Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Neetu Sharma and Version 2 by Conner Chen.
The varied sources of soil pollution include the application of chemical fertilizers and pesticides, industrial discharge, and transformed products of these accumulated chemical residues. These processes may hamper the composition and soil ecosystem. Different types of methodologies ranging from physical, chemical, and biological approaches have been exploited to tackle this challenge. Nanomaterials (NMs) research has contributed to a new dimension for the remediation of polluted soils.
- remediation
- sustainable
- microbe
- metal
- nanoparticles
1. Introduction
The increasing accumulation of heavy metals (HMs) in the food and water supply chain is a major cause of public health concern. Their high density and non-biodegradable nature make HMs such as Hg(II), Cr(VI),Pb(II), and Cd(II), the potent and most challenging environmental contaminants [1][2][1,2]. Soil acts as an important sink for supporting various lifeforms on earth, ranging from organisms as small as microbes to the most complex ones, i.e., animals and humans. The invasion of natural flora by various anthropogenic sources has resulted in the disruption of the natural cycling of nutrients and the accumulation of many undesired components such as HMs in the soil. The concept of sustainable development is not attainable pertaining to the present scenario of soil pollution. Due to rising populations, soil conservation should be a top priority in today’s society, which is facing a challenging situation of diminishing land area and scarcity of food and shelter. The various approaches employed to tackle this situation involve thermal treatment, filtration, adsorption, chemical abstraction, membrane bound separation, microbial degradation, etc. Heavy metal removal can also be efficiently accomplished by employing methylene phosphonic acid (DTPMP) phosphonate intercalated with layered double hydroxide [3]. In another study, lysine intercalated with montmorillonite was reported to remove Pb (II) from wastewater via an adsorption mechanism [4].
The processes documented above consist of a single approach to treatment. It is true that these treatments have been successful, however, they also have certain drawbacks such as inefficiency, high costs, and failure at scale-up [5][6][5,6]. Acid mine drainage (AMD) is a form of pollution caused by drainage water flowing from sulfur-bearing sites into water bodies [7]. The mining of sulfide minerals exposes them to the environment, causing excessive amounts of acid to be produced, which can cause both immediate and long-term environmental harm. Some of the adverse effects of AMD include the corrosion of mining machinery and equipment, degradation of soil quality, and groundwater contamination due to the leaching of HMs present in mine and drainage water [8].
Air or water exposed to iron disulfide or iron pyrite produces acid mine drainage by oxidizing mineral sulfides. Oxygen and water react with metal sulfides to produce metal sulfates and sulfuric acid. Subsequent oxidation of the metals results in increased acidity [9]. In one such example, ferrous sulfide (pyrite) undergoes oxidation on reacting with water and oxygen to form ferrous sulfate and sulfuric acid. Ferrous sulfate oxidizes further to form ferric sulfate, and the rate of this reaction can further be enhanced by the action of certain bacteria such as Acidithiobacillus ferroxidans. Further, ferric sulfate reacts with water to form ferric hydroxide releasing hydrogen ions, which subsequently enhance the acidity of water. The resulting ferric hydroxide formed further reacts with pyrite and produces more acid. The amount of acid produced depends on the amount of iron getting oxidized [10].
FeS
2
+ H
2
O + 3 ½ O
2
→ FeSO
4
+ H
2
SO
4
FeSO4 → Fe2(SO4)3 → 2Fe3+ + 3SO4−2
Fe
3+
+ 3H
2
O → Fe(OH)
3
+ 3H
+
Rainwater or water used in mining operations for dust control, drilling, or other purposes enters the mine as fresh water. Fissures and cracks in underground mines can allow ground water to seep into the mines. Sulfide minerals yield oxidized products that are transported to nearby rivers and other water bodies by flowing into the surrounding aqueous environment [11]. As pyritic sulfur reacts with water and oxygen, sulfuric acid is produced, and iron sulfate is formed. As a result, certain acidophilic bacteria such as Acidithiobacillus ferroxidans thrive and grow in this type of acidic environment created by coal mines. As a result, the acid production reaction is catalyzed by the bacteria and occurs more quickly than chemical oxidation [12]. The acidity in mine drainage water is primarily due to the production of sulfuric acid and hydrolysis of oxidized pyrite products [11].
The selection of any remediation technique employed for the removal of HMs is governed by several factors including the type and nature of the contaminant, its concentration, its form (simpler or complex form), the objective and time frame for treatment, the cost involved, and the environmental impact. Furthermore, treatment techniques are categorized into in situ and ex situ types depending on the nature and location of the site, the degree of contamination, and the treatment strategy to be employed (Figure 1). The former category is the most preferred as it employs the treatment of soil at its natural site by utilizing air, water, microbes, and plants. On the other hand, the latter is based on the excavation of contaminated soil to a point where it can be treated, i.e., into a fermenter, which makes it more complex and ultimately leads to a higher cost. All the conventional methods being employed today have several drawbacks including cost, time frame, and the release of by products, which result in post-treatment challenges involving environmental contamination.
