Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Neetu Sharma | -- | 2650 | 2022-12-12 08:06:42 | | | |
| 2 | Conner Chen | + 1 word(s) | 2651 | 2022-12-12 11:29:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sharma, N.; Singh, G.; Sharma, M.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. Mechanism of Remediation of Contaminated Soil by Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/38553 (accessed on 08 February 2026).
Sharma N, Singh G, Sharma M, Mandzhieva S, Minkina T, Rajput VD. Mechanism of Remediation of Contaminated Soil by Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/38553. Accessed February 08, 2026.
Sharma, Neetu, Gurpreet Singh, Monika Sharma, Saglara Mandzhieva, Tatiana Minkina, Vishnu D. Rajput. "Mechanism of Remediation of Contaminated Soil by Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/38553 (accessed February 08, 2026).
Sharma, N., Singh, G., Sharma, M., Mandzhieva, S., Minkina, T., & Rajput, V.D. (2022, December 12). Mechanism of Remediation of Contaminated Soil by Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/38553
Sharma, Neetu, et al. "Mechanism of Remediation of Contaminated Soil by Nanoparticles." Encyclopedia. Web. 12 December, 2022.
Copy Citation
The varied sources of soil pollution include the application of chemical fertilizers and pesticides, industrial discharge, and transformed products of these accumulated chemical residues. These processes may hamper the composition and soil ecosystem. Different types of methodologies ranging from physical, chemical, and biological approaches have been exploited to tackle this challenge. Nanomaterials (NMs) research has contributed to a new dimension for the remediation of polluted soils.
remediation
sustainable
microbe
metal
nanoparticles
1. Introduction
The increasing accumulation of heavy metals (HMs) in the food and water supply chain is a major cause of public health concern. Their high density and non-biodegradable nature make HMs such as Hg(II), Cr(VI),Pb(II), and Cd(II), the potent and most challenging environmental contaminants [1][2]. Soil acts as an important sink for supporting various lifeforms on earth, ranging from organisms as small as microbes to the most complex ones, i.e., animals and humans. The invasion of natural flora by various anthropogenic sources has resulted in the disruption of the natural cycling of nutrients and the accumulation of many undesired components such as HMs in the soil. The concept of sustainable development is not attainable pertaining to the present scenario of soil pollution. Due to rising populations, soil conservation should be a top priority in today’s society, which is facing a challenging situation of diminishing land area and scarcity of food and shelter. The various approaches employed to tackle this situation involve thermal treatment, filtration, adsorption, chemical abstraction, membrane bound separation, microbial degradation, etc. Heavy metal removal can also be efficiently accomplished by employing methylene phosphonic acid (DTPMP) phosphonate intercalated with layered double hydroxide [3]. In another study, lysine intercalated with montmorillonite was reported to remove Pb (II) from wastewater via an adsorption mechanism [4].
The processes documented above consist of a single approach to treatment. It is true that these treatments have been successful, however, they also have certain drawbacks such as inefficiency, high costs, and failure at scale-up [5][6]. Acid mine drainage (AMD) is a form of pollution caused by drainage water flowing from sulfur-bearing sites into water bodies [7]. The mining of sulfide minerals exposes them to the environment, causing excessive amounts of acid to be produced, which can cause both immediate and long-term environmental harm. Some of the adverse effects of AMD include the corrosion of mining machinery and equipment, degradation of soil quality, and groundwater contamination due to the leaching of HMs present in mine and drainage water [8].
Air or water exposed to iron disulfide or iron pyrite produces acid mine drainage by oxidizing mineral sulfides. Oxygen and water react with metal sulfides to produce metal sulfates and sulfuric acid. Subsequent oxidation of the metals results in increased acidity [9]. In one such example, ferrous sulfide (pyrite) undergoes oxidation on reacting with water and oxygen to form ferrous sulfate and sulfuric acid. Ferrous sulfate oxidizes further to form ferric sulfate, and the rate of this reaction can further be enhanced by the action of certain bacteria such as Acidithiobacillus ferroxidans. Further, ferric sulfate reacts with water to form ferric hydroxide releasing hydrogen ions, which subsequently enhance the acidity of water. The resulting ferric hydroxide formed further reacts with pyrite and produces more acid. The amount of acid produced depends on the amount of iron getting oxidized [10].
