Proton exchange membrane fuel cells (PEMFCs) are an attractive type of fuel cell that have received successful commercialization, benefitted from its unique advantages (including an all solid-state structure, a low operating temperature and low environmental impact). In general, the structure of PEMFCs can be regarded as a sequential stacking of functional layers, among which the gas diffusion layer (GDL) plays an important role in connecting bipolar plates and catalyst layers both physically and electrically, offering a route for gas diffusion and drainage and providing mechanical support to the membrane electrode assemblies.
- proton exchange membrane fuel cell
- gas diffusion layer
- macroporous substrate
- microporous layer
1. Brief Introduction of PEMFCs
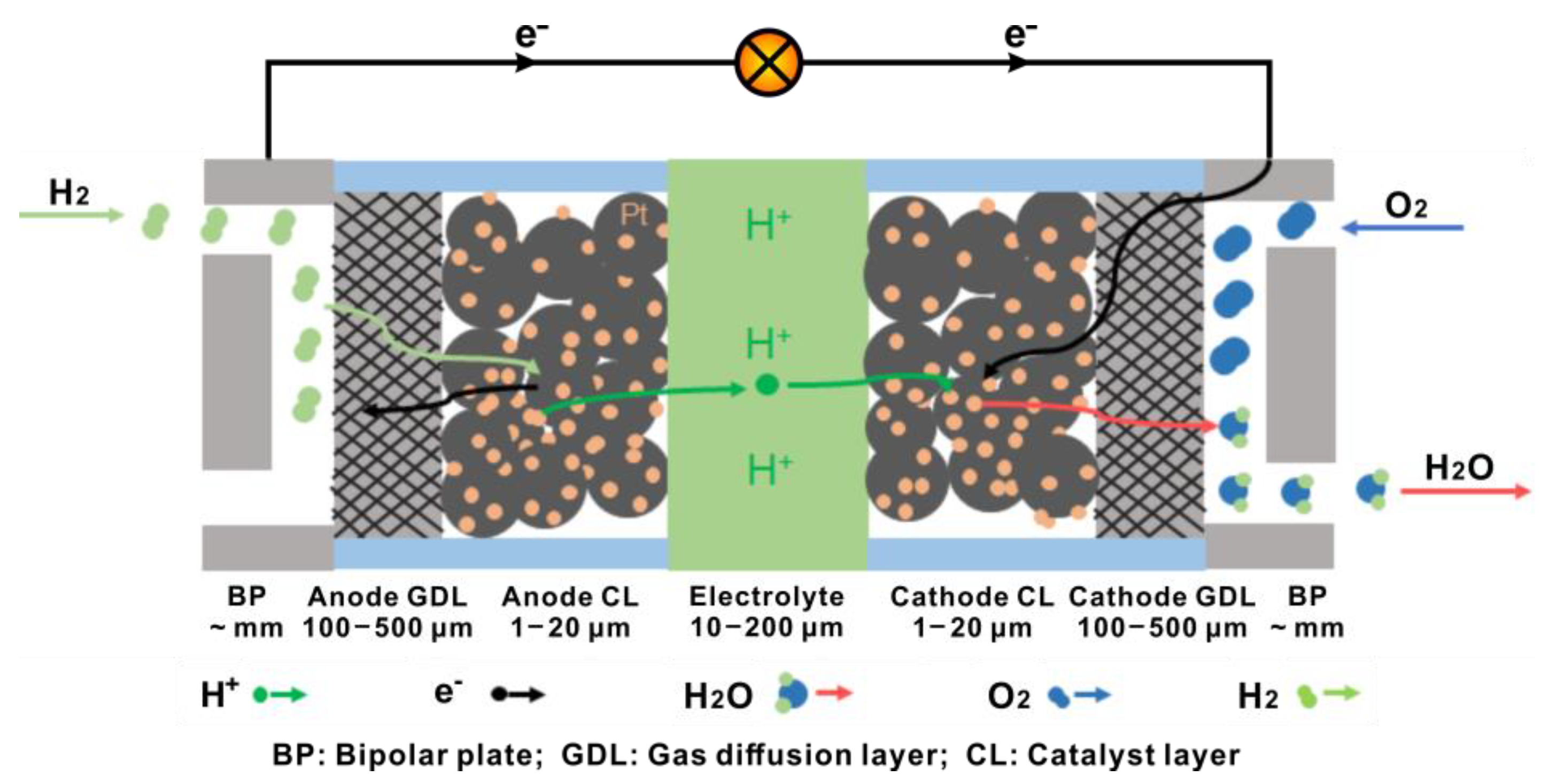
2. General Requirements for GDL
- (1)
-
High gas permeability. The pore structure of GDL impacts the transport properties greatly, since the gas involved in the reaction needs to pass through the GDL to reach the CL to participate in the electrochemical reaction. The electrolyte membrane also needs to be hydrated by being subjected to the wet atmosphere to maintain high proton conductivity. Moreover, water generated from the reaction in PEMFCs needs adequate routes to be drained without flooding the CL and GDL [11][12][13]. Zhou et al. [14] found that the ability of mass transfer was enhanced by reducing the length of the channel for gas flow and increasing the porosity of the anode GDL (A-GDL). Sim et al. [15] reported a new approach to increase the porosity and pore size of GDL by changing the gasket thickness, resulting in a positive effect on the drainage capacity.
