Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Donglin Han | -- | 1560 | 2022-12-12 10:48:41 | | | |
| 2 | Catherine Yang | Meta information modification | 1560 | 2022-12-13 02:36:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guo, H.; Chen, L.; Ismail, S.A.; Jiang, L.; Guo, S.; Gu, J.; Zhang, X.; Li, Y.; Zhu, Y.; Zhang, Z.; et al. GDL for Proton Exchange Membrane Fuel Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/38590 (accessed on 08 March 2026).
Guo H, Chen L, Ismail SA, Jiang L, Guo S, Gu J, et al. GDL for Proton Exchange Membrane Fuel Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/38590. Accessed March 08, 2026.
Guo, Hui, Lubing Chen, Sara Adeeba Ismail, Lulu Jiang, Shihang Guo, Jie Gu, Xiaorong Zhang, Yifeng Li, Yuwen Zhu, Zihan Zhang, et al. "GDL for Proton Exchange Membrane Fuel Cells" Encyclopedia, https://encyclopedia.pub/entry/38590 (accessed March 08, 2026).
Guo, H., Chen, L., Ismail, S.A., Jiang, L., Guo, S., Gu, J., Zhang, X., Li, Y., Zhu, Y., Zhang, Z., & Han, D. (2022, December 12). GDL for Proton Exchange Membrane Fuel Cells. In Encyclopedia. https://encyclopedia.pub/entry/38590
Guo, Hui, et al. "GDL for Proton Exchange Membrane Fuel Cells." Encyclopedia. Web. 12 December, 2022.
Copy Citation
Proton exchange membrane fuel cells (PEMFCs) are an attractive type of fuel cell that have received successful commercialization, benefitted from its unique advantages (including an all solid-state structure, a low operating temperature and low environmental impact). In general, the structure of PEMFCs can be regarded as a sequential stacking of functional layers, among which the gas diffusion layer (GDL) plays an important role in connecting bipolar plates and catalyst layers both physically and electrically, offering a route for gas diffusion and drainage and providing mechanical support to the membrane electrode assemblies.
proton exchange membrane fuel cell
gas diffusion layer
macroporous substrate
microporous layer
1. Brief Introduction of PEMFCs
A general structure of PEMFCs is shown in Figure 1. The main role of BPs is to conduct electrons and transport reactants and products through the gas channels [1]. Currently, the most common materials used for BP are graphite, corrosion-resistant metals and carbon-based or metal-based composites [2]. The flow field design of the BPs determines the diffusion and distribution of the gas in PEMFCs, as well as the rate of chemical reactions, the distribution of current density and the removal of liquid water [3]. The GDL mainly plays a role supporting the cell structure and transmitting electrons, reactants, products and heat [4]. At present, carbon fiber paper and cloth are the GDL materials with developed applications and an extensive research base. In order to obtain sufficient electrode reactivity at the low operating temperature of PEMFCs, catalysts