FeS2 + H2O + 3 ½ O2 → FeSO4 + H2SO4
FeSO4 → Fe2(SO4)3 → 2Fe3+ + 3SO4−2
Fe3+ + 3H2O → Fe(OH)3 + 3H+
Rainwater or water used in mining operations for dust control, drilling, or other purposes enters the mine as fresh water. Fissures and cracks in underground mines can allow ground water to seep into the mines. Sulfide minerals yield oxidized products that are transported to nearby rivers and other water bodies by flowing into the surrounding aqueous environment [11]. As pyritic sulfur reacts with water and oxygen, sulfuric acid is produced, and iron sulfate is formed. As a result, certain acidophilic bacteria such as Acidithiobacillus ferroxidans thrive and grow in this type of acidic environment created by coal mines. As a result, the acid production reaction is catalyzed by the bacteria and occurs more quickly than chemical oxidation [12]. The acidity in mine drainage water is primarily due to the production of sulfuric acid and hydrolysis of oxidized pyrite products [11].
The selection of any remediation technique employed for the removal of HMs is governed by several factors including the type and nature of the contaminant, its concentration, its form (simpler or complex form), the objective and time frame for treatment, the cost involved, and the environmental impact. Furthermore, treatment techniques are categorized into in situ and ex situ types depending on the nature and location of the site, the degree of contamination, and the treatment strategy to be employed (Figure 1). The former category is the most preferred as it employs the treatment of soil at its natural site by utilizing air, water, microbes, and plants. On the other hand, the latter is based on the excavation of contaminated soil to a point where it can be treated, i.e., into a fermenter, which makes it more complex and ultimately leads to a higher cost. All the conventional methods being employed today have several drawbacks including cost, time frame, and the release of by products, which result in post-treatment challenges involving environmental contamination.
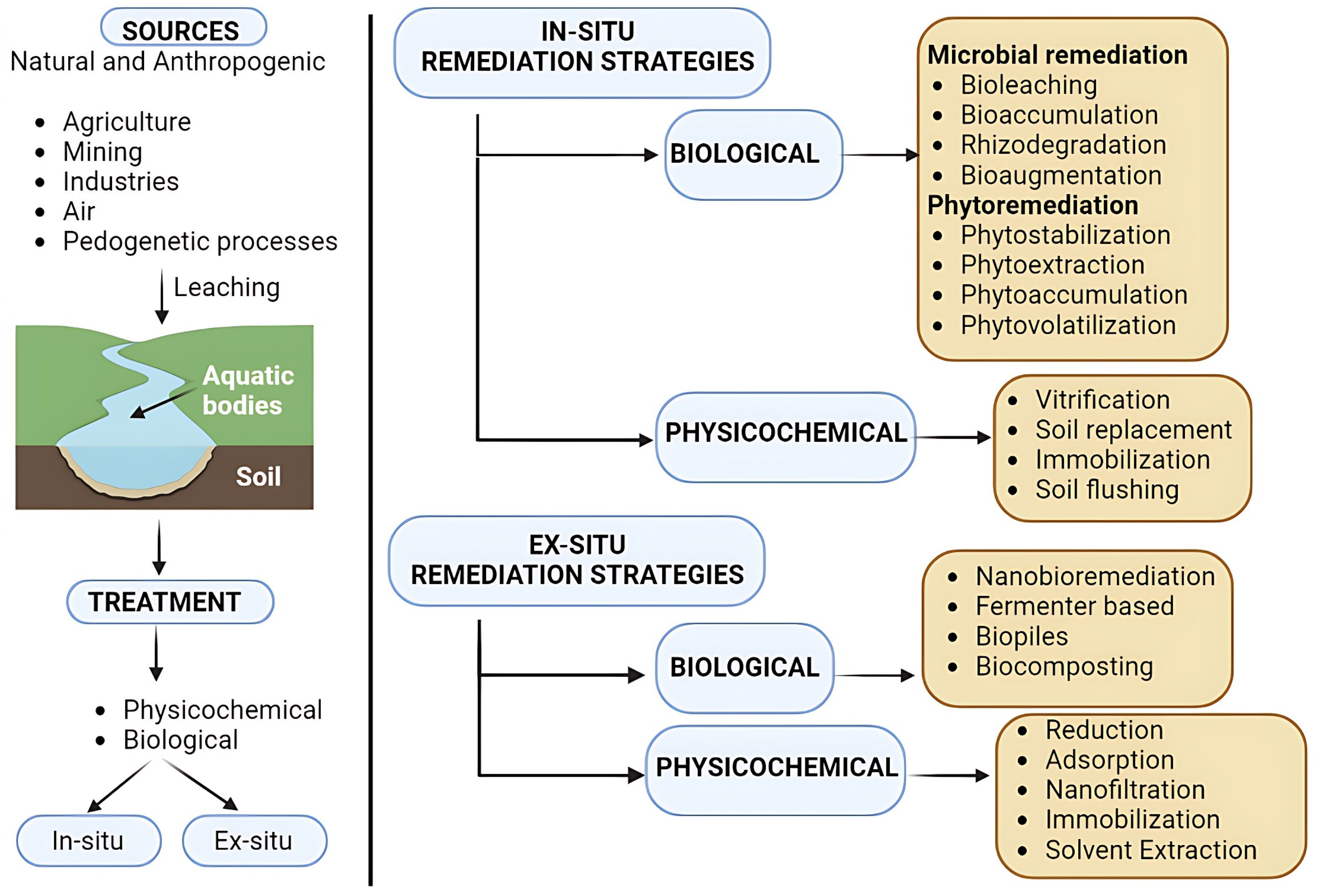
Figure 1. Sources and remediation strategies of water and soil contaminated with heavy metals.
The last two decades have documented a considerable rise in the synthesis and application of nanomaterial (NMs) in several fields, and bioremediation is one of the important areas of their application. Nanoparticles are known to exhibit multiple unique properties owing to their optimum size range and increased surface area making them a preferred choice as environmental remediation agents, which can be employed in various forms such as nanoadsorbents, nanocatalysts, nanofilters, etc., [13]. These