- (2)
-
High electrical conductivity. The GDL provides the electrical connection between the CL and the BP. It delivers electrons from the BP to cathode CL, and collects electrons from anode CL to the BP. Therefore, high electrical conductivity is required for a GDL to decrease its ohmic loss and contact resistance with the adjacent BP and CL [16].
- (3)
-
High thermal conductivity. During the operation of PEMFCs, heat is generated and tends to accumulate locally. If the extra heat is not removed quickly from the system, the increasing temperature shortens the longevity of PEMFCs. Thereby, the GDL must have high thermal conductivity to conduct the extra heat quickly to the BP to ensure a safe system temperature. Botelho et al. [17] applied a resistance network theory to estimate effective thermal contact resistance between the carbon fibers used for composing the GDL. They found that the change in fiber roughness led to a large change in the effective thermal contact resistance, implying a feasible approach to regulate the thermal conduction of the GDL by controlling the morphology of the carbon fibers.
- (4)
-
Good mechanical strength. The GDL must be robust enough to offer mechanical support to the MEA and protect the CL and the electrolyte membrane. Furthermore, a mechanically stable porous structure is vital to construct and maintain channels for gas diffusion and drainage. Csoklich et al. [18] suggested that it was efficient to improve the performance of the GDL by optimizing the structure rather than improving the thermal conduction in order to achieve excellent performance at high current density.
- (5)
-
Good chemical/thermal stability and corrosion resistance. To minimize the degradation rate during long-term operation, the GDL is required to be stable both chemically and thermally, as well as corrosion-resistant in both oxidizing and reducing atmospheres in the chambers of cathode and anode, respectively.
- (6)
-
Facilitation of water removal. To facilitate the drainage at the cathode, the GDL at the cathode is usually processed to be hydrophobic. Moreover, other factors (such as the geometric parameters of the carbon fibers composing the GDL) also effect the hydrophobicity. Wang et al. [19] found that the speed of water discharge was enhanced by increasing the diameter of the carbon fibers in the GDL.
- (7)
-
Low cost. The volumetric ratio of the GDL in PEMFCs is not low (larger than that of the CL and electrolyte), so it is necessary to choose proper materials with both a low cost and a high performance.
3. Structural Parameters for GDL
3.1. Pore Structure
3.2. Thickness
This entry is adapted from the peer-reviewed paper 10.3390/ma15248800
References
- Chiu, L.Y.; Diong, B.; Gemmen, R.S. An Improved Small-Signal Model of the Dynamic Behavior of PEM Fuel Cells. IEEE Trans. Ind. Appl. 2004, 40, 970–977.
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53.
- Manso, A.P.; Marzo, F.F.; Barranco, J.; Garikano, X.; Garmendia Mujika, M. Influence of geometric parameters of the flow fields on the performance of a PEM fuel cell. A review. Int. J. Hydrogen Energy 2012, 37, 15256–15287.
- Ge, J.; Higier, A.; Liu, H. Effect of gas diffusion layer compression on PEM fuel cell performance. J. Power Sources 2006, 159, 922–927.
- Acres, G.J.K.; Frost, J.C.; Hards, G.A.; Potter, R.J.; Ralph, T.R.; Thompsett, D.; Burstein, G.T.; Hutchings, G.J. Electrocatalysts for fuel cells. Catal. Today 1997, 38, 393–400.
