Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kajol J Shah | -- | 1370 | 2022-12-11 17:15:30 | | | |
| 2 | Catherine Yang | Meta information modification | 1370 | 2022-12-12 03:32:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shah, K.J.; Roy, T.L. Treatment of Lower Extremity Deep Vein Thrombosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/38502 (accessed on 09 March 2026).
Shah KJ, Roy TL. Treatment of Lower Extremity Deep Vein Thrombosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/38502. Accessed March 09, 2026.
Shah, Kajol J., Trisha L. Roy. "Treatment of Lower Extremity Deep Vein Thrombosis" Encyclopedia, https://encyclopedia.pub/entry/38502 (accessed March 09, 2026).
Shah, K.J., & Roy, T.L. (2022, December 11). Treatment of Lower Extremity Deep Vein Thrombosis. In Encyclopedia. https://encyclopedia.pub/entry/38502
Shah, Kajol J. and Trisha L. Roy. "Treatment of Lower Extremity Deep Vein Thrombosis." Encyclopedia. Web. 11 December, 2022.
Copy Citation
Lower extremity deep vein thrombosis (DVT) leads to significant morbidity including pain, swelling, and difficulty walking in the affected limb. If left untreated, DVT increases the risk of pulmonary embolism (PE), recurrent venous thromboembolism (VTE), and post thrombotic syndrome (PTS). The objective of this overview is to identify catheter-directed interventions for the treatment of lower extremity DVT. Percutaneous mechanical thrombectomy (PMT), catheter-directed thrombolysis (CDT), and pharmacomechanical CDT (PCDT) devices are discussed in detail with a focus on their mechanism of action and indication of use.
deep vein thrombosis (DVT)
thrombectomy
thrombolytic therapy
anti-thrombotic therapy
endovascular therapy
1. Systemic Anticoagulation
Anticoagulants are considered the standard of care for treating DVT and assist in preventing thrombus growth [1][2][3]. Due to the variability in thrombus age and composition, however, they are ineffective for complete clot dissolution [4][5]. Anticoagulation can pose significant systemic bleeding risks, and residual thrombi can increase the risk of the recurrent DVT [5][6][7]. Furthermore, anticoagulant therapy is not always effective in preventing chronic complications of acute DVTs such as valvular insufficiency and PTS [8][9][10]. PTS impacts quality of life and induces significant clinical burden on patients including pain, swelling, cramping, skin discoloration, and ulcers in the affected leg [11][12].
The risk of major bleeding complications from prolonged anticoagulation should be considered in the decision criteria for patients over the age of 65, with a history of bleeding disorders, recent surgery, frequent falls, as well as those with comorbid conditions such as cancer, renal insufficiency, and impaired hepatic function [13][14][15][16]. Anticoagulants are also known to cause differences in bleeding complications between genders [17][18]. In a meta-analysis comparing the risk factors associated with Direct Oral Anticoagulants (DOAC), females were likely to have higher incidences of major and clinically relevant non-major bleeding complications than males when treated with DOACs for venous thromboembolism (VTE) [17].
Furthermore, anticoagulants such as warfarin require frequent monitoring and dose adjustment, take time in reaching target levels in the body, and can have adverse interactions with certain foods and medications [19][20]. DOACs used for DVT treatment do not require frequent monitoring; however, they are shorter acting and lack of adherence to medication regime can result in greater risk of recurrent thrombosis [19][21].
2. Catheter-Directed Intervention
Catheter-directed interventions, including percutaneous mechanical thrombectomy (PMT), catheter-directed thrombolysis (CDT), and pharmacomechanical CDT (PCDT), have been used as alternatives or adjunctive strategies of systemic anticoagulation for the treatment of DVT. The goal of these interventions is to reduce clot burden, alleviate acute symptoms, restore venous flow, and retain valvular functionality to prevent PTS in the future [22][23][24]. A summary of available catheter-directed interventions is presented in Figure 1.