novel candidates still pose a risk to the environment due to their unnecessary build up in the environment and then causing toxicity to plants and other living systems in the ecosystem. In view of their technological importance, there is a need to understand their post-treatment behavior and the movement of nanoparticles in soil or aquatic ecosystems. An emphasis must be placed on their design during the developmental phase, their effective management during application, and the disposal pathways post application in the environment in order to avoid and overcome the risks posed by them and ensure environmental safety. Another option is to utilize plant systems for tackling the issues by exploiting their hyperaccumulator potential for the removal of nanoparticle residues and thus attributing beneficial aspects to the use of nanoparticles [14]. Additionally, NMs provide a means of detecting contaminants in addition to removing them. NMs have been found to have a wide range of applications, which have been the subject of extensive research.
2. Mechanism of Action of Nanoparticles
To work as bioremediation agents, NMs should possess the following characteristics: (1) be deliverable to the target site and (2) be confined to the site without getting aggregated [15]. These challenges can be overcome by employing organic stabilizers such as collagen, starch, etc., [16]. The conventional methods employed for the removal of HMs suffer from a variety of drawbacks, hence the consortia of nanotechnology along with the available methods can offer a solution to the existing associated challenges [17]. Various NMs have been explored for the removal of a wide variety of contaminants including HMs via various modes such as precipitation, catalysis, conjugation, adsorption, and redox properties [18]. They can be further employed in a variety of forms, i.e., based on sensors, nanotubes, oxides, catalysts, and membranes, and the most commonly used NMs are magnetic-based NMs, which can be easily recovered and reused [19].
The enormous specific surface area of NMs makes them ideal for removing contaminants through several physicochemical and biological methods based on redox reactions, precipitation, co-precipitation, adsorption, ion-exchange, bioremediation, and phytoremediation [18].
Following the entry of NMs into the system, pollutants are subjected to a variety of physicochemical processes and alterations representing abiotic mechanisms, which include absorption, dissolution, adsorption, and photocatalysis [20]. In the next phase, biotic processes are used to remove the pollutants, including biocides, biostimulation, bioaccumulation, and biotransformation [21][22].