containing Pt (e.g., Pt/C) are commonly used in the CL. Moreover, some alloy catalysts like PtRu/C and SnPt/C also show excellent carbon monoxide tolerance and are suitable for application in direct methanol or ethanol PEMFCs [5][6]. The electrolytes used are commonly perfluorinated polymers, which are proton-conducting and electron-insulating and act as a separator for the reactants and the products between the cathode and anode as well [7].
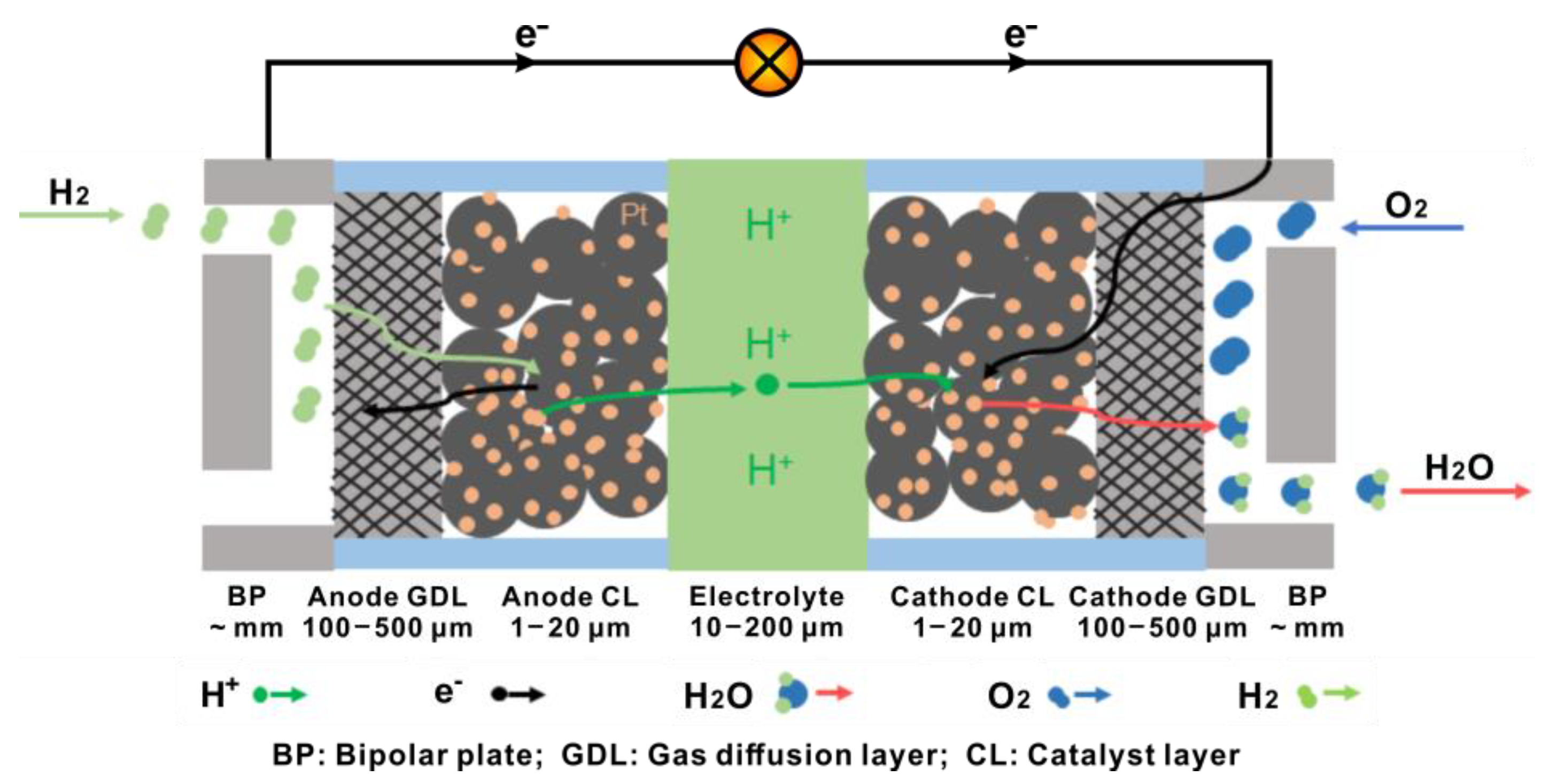
Figure 1. Schematic diagram of the structure of a PEMFC.
Referring to the working mechanism of PEMFCs, as shown in Figure 1, hydrogen reaches the anode CL by passing through the anode GDL and dissociates into protons and electrons, following
Protons move across the electrolyte and react with oxygen gas at the cathode CL, following
The GDL at the cathode side leads the oxygen passing through it to the cathode CL and expels the water produced from the cathode CL out of the system. Since the operating temperature of PEMFCs is usually low (typically around 80 °C), water generated at the cathode is easy to condense into liquid water. If this liquid water is not drained away in time, it occupies the open space in the cathode CL and GDL and causes flooding. This blocks the transport channels for oxygen, leading to a limited current and a dramatic degradation in the performance of PEMFCs during operation [8][9][10].
2. General Requirements for GDL
The GDL is one of the core components of PEMFCs, located between and connecting the BP and CL. It provides support not only for the MEA, but also for the route for reactants and drainage, and it has the following requirements.
- (1)
-
High gas permeability. The pore structure of GDL impacts the transport properties greatly, since the gas involved in the reaction needs to pass through the GDL to reach the CL to participate in the electrochemical reaction. The electrolyte membrane also needs to be hydrated by being subjected to the wet atmosphere to maintain high proton conductivity. Moreover, water generated from the reaction in PEMFCs needs adequate routes to be drained without flooding the CL and GDL [11][12][13]. Zhou et al. [14] found that the ability of mass transfer was enhanced by reducing the length of the channel for gas flow and increasing the porosity of the anode GDL (A-GDL). Sim et al. [15] reported a new approach to increase the porosity and pore size of GDL by changing the gasket thickness, resulting in a positive effect on the drainage capacity.
- (2)
-
High electrical conductivity. The GDL provides the electrical connection between the CL and the BP. It delivers electrons from the BP to cathode CL, and collects electrons from anode CL to the BP. Therefore, high electrical conductivity is required for a GDL to decrease its ohmic loss and contact resistance with the adjacent BP and CL [16].
