
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gregory Barshtein | -- | 1907 | 2022-12-06 09:20:41 | | | |
| 2 | Beatrix Zheng | + 1 word(s) | 1908 | 2022-12-13 02:30:11 | | |
Video Upload Options
The potential use of nanomaterials in medicine offers opportunities for novel therapeutic approaches to treating complex disorders. For that reason, a new branch of science, named nanotoxicology, which aims to study the dangerous effects of nanomaterials on human health and on the environment, has recently emerged. However, the toxicity and risk associated with nanomaterials are unclear or not completely understood. The development of an adequate experimental strategy for assessing the toxicity of nanomaterials may include a rapid/express method that will reliably, quickly, and cheaply make an initial assessment. One possibility is the characterization of the hemocompatibility of nanomaterials, which includes their hemolytic activity as a marker.
1. Introduction
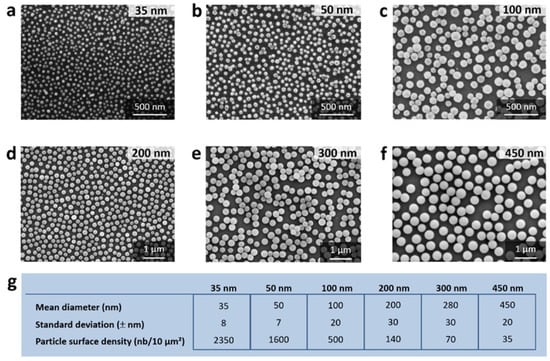
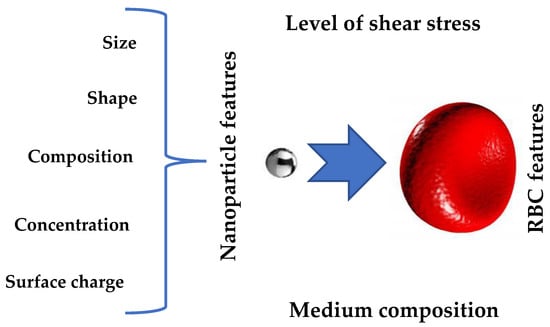
2. Methods for Assessment of Nanomaterials’ Hemotoxicity
References
- Ding, H.; Zhang, J.; Zhang, F.; Xu, Y.; Liang, W.; Yu, Y. Nanotechnological approaches for diagnosis and treatment of ovarian cancer: A review of recent trends. Drug Deliv. 2022, 29, 3218–3232.
- Parhiz, H.; Khoshnejad, M.; Myerson, J.W.; Hood, E.; Patel, P.N.; Brenner, J.S.; Muzykantov, V.R. Unintended effects of drug carriers: Big issues of small particles. Adv. Drug Deliv. Rev. 2018, 130, 90–112.
- Ding, Y.N.; Xue, M.; Tang, Q.S.; Wang, L.J.; Ding, H.Y.; Li, H.; Gao, C.C.; Yu, W.P. Immunotherapy-based novel nanoparticles in the treatment of gastrointestinal cancer: Trends and challenges. World J. Gastroenterol. 2022, 28, 5403–5419.
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71.
- Malatesta, M. Transmission Electron Microscopy as a Powerful Tool to Investigate the Interaction of Nanoparticles with Subcellular Structures. Int. J. Mol. Sci. 2021, 22, 12789.
- Yohan, D.; Chithrani, B.D. Applications of nanoparticles in nanomedicine. J. Biomed. Nanotechnol. 2014, 10, 2371–2392.
- Chen, F.; Hong, H.; Shi, S.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Engineering of Hollow Mesoporous Silica Nanoparticles for Remarkably Enhanced Tumor Active Targeting Efficacy. Sci. Rep. 2014, 4, 5080.
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912.
- Jiang, Y.; Zheng, W.; Tran, K.; Kamilar, E.; Bariwal, J.; Ma, H.; Liang, H. Hydrophilic nanoparticles that kill bacteria while sparing mammalian cells reveal the antibiotic role of nanostructures. Nat. Commun. 2022, 13, 197.
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78.
- Meen, T.-H.; Tsai, J.-K.; Chao, S.-M.; Lin, Y.-C.; Wu, T.-C.; Chang, T.-Y.; Ji, L.-W.; Water, W.; Chen, W.-R.; Tang, I.-T.; et al. Surface plasma resonant effect of gold nanoparticles on the photoelectrodes of dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8, 450.
- Petithory, T.; Pieuchot, L.; Josien, L.; Ponche, A.; Anselme, K.; Vonna, L. Size-Dependent Internalization Efficiency of Macrophages from Adsorbed Nanoparticle-Based Monolayers. Nanomaterials 2021, 11, 1963.
- Kher, C.; Kumar, S. The Application of Nanotechnology and Nanomaterials in Cancer Diagnosis and Treatment: A Review. Cureus 2022, 14, e29059.
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206.
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141.
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627.
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134.
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96.
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339.