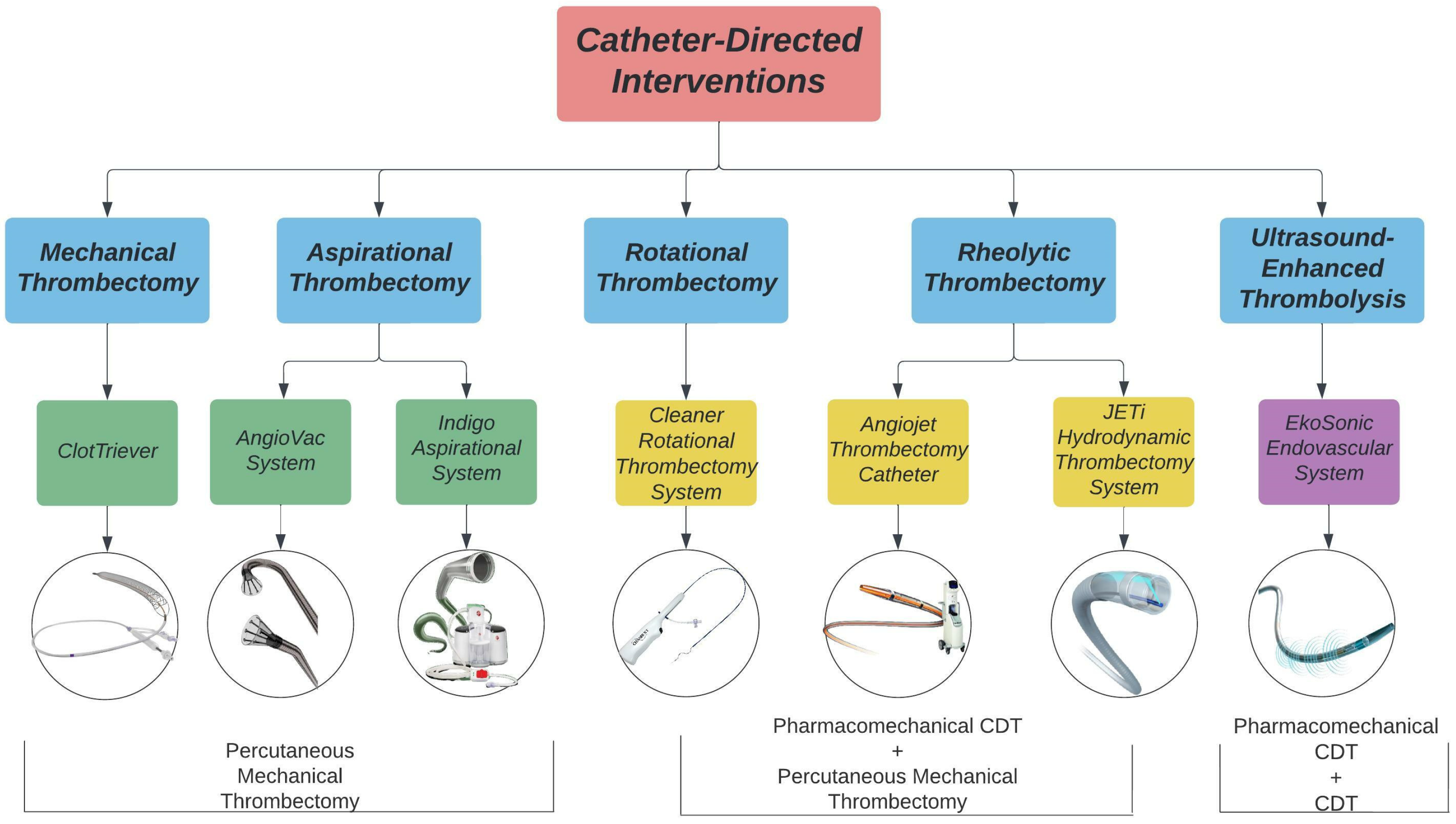
Figure 1. Catheter-directed interventions for lower extremity DVT treatment. Images acquired from Inari Medical, AngioDynamics, Penumbra Inc., Argon Medical, Abbott, and Boston Scientific.
PMT devices are designed to mechanically break apart and remove thrombi from the deep veins without the use of thrombolytics. PMT devices are categorized into rotational, aspirational, rheolytic, and mechanical fragmentation devices. Rotational thrombectomy devices utilize a sinusoidal wire to spin and macerate thrombi while collecting the fragments in a built-in or separate aspiration system. Aspirational thrombectomy devices generate negative pressure to suction clots from the venous system. Rheolytic thrombectomy devices inject high pressure saline jets to break apart thrombi and create a low-pressure zone to vacuum the fragments into the catheter. Mechanical fragmentation devices manually core and separate thrombi from the venous wall.
CDT procedures allow for infusion of lytic drugs to the thrombus site through side ports within the catheter. The Ekosonic Endovascular system can act as a CDT device alone or as a PCDT device by combining the use of thrombolytics and ultrasound to remove thrombi. Ultrasound-enhanced devices use high-frequency, low energy ultrasound waves to penetrate and dissolve clots. Rheolytic devices such as the Angiojet and JETi System and rotational devices such as the Cleaner system are additionally categorized as PCDT devices as they can infuse thrombolytics to the site of the thrombus.
3. Rotational Thrombectomy
Cleaner Rotational Thrombectomy System
The Cleaner XTTM (6 Fr) and Cleaner 15TM (7 Fr) Rotational Thrombectomy System (Argon Medical Devices; Plano, TX, USA) are PMT catheters used for mechanical declotting and removal of wall-adherent thrombi in peripheral vasculature. A single sinusoidal-shaped wire macerates thrombi through a rotational motion at 4000 RPM. The clot is then aspirated through an introducer sheath. The Cleaner XTTM uses a 9 mm sinusoidal wire for maceration of thrombi in smaller lumens whereas the Cleaner 15TM uses a 15 mm sinusoidal wire for maceration of thrombi in larger lumens. The Cleaner system also has an infusion port for the delivery of thrombolytics.