- (3)
-
High thermal conductivity. During the operation of PEMFCs, heat is generated and tends to accumulate locally. If the extra heat is not removed quickly from the system, the increasing temperature shortens the longevity of PEMFCs. Thereby, the GDL must have high thermal conductivity to conduct the extra heat quickly to the BP to ensure a safe system temperature. Botelho et al. [17] applied a resistance network theory to estimate effective thermal contact resistance between the carbon fibers used for composing the GDL. They found that the change in fiber roughness led to a large change in the effective thermal contact resistance, implying a feasible approach to regulate the thermal conduction of the GDL by controlling the morphology of the carbon fibers.
- (4)
-
Good mechanical strength. The GDL must be robust enough to offer mechanical support to the MEA and protect the CL and the electrolyte membrane. Furthermore, a mechanically stable porous structure is vital to construct and maintain channels for gas diffusion and drainage. Csoklich et al. [18] suggested that it was efficient to improve the performance of the GDL by optimizing the structure rather than improving the thermal conduction in order to achieve excellent performance at high current density.
- (5)
-
Good chemical/thermal stability and corrosion resistance. To minimize the degradation rate during long-term operation, the GDL is required to be stable both chemically and thermally, as well as corrosion-resistant in both oxidizing and reducing atmospheres in the chambers of cathode and anode, respectively.
- (6)
-
Facilitation of water removal. To facilitate the drainage at the cathode, the GDL at the cathode is usually processed to be hydrophobic. Moreover, other factors (such as the geometric parameters of the carbon fibers composing the GDL) also effect the hydrophobicity. Wang et al. [19] found that the speed of water discharge was enhanced by increasing the diameter of the carbon fibers in the GDL.
- (7)
-
Low cost. The volumetric ratio of the GDL in PEMFCs is not low (larger than that of the CL and electrolyte), so it is necessary to choose proper materials with both a low cost and a high performance.
3. Structural Parameters for GDL
3.1. Pore Structure
The water management is one of the main functions of the MPL, whose porosity and pore size distribution impose significant influence. Chun et al. [20] regulated the condition for drying to change the distribution of pore size in the MPL, and found that higher drying temperatures led to rapid decomposition of the pore-forming agent, resulting in the formation of macropores. By lowering the drying temperature, the decomposition of pore-forming agent slowed down, giving rise to the formation of more micropores. The micropores showed a better capillary effect to remove water, while the macropores can provide better gas transport. So, a proper combination and distribution of the pores with different scales is necessary to optimize the performance of the MPL. Furthermore, different humidity conditions during the operation of PEMFCs also ask for different pore distribution. Hou et al. [21] established a three-dimensional Boltzmann model to simulate the porous transport layer by adjusting the porosity and distribution of pore size in MPL. It was found that even with the same porosity, the distribution of pore size influences the water transport significantly, suggesting that the porosity and distribution of pore size must be carefully determined to match the working conditions.
Lin et al. [22] prepared a gradient double-layer MPL structures by using conductive carbon black with different particle size, so as to achieve gradual change of pore size from GDL to CL, with the aim to improve the water management ability. They therefore designed a GDL with an MPL containing two layers that were different in pore size by using two types of conductive carbon blacks—one is acetylene carbon black (7–20 μm), the other is Vulcan XC-72 (20–100 μm).
3.2. Thickness
MPL must has proper thickness to regulate the water balance between the CL and GDL. If the MPL is too thin, it cannot collect water efficiently from the CL. Tseng et al. [23] compared the performance of three PEMFCs with different MPL thicknesses and found that the MPL with a thickness of 84 μm showed the best performance over a certain range of current density (300–1300 mAcm−2). They also studied the influence of the distribution of pore size in MPLs with three different thicknesses and found that when the MPL was too thin and the pore size was too small, the gas transport was hindered. On the other hand, when MPL was too thick, the diffusion path turned to be too long, resulting in greater resistance. Therefore, it can be concluded that the MPL with an appropriate thickness is essential to ensure the overall transport of gas and water. Lin et al. [24] investigated the effect of the MPL thickness on the performance of PEMFCs. They prepared a bilayer MPL by spraying two slurries containing the conductive carbon black with a different size. It was found that the performance of the single cell changed with the adjustment of the thickness of the two MPL layers (MPL1 and MPL2). The authors tested the performances of three fuel cells using GDL24 (thickness of MPL1: 20 ± 2.0 μm, MPL2: 40 ± 1.6 μm), GDL33 (MPL1: 30 ± 1.5 μm, MPL2: 30 ± 2.5 μm) and GDL42 (MPL1: 40 ± 1.8 μm, MPL2: 20 ± 3.1 μm).
References
- Chiu, L.Y.; Diong, B.; Gemmen, R.S. An Improved Small-Signal Model of the Dynamic Behavior of PEM Fuel Cells. IEEE Trans. Ind. Appl. 2004, 40, 970–977.
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53.
- Manso, A.P.; Marzo, F.F.; Barranco, J.; Garikano, X.; Garmendia Mujika, M. Influence of geometric parameters of the flow fields on the performance of a PEM fuel cell. A review. Int. J. Hydrogen Energy 2012, 37, 15256–15287.