- De La Cruz, G.G.; Rodríguez-Fragoso, P.; Reyes-Esparza, J.; Rodríguez-López, A.; Gómez-Cansino, R.; Rodriguez-Fragoso, L. Interaction of Nanoparticles with Blood Components and Associated Pathophysiological Effects. In Unraveling the Safety Profile of Nanoscale Particles and Materials; de Casto Gomez, A.F.S.M., Ed.; IntechOpen: London, UK, 2018; pp. 168–180.
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454.
- Greish, K.; Thiagarajan, G.; Ghandehari, H. In Vivo Methods of Nanotoxicology. Methods Mol. Biol. 2012, 926, 235–253.
- Santamaria, A. Historical Overview of Nanotechnology and Nanotoxicology. Methods Mol. Biol. 2012, 926, 1–12.
- Luyts, K.; Napierska, D.; Nemery, B.; Hoet, P.H.M. How physico-chemical characteristics of nanoparticles cause their toxicity: Complex and unresolved interrelations. Environ. Sci. Process. Impacts 2013, 15, 23–38.
- Wu, Y.-L.; Putcha, N.; Ng, K.W.; Leong, D.T.; Lim, C.T.; Loo, S.C.J.; Chen, X. Biophysical Responses upon the Interaction of Nanomaterials with Cellular Interfaces. Accounts Chem. Res. 2012, 46, 782–791.
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8.
- de la Harpe, K.M.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209.
- Stone, V.; Johnston, H.; Schins, R.P.F. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit. Rev. Toxicol. 2009, 39, 613–626.
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. Methods Mol. Biol. 2019, 1894, 1–29.
- Odeyemi, S.W.; De La Mare, J.; Edkins, A.L.; Afolayan, A.J. In vitro and in vivo toxicity assessment of biologically synthesized silver nanoparticles from Elaeodendron croceum. J. Complement. Integr. Med. 2019, 16.
- Pardeshi, S.R.; More, M.P.; Patil, P.B.; Mujumdar, A.; Naik, J.B. Statistical optimization of voriconazole nanoparticles loaded carboxymethyl chitosan-poloxamer based in situ gel for ocular delivery: In vitro, ex vivo, and toxicity assessment. Drug Deliv. Transl. Res. 2022, 12, 3063–3082.
- Yazhiniprabha, M.; Vaseeharan, B. In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109763.
- Yazhiniprabha, M.; Vaseeharan, B.; Sonawane, A.; Behera, A. In vitro and In vivo toxicity assessment of phytofabricated ZnO nanoparticles showing bacteriostatic effect and larvicidal efficacy against Culex quinquefasciatus. J. Photochem. Photobiol. B Biol. 2019, 192, 158–169.
- Basith, S.; Manavalan, B.; Shin, T.H.; Park, C.B.; Lee, W.-S.; Kim, J.; Lee, G. The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials 2022, 12, 2656.
- Nemmar, A.; Hoet, P.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of Inhaled Particles Into the Blood Circulation in Humans. Circulation 2002, 105, 411–414.
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.M.; Verbruggen, A.; Nemery, B. Passage of Intratracheally Instilled Ultrafine Particles from the Lung into the Systemic Circulation in Hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668.
- Blank, F.; von Garnier, C.; Gehr, P.; Rothen-Rutishauser, B. Translocation across the Air–Blood Tissue Barrier. In Nanoparticles in the Lung, 1st ed.; Tsuda, A., Gehr, P., Eds.; CRC Press: Boca Raton, FL, USA, 2015.
- Barshtein, G.; Livshits, L.; Shvartsman, L.D.; Shlomai, N.O.; Yedgar, S.; Arbell, D. Polystyrene Nanoparticles Activate Erythrocyte Aggregation and Adhesion to Endothelial Cells. Cell Biophys. 2015, 74, 19–27.
- Han, Y.; Wang, X.; Dai, H.; Li, S. Nanosize and Surface Charge Effects of Hydroxyapatite Nanoparticles on Red Blood Cell Suspensions. ACS Appl. Mater. Interfaces 2012, 4, 4616–4622.
- Guo, S.; Shi, Y.; Liang, Y.; Liu, L.; Sun, K.; Li, Y. Relationship and improvement strategies between drug nanocarrier characteristics and hemocompatibility: What can we learn from the literature. Asian J. Pharm. Sci. 2021, 16, 551–576.
- Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes. Nanomaterials 2022, 12, 1632.
- Shimizu, Y.; Isoda, K.; Tezuka, E.; Yufu, T.; Nagai, Y.; Ishida, I.; Tezuka, M. Influence of 50-nm polystyrene particles in inducing cytotoxicity in mice co-injected with carbon tetrachloride, cisplatin, or paraquat. Die Pharm. 2012, 67, 712–714.
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.; Dawson, K.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40.
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized polystyrene nanoparticles as a platform for studying bio–nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412.
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.-K. In Vivo Biodistribution and Toxicology of Carboxylated Graphene Quantum Dots. ACS Nano 2013, 7, 6858–6867.
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428.
- Chen, G.; Xiong, S.; Jing, Q.; van Gestel, C.A.; van Straalen, N.M.; Roelofs, D.; Sun, L.; Qiu, H. Maternal exposure to polystyrene nanoparticles retarded fetal growth and triggered metabolic disorders of placenta and fetus in mice. Sci. Total Environ. 2022, 854, 158666.