2.1. Remediation Techniques
There are numerous processes such as clarification, de-aeration, de-carbonation, sludge densification, or the high-density sludge (HDS) process being extensively employed to treat acid mine drainage water, but most of them are not sustainable and lead to the production of secondary waste in the form of end-products such as methane (CH4) and non-soluble metal oxides or hydroxides, which need to be treated further and hence may not be cost-effective [23]. Acid mine drainage must therefore be properly remedied by integrating novel emerging techniques. Phytoremediation and nanoremediation are two of the most promising techniques for the remediation of acid mine drainage water (Figure 2). The former involves using plants to decontaminate mine drainage water infested with various toxic metals and pollutants. In contrast, the latter reduces the load of pollutants in such water by using NMs with diameters below 100 nm [10]. Both these techniques are effective in revegetating soils contaminated with heavy metals and have gained a high degree of public acceptance as sustainable alternatives to eliminate emerging pollutants such as heavy metals, chlorinated solvents, halogenated chemicals, or pesticides. Furthermore, the synergistic application of these techniques can result in improved heavy metal removal, reducing environmental stress as a result of the application of nanomaterials in low concentrations due to the inculcation of plants as additional remediation agents [24].
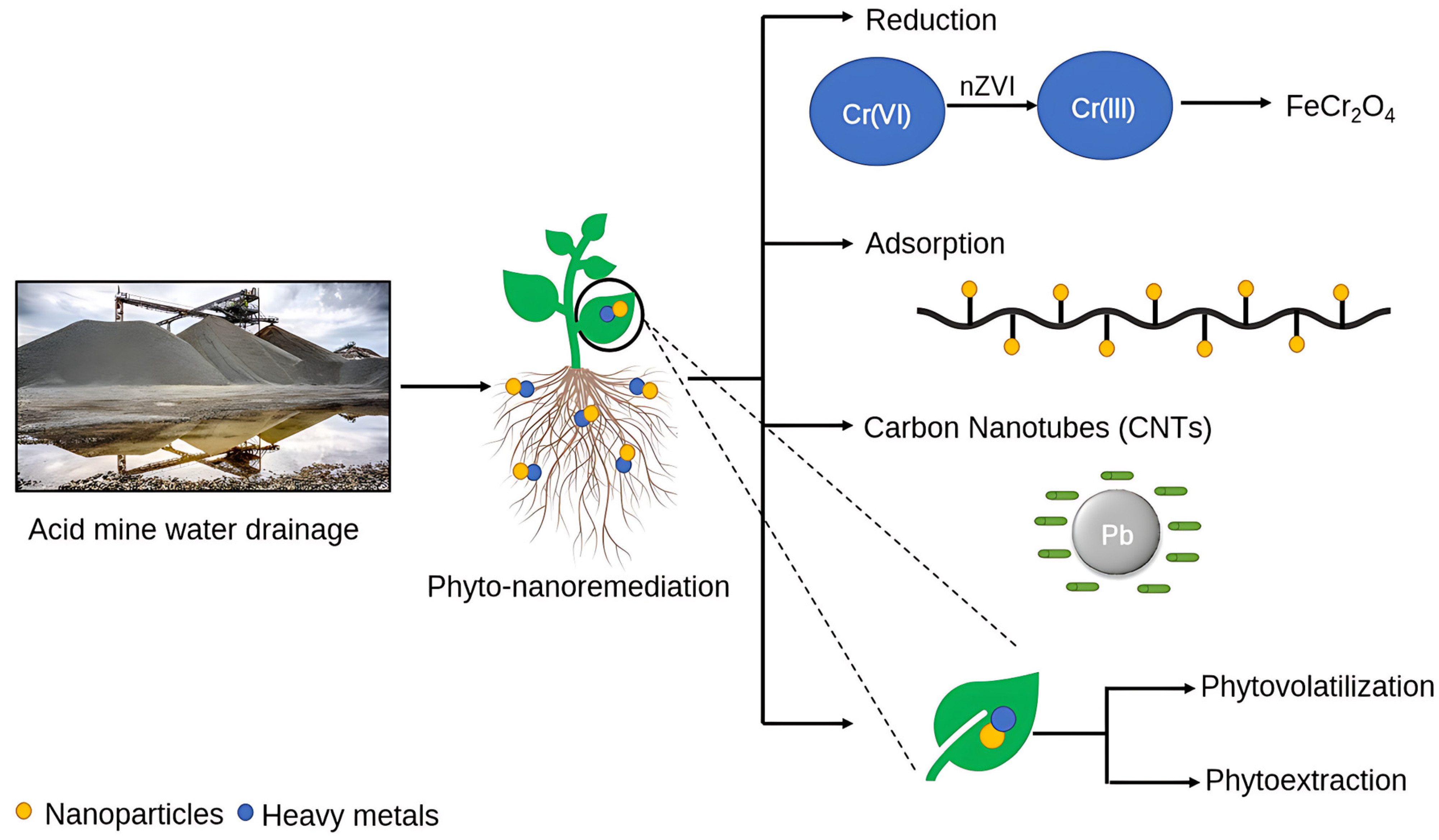
Figure 2. Remediation strategy for acid mine drainage (AMD).