- Ross, P.N.; Kinoshita, K.; Scarpellino, A.J.; Stonehart, P. Electrocatalysis on Binary-Alloys.1. Oxidation of Molecular-Hydrogen on Supported Pt-Rh Alloys. J. Electroanal. Chem. 1975, 59, 177–189.
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384.
- Gurau, V.; Zawodzinski, T.A.; Mann, J.A. Two-phase transport in PEM fuel cell cathodes. J. Fuel Cell Sci. Technol. 2008, 5, 021009.
- Masuda, H.; Ito, K.; Oshima, T.; Sasaki, K. Comparison between numerical simulation and visualization experiment on water behavior in single straight flow channel polymer electrolyte fuel cells. J. Power Sources 2008, 177, 303–313.
- Passalacqua, E.; Squadrito, G.; Lufrano, F.; Patti, A.; Giorgi, L. Effects of the diffusion layer characteristics on the performance of polymer electrolyte fuel cell electrodes. J. Appl. Electrochem. 2001, 31, 449–454.
- Zenyuk, I.V.; Parkinson, D.Y.; Hwang, G.; Weber, A.Z. Probing water distribution in compressed fuel-cell gas-diffusion layers using X-ray computed tomography. Electrochem. Commun. 2015, 53, 24–28.
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880.
- Deevanhxay, P.; Sasabe, T.; Tsushima, S.; Hirai, S. Observation of dynamic liquid water transport in the microporous layer and gas diffusion layer of an operating PEM fuel cell by high-resolution soft X-ray radiography. J. Power Sources 2013, 230, 38–43.
- Zhou, H.R.; Chen, B.; Meng, K.; Luo, M.J.; Li, P.; Tu, Z.K. Combination effect of flow channel configuration and anode GDL porosity on mass transfer and performance of PEM water electrolyzers. Sustain. Energy Fuels 2022, 6, 3944–3960.
- Sim, J.; Kang, M.; Min, K. Effects of porosity gradient and average pore size in the in-plane direction and disposition of perforations in the gas diffusion layer on the performance of proton exchange membrane fuel cells. J. Power Sources 2022, 544, 231912.
- Owejan, J.P.; Trabold, T.A.; Mench, M.M. Oxygen transport resistance correlated to liquid water saturation in the gas diffusion layer of PEM fuel cells. Int. J. Heat Mass Transf. 2014, 71, 585–592.
- Botelho, S.J.; Bazylak, A. The impact of fibre surface morphology on the effective thermal conductivity of a polymer electrolyte membrane fuel cell gas diffusion layer. J. Power Sources 2014, 269, 385–395.
- Csoklich, C.; Sabharwal, M.; Schmidt, T.J.; Büchi, F.N. Does the thermal conductivity of gas diffusion layer matter in polymer electrolyte fuel cells? J. Power Sources 2022, 540, 231539.
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Sources 2022, 525, 231121.
- Chun, J.H.; Park, K.T.; Jo, D.H.; Lee, J.Y.; Kim, S.G.; Lee, E.S.; Jyoung, J.-Y.; Kim, S.H. Determination of the pore size distribution of micro porous layer in PEMFC using pore forming agents under various drying conditions. Int. J. Hydrogen Energy 2010, 35, 11148–11153.
- Hou, Y.; Li, X.; Du, Q.; Jiao, K.; Zamel, N. Pore-Scale Investigation of the Effect of Micro-Porous Layer on Water Transport in Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2020, 167, 144504.
- Lin, G.; Liu, S.; Yu, B.; Wang, H.; Yu, K.; Hu, Y. Preparation of graded microporous layers for enhanced water management in fuel cells. J. Appl. Polym. Sci. 2020, 137, 49564.
- Tseng, C.-J.; Lo, S.-K. Effects of microstructure characteristics of gas diffusion layer and microporous layer on the performance of PEMFC. Energy Convers. Manag. 2010, 51, 677–684.
- Lin, G.; Liu, S.; Qu, S.; Qu, G.; Li, T.; Liang, Z.; Hu, Y.; Liu, F. Effects of thickness and hydrophobicity of double microporous layer on the performance in proton exchange membrane fuel cells. J. Appl. Polym. Sci. 2020, 138, 50355.