- Ge, J.; Higier, A.; Liu, H. Effect of gas diffusion layer compression on PEM fuel cell performance. J. Power Sources 2006, 159, 922–927.
- Acres, G.J.K.; Frost, J.C.; Hards, G.A.; Potter, R.J.; Ralph, T.R.; Thompsett, D.; Burstein, G.T.; Hutchings, G.J. Electrocatalysts for fuel cells. Catal. Today 1997, 38, 393–400.
- Ross, P.N.; Kinoshita, K.; Scarpellino, A.J.; Stonehart, P. Electrocatalysis on Binary-Alloys.1. Oxidation of Molecular-Hydrogen on Supported Pt-Rh Alloys. J. Electroanal. Chem. 1975, 59, 177–189.
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384.
- Gurau, V.; Zawodzinski, T.A.; Mann, J.A. Two-phase transport in PEM fuel cell cathodes. J. Fuel Cell Sci. Technol. 2008, 5, 021009.
- Masuda, H.; Ito, K.; Oshima, T.; Sasaki, K. Comparison between numerical simulation and visualization experiment on water behavior in single straight flow channel polymer electrolyte fuel cells. J. Power Sources 2008, 177, 303–313.
- Passalacqua, E.; Squadrito, G.; Lufrano, F.; Patti, A.; Giorgi, L. Effects of the diffusion layer characteristics on the performance of polymer electrolyte fuel cell electrodes. J. Appl. Electrochem. 2001, 31, 449–454.
- Zenyuk, I.V.; Parkinson, D.Y.; Hwang, G.; Weber, A.Z. Probing water distribution in compressed fuel-cell gas-diffusion layers using X-ray computed tomography. Electrochem. Commun. 2015, 53, 24–28.
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880.
- Deevanhxay, P.; Sasabe, T.; Tsushima, S.; Hirai, S. Observation of dynamic liquid water transport in the microporous layer and gas diffusion layer of an operating PEM fuel cell by high-resolution soft X-ray radiography. J. Power Sources 2013, 230, 38–43.
- Zhou, H.R.; Chen, B.; Meng, K.; Luo, M.J.; Li, P.; Tu, Z.K. Combination effect of flow channel configuration and anode GDL porosity on mass transfer and performance of PEM water electrolyzers. Sustain. Energy Fuels 2022, 6, 3944–3960.
- Sim, J.; Kang, M.; Min, K. Effects of porosity gradient and average pore size in the in-plane direction and disposition of perforations in the gas diffusion layer on the performance of proton exchange membrane fuel cells. J. Power Sources 2022, 544, 231912.
- Owejan, J.P.; Trabold, T.A.; Mench, M.M. Oxygen transport resistance correlated to liquid water saturation in the gas diffusion layer of PEM fuel cells. Int. J. Heat Mass Transf. 2014, 71, 585–592.
- Botelho, S.J.; Bazylak, A. The impact of fibre surface morphology on the effective thermal conductivity of a polymer electrolyte membrane fuel cell gas diffusion layer. J. Power Sources 2014, 269, 385–395.
- Csoklich, C.; Sabharwal, M.; Schmidt, T.J.; Büchi, F.N. Does the thermal conductivity of gas diffusion layer matter in polymer electrolyte fuel cells? J. Power Sources 2022, 540, 231539.
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Sources 2022, 525, 231121.
- Chun, J.H.; Park, K.T.; Jo, D.H.; Lee, J.Y.; Kim, S.G.; Lee, E.S.; Jyoung, J.-Y.; Kim, S.H. Determination of the pore size distribution of micro porous layer in PEMFC using pore forming agents under various drying conditions. Int. J. Hydrogen Energy 2010, 35, 11148–11153.
- Hou, Y.; Li, X.; Du, Q.; Jiao, K.; Zamel, N. Pore-Scale Investigation of the Effect of Micro-Porous Layer on Water Transport in Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2020, 167, 144504.
- Lin, G.; Liu, S.; Yu, B.; Wang, H.; Yu, K.; Hu, Y. Preparation of graded microporous layers for enhanced water management in fuel cells. J. Appl. Polym. Sci. 2020, 137, 49564.
- Tseng, C.-J.; Lo, S.-K. Effects of microstructure characteristics of gas diffusion layer and microporous layer on the performance of PEMFC. Energy Convers. Manag. 2010, 51, 677–684.
- Lin, G.; Liu, S.; Qu, S.; Qu, G.; Li, T.; Liang, Z.; Hu, Y.; Liu, F. Effects of thickness and hydrophobicity of double microporous layer on the performance in proton exchange membrane fuel cells. J. Appl. Polym. Sci. 2020, 138, 50355.
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
13 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





