride as the IL. The highest reduction peak occurred at −1.0 V, which favored the diffusional process deposited with 20 wt.% of Co
[15]. However, by adding ethylene glycol to form a eutectic solvent, the diffusion process reduced the Co content to about 12 wt.%. This reduction indicated a worsening in corrosion protection.
[MOFIM]I is considered to be a prominent additive for Watts baths of Ni–Mo alloys
[80][20]. In contrast, [FPIM]Br was an effective bleach for smooth and uniform Ni, Co, and Ni–Co deposits due to the reduced grain sizes imposed by IL
[81][21]. Likewise, [FPIM]Br, compared to [MOFIM]I, resulted in deposits with less improved morphology, obtaining an atomic radius of 51.1 nm, almost twice the atomic radius using [MOFIM]I (28 nm)
[82][19]. In addition, there was a better inhibition efficiency, obtaining a Co content of up to 30 wt.%.
2.5. Copper
Due to its high electrical conductivity, copper is the most applicable metal in the electronics industry. In galvanizing processes, copper-based films have a wide application as an undercoat for other metallic finishes as they cover minor imperfections in the substrate
[83][84][67,174]. Due to these properties, Cu electrodeposition is one of the preferred methods for introducing ILs into electrolyte solutions, second only to Al
[85][86][100,109]. As with Al, the major Cu source for electrodeposition using ILs is metal chloride
[87][88][32,175].
As with most studies with other metals, choline chloride-based ILs also promote a homogeneous, smooth, shiny, and corrosion-resistant deposit due to their ability to generate hydrophobicity on the metal surface
[89][90][113,118]. However, the addition of nicotinic acid (NA) in the electrolyte can inhibit the electrochemical processes due to the formation of transient species near the electrode surface, influencing the nucleation kinetics and the faster growth of Cu deposits
[91][140]. In contrast, sodium bromide modified the morphology of the deposits, not only of Cu, but also of Al, Sn, Ag, and Ni
[92][136]. However, the electrochemical results did not indicate significant differences compared to the deposits formed by ChCl–EG without additives.
Recent studies highlight the use of Cu
2O as a source of Cu for its deposition under suitable dissolution conditions
[85][93][100,112]. According to He et al.
[93][112], Cu
2O dissolved in an electrolyte containing [EMIM]F–urea–H
2O at 353 K. Likewise, in another study, theu se of urea–EMIC as an electrolyte at 363 K increased the diffusion coefficient of the reaction to 1.02 × 10
−10 m
2/s. In addition, urea–EMIC efficiently dissolved Cu
2O, improving the diffusion reaction, nuclei formation capacity, and Cu grain growth.
In contrast to pure copper deposition studies, for Cu–Pb alloys, the use of [Hbet][TFSA] promoted a complete dissolution of a mixture of water and CuO/PbO. The alloy morphology was altered by the nitrate reduction capacity
[94][127] and the glucose oxidation
[95][128]. Therefore, the system showed greater flexibility for electrocatalytic reduction or oxidation activities. The Cu–Sn alloy, popularly known as bronze, has good anti-corrosion properties. However, studies seek to improve the ratio of Cu:Sn using IL based on imidazolium cation, such as [Bmim]Cl
[96][97][65,66], [EMIm]DCA
[98][76], [EMIM]Cl
[99][101], and [EMIm][TFSA]
[100][114], with an Sn content of 13.7 at.%
[96][97][65,66], 15 at.%
[99][101], and 25 at.%, respectively
[100][114].
In addition to improving anticorrosive properties, Jie et al.
[96][65] reported that [BMIM]Cl in the Cu–Sn deposition system resulted in a crystalline plane (111). Due to the IL, the coloration became darker, making it interesting for industries that produce products with decorative aspects. The cyclic voltammetry peaks of Cu with [BMIM]Cl occur in two steps, 0.45~0.55 V for Cu
2+ → Cu
+ and 1.25~−1.5 V for Cu
+ → Cu
0, which could change the current density of the system during the process
[96][97][65,66]. However, the reduction of the Cu–Sn alloy covered an intermediate reduction peak between −0.5 and −1.0 V, without interference in the alloy synthesis, with the reduction process occurring spontaneously and irreversibly. On the other hand, a system with [EMIm]DCA, polycarbonate membranes, and polystyrene models did not obtain a Cu–Sn alloy in the plane (111)
[98][76]. The authors reported an oriented Cu
6Sn
5 alloy with nanofibers in the structure (221), being interesting for application in Li-ion batteries as anodic hosts.
2.6. Zinc
Zn is a low-cost material widely used in electroplating for application in the automotive and aerospace industries
[101][176]. In addition, it has applications for energy storage due to the reversible capacity of its deposits
[102][103][115,167]. However, the control of Zn alloys can be improved in terms of morphology
[104][75], resulting in nanotubes
[105][116]. In this context, IL-based electrolytes, instead of conventional aqueous-based electrolytes, have been promising in reducing harmful morphologies and forming a uniform metallic film.
The main dissolution of Zn for electrodeposition in ILs uses ZnCl
2 [17][106][17,80]. The IL control variables, such as viscosity, density, and conductivity for Zn electrodeposition, are analyzed with ZnCl
2 as the Zn source
[107][27]. This control of the properties of an electrolyte containing ChCl–EG in a 1:2 ratio can result in Zn–Co alloys with corrosion resistivity up to 30 times higher than uncoated Cu
[79][141]. ChCl–EG also improved charge transfer by agglomerating Zn–Ce alloy particles
[108][142]. The Ce deposit occurred in cathodic and higher pH areas, demonstrating a tendency to improve the self-repair capacity of the alloy due to the Ce content.
Corrosion resistance was also evaluated for the ternary Zn–Mn–Sn alloy synthesized from an IL
[109][143]. The control of the Sn content occurred due to the increase of the concentration and the potential. In addition, the ease of dissolution in Sn
2+ and ChCl–EG improves the conductivity of the electrolyte solution. In this sense, both studies demonstrated the affinity of Zn and its alloys with ChCl–EG, encouraging the synthesis of new materials.