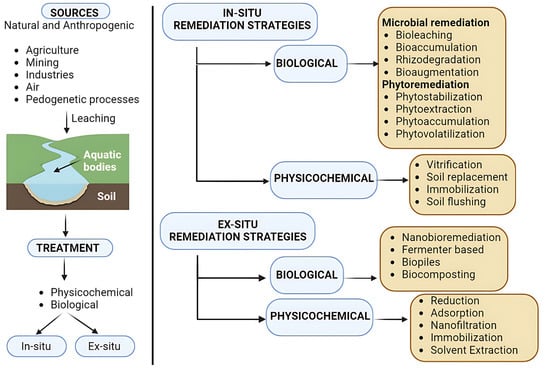
Figure 1.
Sources and remediation strategies of water and soil contaminated with heavy metals.
The last two decades have documented a considerable rise in the synthesis and application of nanomaterial (NMs) in several fields, and bioremediation is one of the important areas of their application. Nanoparticles are known to exhibit multiple unique properties owing to their optimum size range and increased surface area making them a preferred choice as environmental remediation agents, which can be employed in various forms such as nanoadsorbents, nanocatalysts, nanofilters, etc., [13]. These novel candidates still pose a risk to the environment due to their unnecessary build up in the environment and then causing toxicity to plants and other living systems in the ecosystem. In view of their technological importance, there is a need to understand their post-treatment behavior and the movement of nanoparticles in soil or aquatic ecosystems. An emphasis must be placed on their design during the developmental phase, their effective management during application, and the disposal pathways post application in the environment in order to avoid and overcome the risks posed by them and ensure environmental safety. Another option is to utilize plant systems for tackling the issues by exploiting their hyperaccumulator potential for the removal of nanoparticle residues and thus attributing beneficial aspects to the use of nanoparticles [14]. Additionally, NMs provide a means of detecting contaminants in addition to removing them. NMs have been found to have a wide range of applications, which have been the subject of extensive research.
2. Mechanism of Action of Nanoparticles
To work as bioremediation agents, NMs should possess the following characteristics: (1) be deliverable to the target site and (2) be confined to the site without getting aggregated [15]. These challenges can be overcome by employing organic stabilizers such as collagen, starch, etc., [16]. The conventional methods employed for the removal of HMs suffer from a variety of drawbacks, hence the consortia of nanotechnology along with the available methods can offer a solution to the existing associated challenges [17]. Various NMs have been explored for the removal of a wide variety of contaminants including HMs via various modes such as precipitation, catalysis, conjugation, adsorption, and redox properties [18]. They can be further employed in a variety of forms, i.e., based on sensors, nanotubes, oxides, catalysts, and membranes, and the most commonly used NMs are magnetic-based NMs, which can be easily recovered and reused [19]. The enormous specific surface area of NMs makes them ideal for removing contaminants through several physicochemical and biological methods based on redox reactions, precipitation, co-precipitation, adsorption, ion-exchange, bioremediation, and phytoremediation [18]. Following the entry of NMs into the system, pollutants are subjected to a variety of physicochemical processes and alterations representing abiotic mechanisms, which include absorption, dissolution, adsorption, and photocatalysis [20]. In the next phase, biotic processes are used to remove the pollutants, including biocides, biostimulation, bioaccumulation, and biotransformation [21][22][21,22].2.1. Remediation Techniques
There are numerous processes such as clarification, de-aeration, de-carbonation, sludge densification, or the high-density sludge (HDS) process being extensively employed to treat acid mine drainage water, but most of them are not sustainable and lead to the production of secondary waste in the form of end-products such as methane (CH4) and non-soluble metal oxides or hydroxides, which need to be treated further and hence may not be cost-effective [23]. Acid mine drainage must therefore be properly remedied by integrating novel emerging techniques. Phytoremediation and nanoremediation are two of the most promising techniques for the remediation of acid mine drainage water (Figure 2). The former involves using plants to decontaminate mine drainage water infested with various toxic metals and pollutants. In contrast, the latter reduces the load of pollutants in such water by using NMs with diameters below 100 nm [10]. Both these techniques are effective in revegetating soils contaminated with heavy metals and have gained a high degree of public acceptance as sustainable alternatives to eliminate emerging pollutants such as heavy metals, chlorinated solvents, halogenated chemicals, or pesticides. Furthermore, the synergistic application of these techniques can result in improved heavy metal removal, reducing environmental stress as a result of the application of nanomaterials in low concentrations due to the inculcation of plants as additional remediation agents [24].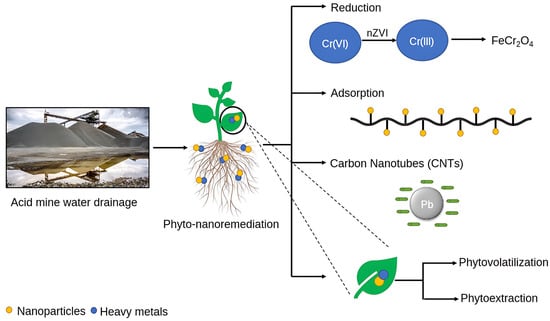
Figure 2.
Remediation strategy for acid mine drainage (AMD).