2.2. Reduction
A reduction reaction using nano-zero valent iron (nZVI) NMs can effectively remove both HMs and organic compounds from contaminated soils as well as from polluted groundwater and water [25]. There has been a wide application of nZVI NMs in wide fields. Their large surface area and small size facilitate the direct contact of nZVI particles with contaminants for an improved remediation efficiency. In addition to having a strong reduction capacity and superior adsorption ability, nZVI particles are competent in transforming toxic contaminants into less noxious compounds such as transforming chromium(VI) into chromium(III) and forming ferrous chromite [26]. Moreover, it has been demonstrated that biochar added to nano-zero valent iron nanoparticles (nZVI NPs) enhances the reduction reaction capacity of nZVI and increases its removal efficiency as well as reducing the movement of mixture in the soil by strengthening the disparity of iron particles. For instance, combining nZVI NMs with biochar has been found to remove 66% of the chromium (VI) content in soil [25]. It has been found that one gram of nZVI injection into contaminated soil reduces 28% of the mass of 1 kg chromium(VI). Additionally, in a treatment condition with a pH level of 5, 98% of the chromium(VI) was removed within 24 h [27]. Another study reported the successful application of biochar and NPs for the restoration of soils contaminated with potentially toxic elements [28]. Biochar prepared using low-cost raw materials such as rice husk, water hyacinth, and black tea waste showed the removal of copper, nickel, cadmium, and zinc from affected soils [29][30][31]. Burachevskaya et al. [32] documented the decreased absorption of highly concentrated copper and zinc in Hordeum sativum upon augmentation with biochar and granular activated carbon.
Moreover, it has also been shown that combining carboxymethyl cellulose (CMC) stabilizer and nZVI significantly reduces the amount of chromium(VI) contaminants that can be converted into carbonates as well as iron-manganese oxides, which will increase chromium bioavailability and leachability by 50% when 1 g to 10 mL of soil is added [33]. It has also been reported that nZVI combined with a carboxymethyl cellulose stabilizer removes organic contaminants from soil columns such as trichloroethylene (TCE), dichlorodiphenyltrichloroethane (DDT), and pesticides. For example, an injection of nZVI stabilized with CMC into potting soil containing 9.2% organic matter dechlorinated 44% of the TCE in the soil within 30 h of treatment. One kg of soil containing 24 mg of DDT was effectively treated with 20% aqueous nZVI within 72 h, thereby removing 25% of the DDT. To remediate soils that have been contaminated for prolonged periods, a higher concentration of nZVI was required to enhance its reaction activity [34].
2.3. Phytoremediation
Rhizofiltration and avoidance mechanisms for HM uptake have enabled a few plants to survive at an optimal level of HMs, including Amaranthus spinosus, Pedioplanis burchelli, and Alternanthera pungens [35]. Plant growth and human health are adversely impacted by HMs at concentrations above the optimum [36]. Despite this, metals are ingested in high concentrations by hyperaccumulating plant species and are then transported and accumulated in different parts at much higher concentrations than non-hyperaccumulators without showing apparent phytotoxicity [37][38].