- Fan, X.; Wei, X.; Hu, H.; Zhang, B.; Yang, D.; Du, H.; Zhu, R.; Sun, X.; Oh, Y.; Gu, N. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere 2021, 288, 132607.
- Li, D.; Sun, W.; Jiang, X.; Yu, Z.; Xia, Y.; Cheng, S.; Mao, L.; Luo, S.; Tang, S.; Xu, S.; et al. Polystyrene nanoparticles enhance the adverse effects of di-(2-ethylhexyl) phthalate on male reproductive system in mice. Ecotoxicol. Environ. Saf. 2022, 245, 114104.
- Yasin, N.A.; El-Naggar, M.E.; Ahmed, Z.S.O.; Galal, M.K.; Rashad, M.M.; Youssef, A.M.; Elleithy, E.M. Exposure to Polystyrene nanoparticles induces liver damage in rat via induction of oxidative stress and hepatocyte apoptosis. Environ. Toxicol. Pharmacol. 2022, 94, 103911.
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44.
- Cheng, L.-C.; Jiang, X.; Wang, J.; Chen, C.; Liu, R.-S. Nano–bio effects: Interaction of nanomaterials with cells. Nanoscale 2013, 5, 3547–3569.
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of Silica Nanoparticle Design on Cellular Toxicity and Hemolytic Activity. ACS Nano 2011, 5, 5717–5728.
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84.
- Yedgar, S.; Hovav, T.; Barshtein, G. Red blood cell intercellular interactions in oxidative stress states. Clin. Hemorheol. Microcirc. 1999, 21, 189–193.
- Ben-Hur, E.; Barshtein, G.; Chen, S.; Yedgar, S. Photodynamic Treatment of Red Blood Cell Concentrates For Virus Inactivation Enhances Red Blood Cell Aggregation: Protection with Antioxidants. Photochem. Photobiol. 1997, 66, 509–512.
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemolytic Effect of Polymeric Nanoparticles: Role of Albumin. IEEE Trans. NanoBiosci. 2011, 10, 259–261.
- Ferenc, M.; Katir, N.; Miłowska, K.; Bousmina, M.; Majoral, J.-P.; Bryszewska, M.; El Kadib, A. Haemolytic activity and cellular toxicity of SBA-15-type silicas: Elucidating the role of the mesostructure, surface functionality and linker length. J. Mater. Chem. B 2015, 3, 2714–2724.
- ASTM E2524-08; Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles. ASTM: West Conshohocken, PE, USA, 2008.
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466.
- Luna, L.A.V.; Martinez, D.S.T.; Alves, O.L. Nanomaterials: From Current Methods to Biomolecular Surface Chemistry Interactions. In Nanotoxicology: Materials, Methodologies and Assessments; Durán, N., Guterres, S.S., Alves, O.L., Eds.; Springer: Sao Paulo, Brazil, 2014.
- Martinez, D.S.T.; Paula, A.J.; Fonseca, L.C.; Luna, L.A.V.; Silveira, C.P.; Durán, N.; Alves, O.L. Monitoring the Hemolytic Effect of Mesoporous Silica Nanoparticles after Human Blood Protein Corona Formation. Eur. J. Inorg. Chem. 2015, 2015, 4595–4602.
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical Characterization and In Vitro Hemolysis Evaluation of Silver Nanoparticles. Toxicol. Sci. 2011, 123, 133–143.
- A Love, S.; Thompson, J.W.; Haynes, C.L. Development of screening assays for nanoparticle toxicity assessment in human blood: Preliminary studies with charged Au nanoparticles. Nanomedicine 2012, 7, 1355–1364.
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. ACS Nano 2011, 5, 1366–1375.
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; An, Y.-L.; Lin, M.; Gao, Z.; Zhang, J. Biocompatibility of composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819.
- Lu, S.; Duffin, R.; Poland, C.; Daly, P.; Murphy, F.; Drost, E.; MacNee, W.; Stone, V.; Donaldson, K. Efficacy of Simple Short-Term in Vitro Assays for Predicting the Potential of Metal Oxide Nanoparticles to Cause Pulmonary Inflammation. Environ. Health Perspect. 2009, 117, 241–247.
- Cho, W.-S.; Duffin, R.; Bradley, M.; Megson, I.L.; MacNee, W.; Lee, J.K.; Jeong, J.; Donaldson, K. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part. Fibre Toxicol. 2013, 10, 55.
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27.
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life–Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656.
- Pan, D.; Vargas-Morales, O.; Zern, B.; Anselmo, A.C.; Gupta, V.; Zakrewsky, M.; Mitragotri, S.; Muzykantov, V. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PLoS ONE 2016, 11, e0152074.
- Kim, M.J.; Shin, S. Toxic effects of silver nanoparticles and nanowires on erythrocyte rheology. Food Chem. Toxicol. 2014, 67, 80–86.
- Lin, Y.-S.; Haynes, C.L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J. Am. Chem. Soc. 2010, 132, 4834–4842.