The mechanism of phytostabilization and phytoextraction can account for HMs with a bioconcentration factor (BCF) more than one [39]. A TF (translocation factor) and BCF of more than one demonstrates phytostabilization traits [40]. A similar study by Kisku et al. [41] found that Sacrum munja, Parthenium hysterophorus, and Ipomoea carnea had both phytostabilization and phytoextraction activities, and the authors found that Cr, Ni, Cd, and Pb had at least one BCF and TF, indicating a phytostabilization mechanism, while Zn and Mn had more than one BCF and less than one TF, indicating a phytoextraction mechanism. On the other hand, there is a need to understand the exact mechanism of the interaction of NPs with plants as the studies are still in their initial stages, and this will pave the way for better understanding of the synergistic potential of plants and NPs in the remediation of contaminants [14].
2.4. Rhizodegradation of Heavy Metals
The bioavailability of metals in the rhizosphere is governed by several factors such as the pH of the native soil, the ionic state and concentration of metal ions, the nature of the microbial population, the plant species and their root secretions, etc. The rhizosphere facilitates the degradation of contaminants through symbiotic relationships between plants and soil microbes [42][43]. The process of rhizodegradation involves pollutants being accumulated in the rhizosphere of soil by the action of microbes and their breakdown for getting energy and nutrition. Through this mechanism, microbes can decompose hazardous pollutants into harmless and nontoxic substances [44]. The root systems of plants release natural carbon compounds such as alcohols, sugars, and acids, thus providing microorganisms with additional nutrients and stimulating the process of rhizodegradation [45]. The secretions of root exudates may result in a decreased pH of the rhizosphere, which further facilitates the absorption of HMs [46]. It has been found that Zea mays is more capable of bioaccumulating mercury than other plants [47]. There are some plants that provide the most favorable conditions for mycorrhizae and bacteria to associate and degrade toxins effectively. This degradation results in the volatilization or incorporation of components into the soil matrix [48]. Sugars and organic acids released by plants promote the growth of bacteria and fungi [49]. It is possible to enhance rhizodegradation by improving soil characteristics such as moisture content and soil aeration [49]. It was recently found that rhizomes of Typha latifolia are capable of phytodegrading terbuthylazine (TER) in a wetland contaminated with terbuthylazine (TER) [50]. A study by Sampaio et al. found that a Rhizophora mangle mangrove under the influence of plant-growth-promoting rhizobacteria (Bacillus sp. and Pseudomonas aeruginosa) was capable of degrading polycyclic aromatic hydrocarbons (PAHs) in contaminated sediment [51]. As a result of rhizodegradation, contaminants are dissolved in their natural environment, which is its most significant benefit. Further, plant species related to the oil family have been found to have a positive effect on the removal of heavy metals from contaminated soils. In one such study, the application of nZVI particles in a rhizospheric region of sunflowers resulted in a positive impact on the arsenic mobility in the plant, which was due to a decreased percentage of accumulation into the roots and shoots of the test plants as compared to the control plants [52]. The rhizospheric regions of plants grown in heavy-metal-contaminated soils are inhabited by heavy-metal-tolerant microflora such as arbuscular mycorrhizal fungi (AMF), mycorrhizal-helping bacteria (MHB), and plant-growth-promoting rhizobial microbes (PGPR), which have been reported to be beneficial for the process of nano-phytoremediation [53]. Hence, the fundamental mechanism of rhizodegradation-assisted heavy metal removal from contaminated water and soil relies on the synthesis and secretion of HM-affinity transporter nanomaterials by inhabitant microflora, which can further bind and mobilize the available HMs into root cells [54].
References
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; Di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210.
- Yu, G.; Liu, J.; Long, Y.; Chen, Z.; Sunahara, G.I.; Jiang, P.; You, S.; Lin, H.; Xiao, H. Phytoextraction of cadmium-contaminated soils: Comparison of plant species and low molecular weight organic acids. Int. J. Phytoremediation 2019, 22, 383–391.
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mater. 2022, 426, 128062.
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the simulation of Interlayer Structure. Appl. Clay Sci. 2018, 169, 40–47.
- Volesky, B.; Holan, Z.R. Biosorption of Heavy Metals. Biotechnol. Prog. 1995, 11, 235–250.
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 66.
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14.
- Gaikwad, R. REVIEW ON REMOVAL OF HEAVY METALS FROM ACID MINE DRAINAGE. Appl. Ecol. Environ. Res. 2008, 6, 81–98.
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation. Rev. Mineral. Geochem. 1997, 35, 361–390.
- Das, P.K. Phytoremediation and Nanoremediation: Emerging Techniques for Treatment of Acid Mine Drainage Water. Def. Life Sci. J. 2018, 3, 190–196.
- Singh, G. Mine water quality deterioration due to acid mine drainage. Mine Water Environ. 1987, 6, 49–61.
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; BlakeII, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597.
- Chauhan, G.; González-González, R.B.; Iqbal, H.M. Bioremediation and decontamination potentials of metallic nanoparticles loaded nanohybrid matrices—A review. Environ. Res. 2021, 204, 112407.
- Sharma, T.; Sharma, N. Nanoparticles: Uptake, Translocation, Physiological, Biochemical Effects in Plants and their Molecular Aspects; Springer: Cham, Switzerland, 2022; pp. 103–116.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Nogueira, S.S.; de Araujo-Nobre, A.R.; Mafud, A.C.; Guimarães, M.A.; Alves, M.M.M.; Plácido, A.; Carvalho, F.A.A.; Arcanjo, D.D.R.; Mascarenhas, Y.; Costa, F.G.; et al. Silver nanoparticle stabilized by hydrolyzed collagen and natural polymers: Synthesis, characterization and antibacterial-antifungal evaluation. Int. J. Biol. Macromol. 2019, 135, 808–814.
- Cecchin, I.; Reddy, K.R.; Thomé, A.; Tessaro, E.F.; Schnaid, F. Nanobioremediation: Integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int. Biodeterior. Biodegrad. 2017, 119, 419–428.
- Raffa, C.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134.
- Gong, Z.; Chan, H.; Chen, Q.; Chen, H. Application of Nanotechnology in Analysis and Removal of Heavy Metals in Food and Water Resources. Nanomaterials 2021, 11, 1792.
- Abebe, B.; Murthy, H.C.A.; Amare, E. Summary on Adsorption and Photocatalysis for Pollutant Remediation: Mini Review. J. Encapsul. Adsorpt. Sci. 2018, 08, 225–255.
- Desiante, W.L.; Minas, N.S.; Fenner, K. Micropollutant biotransformation and bioaccumulation in natural stream biofilms. Water Res. 2021, 193, 116846.
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable Application of Biosorption and Bioaccumulation of Persistent Pollutants in Wastewater Treatment: Current Practice. Processes 2021, 9, 1696.
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr. Pollut. Rep. 2015, 1, 131–141.
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359.
- Qian, Y.; Qin, C.; Chen, M.; Lin, S. Nanotechnology in soil remediation—Applications vs. implications. Ecotoxicol. Environ. Saf. 2020, 201, 110815.
- Rabbani, M.M.; Ahmed, I.; Park, S.-J. Application of Nanotechnology to Remediate Contaminated Soils. In Environmental Remediation Technologies for Metal-Contaminated Soils; Springer: Tokyo, Japan, 2016; pp. 219–229.
- Chrysochoou, M.; Johnston, C.P.; Dahal, G. A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J. Hazard. Mater. 2012, 201–202, 33–42.
- Rajput, V.D.; Kumari, A.; Minkina, T.; Barakhov, A.; Singh, S.; Mandzhieva, S.S.; Sushkova, S.; Ranjan, A.; Rajput, P.; Garg, M.C. A practical evaluation on integrated role of biochar and nanomaterials in soil remediation processes. Environ. Geochem. Health 2022, 1–15.
- Lobzenko, I.; Burachevskaya, M.; Zamulina, I.; Barakhov, A.; Bauer, T.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Tereschenko, A.; Kalinichenko, V.; et al. Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation. Agriculture 2022, 12, 1689.
- Elbehiry, F.; Darweesh, M.; Al-Anany, F.S.; Khalifa, A.M.; Almashad, A.A.; El-Ramady, H.; El-Banna, A.; Rajput, V.D.; Jatav, H.S.; Elbasiouny, H. Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability 2022, 14, 10118.
- Awasthi, G.; Nagar, V.; Mandzhieva, S.; Minkina, T.; Sankhla, M.S.; Pandit, P.P.; Aseri, V.; Awasthi, K.K.; Rajput, V.D.; Bauer, T.; et al. Sustainable Amelioration of Heavy Metals in Soil Ecosystem: Existing Developments to Emerging Trends. Minerals 2022, 12, 85.
- Burachevskaya, M.; Mandzhieva, S.; Bauer, T.; Minkina, T.; Rajput, V.; Chaplygin, V.; Fedorenko, A.; Chernikova, N.; Zamulina, I.; Kolesnikov, S.; et al. The Effect of Granular Activated Carbon and Biochar on the Availability of Cu and Zn to Hordeum sativum Distichum in Contaminated Soil. Plants 2021, 10, 841.
- Zhang, R.; Zhang, N.; Fang, Z. In situ remediation of hexavalent chromium contaminated soil by CMC-stabilized nanoscale zero-valent iron composited with biochar. Water Sci. Technol. 2018, 77, 1622–1631.
- El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228.
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367.
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711.
- Jabeen, R.; Ahmad, A.; Iqbal, M.F. Phytoremediation of Heavy Metals: Physiological and Molecular Mechanisms. Bot. Rev. 2009, 75, 339–364.
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181.
- Upadhyay, S.K.; Ahmad, M.; Srivastava, A.K.; Abhilash, P.C.; Sharma, B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere 2021, 267, 129216.
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658.
- Kisku, G.C.; Kumar, V.; Sahu, P.; Kumar, P.; Kumar, N. Characterization of coal fly ash and use of plants growing in ash pond for phytoremediation of metals from contaminated agricultural land. Int. J. Phytoremediation 2018, 20, 330–337.
- Das, S.; Chou, M.-L.; Jean, J.-S.; Yang, H.-J.; Kim, P.J. Arsenic-enrichment enhanced root exudates and altered rhizosphere microbial communities and activities in hyperaccumulator Pteris vittata. J. Hazard. Mater. 2017, 325, 279–287.
- Caracciolo, A.B.; Grenni, P.; Garbini, G.L.; Rolando, L.; Campanale, C.; Aimola, G.; Fernandez-Lopez, M.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Ancona, V. Characterization of the Belowground Microbial Community in a Poplar-Phytoremediation Strategy of a Multi-Contaminated Soil. Front. Microbiol. 2020, 11, 2073.
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504.
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Dar, T.U.H.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. Physiol. Plant. 2021, 137, 287–304.
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ. Geochem. Health 2022, 1–24.
- Benavides, L.C.L.; Pinilla, L.A.C.; Serrezuela, R.R.; Serrezuela, W.F.R. Extraction in laboratory of heavy metals through rhizofiltration using the plant Zea mays (maize). Int. J. Appl. Environ. Sci. 2018, 13, 9–26.
- De Farias, V.; Maranho, L.T.; De Vasconcelos, E.C.; Filho, M.A.D.S.C.; Lacerda, L.G.; Azevedo, J.A.M.; Pandey, A.; Soccol, C.R. Phytodegradation Potential of Erythrina crista-galli L., Fabaceae, in Petroleum-Contaminated Soil. Appl. Biochem. Biotechnol. 2009, 157, 10–22.
- Kirk, J.L.; Klironomos, J.N.; Lee, H.; Trevors, J.T. The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environ. Pollut. 2005, 133, 455–465.
- Papadopoulos, N.; Zalidis, G. The Use of Typha Latifolia L. in Constructed Wetland Microcosms for the Remediation of Herbicide Terbuthylazine. Environ. Process. 2019, 6, 985–1003.
- Sampaio, C.J.S.; de Souza, J.R.B.; Damião, A.O.; Bahiense, T.C.; Roque, M.R.A. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in a diesel oil-contaminated mangrove by plant growth-promoting rhizobacteria. 3 Biotech 2019, 9, 155.
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226.
- Khan, A.; Kuek, C.; Chaudhry, T.; Khoo, C.; Hayes, W. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207.
- Khan, A.G. In Situ Phytoremediation of Uranium Contaminated Soils. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–151.
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
12 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