4. Aspiration Thrombectomy
4.1. Indigo Aspiration System
The Indigo Aspiration System (3.4–12 Fr) (Penumbra, Inc.; Alameda, CA, USA) is an aspiration mechanical thrombectomy system composed of a catheter, separator, and vacuum pump. The Penumbra ENGINE delivers continuous suction for thrombus removal. The Indigo SeparatorTM aids in thrombus fragmentation and cleaning of the catheter. Through its dual pressure sensors, The LightningTM Intelligent Aspiration Tubing monitors blood flow in real time. This allows for the system to automatically control the degree of aspiration and reduce blood loss by providing intermittent aspiration around the venous segment and continuous aspiration near the clot.
4.2. AngioVac System
The AngioVac System (18, 22 Fr) (AngioDynamics; Latham, NY, USA) is a vacuum-assisted thrombectomy device indicated for removing fresh, soft thrombi or emboli through a veno–venous bypass circuit. The system utilizes a suction cannula to collect clot fragments and filters the fragments through a canister before returning the blood into the reinfusion cannula. The system has a self-expanding funnel shape tip to enhance venous drainage flow and prevent clogging of the cannula. The device is available with a 20- or 180-degree angled tip to facilitate navigation through the vasculature.
5. Mechanical Fragmentation
ClotTreiver
The ClotTriever BOLDTM (16 Fr) (Inari Medical; Irvine, CA, USA) is a mechanical thrombectomy device that is designed to remove large thrombi from peripheral vasculature. The system includes two elements: the ClotTriever catheter, which contains a nitinol coring element and braided collection bag, as well as the ClotTriever sheath, which contains a self-expanding nitinol funnel and a side port for aspiration. Once the guidewire is inserted beyond the clot, the ClotTriever sheath is positioned over the guidewire distal to the clot and the funnel is expanded for clot capture. The ClotTriever catheter is advanced through the sheath beyond the clot where the nitinol coring element and collection bag are deployed. As the coring element is retracted back towards the sheath, the thrombus is separated from the venous wall and captured in the collection bag. The side port can be used for aspiration of remaining clot fragments in the sheath. The device can be reinserted multiple times to allow for extensive thrombus capture.
6. Ultrasound-Enhanced Thrombolysis
EkoSonic Endovascular System
The EkoSonic Endovascular System (EKOS Corporation, Bothell, WA, USA) is an ultrasound-enhanced device that aids in the controlled and selective infusion of thrombolytics to peripheral vasculature to dissolve thrombi. The catheter has two parts: the infusion catheter and the ultrasonic core. The infusion catheter uses an infusion pump to deliver lytic agents through its side holes and allows for the flow of saline to cool the ultrasound core while activated. The ultrasound core generates radial pressure that increases access to receptor sites for lytic agents and accelerates lytic dispersion into the thrombus.
7. Rheolytic Thrombectomy System
7.1. AngioJet Thrombectomy Catheter
The AngioJet Thrombectomy Catheter (Boston Scientific; Natick, MA, USA) delivers high pressure saline jets through the tip of the catheter. The saline jets travel backwards to create a low-pressure zone, which allows for the thrombus to be suctioned into the in-flow windows. ZelanteDVT Catheter (8 Fr) is designed to treat upper and lower peripheral veins with a diameter of ≥6 mm. The Solent Omni and Proxi Catheters (6 Fr) are used for treating veins that are ≥3 mm in diameter. The AngioJet system also has a Power Pulse feature which allows for the infusion of thrombolytics at the site of the thrombi.
7.2. JETi Hydrodynamic Thrombectomy System
The JETiTM Hydrodynamic Thrombectomy System (6F and 8F) (Abbott; Irvine, CA, USA) uses a combination of aspiration and high-pressure saline jets to suction and fragment clots from the venous system. The outer conduit allows for continuous thrombus aspiration while the inner catheter tip utilizes saline jets to break up the aspirated thrombus and reduce catheter clogging. The JETi device also has a HyperPulseTM Fluid Delivery feature which allows for selective infusion and delivery of thrombolytics.
References
- Tritschler, T.; Kraaijpoel, N.; Le Gal, G.; Wells, P.S. Venous Thromboembolism: Advances in Diagnosis and Treatment. Jama 2018, 320, 1583–1594.
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738.
- Hirsh, J.; Hoak, J. Management of Deep Vein Thrombosis and Pulmonary Embolism. Circulation 1996, 93, 2212–2245.
- Hendley, S.A.; Dimov, A.; Bhargava, A.; Snoddy, E.; Mansour, D.; Afifi, R.O.; Wool, G.D.; Zha, Y.; Sammet, S.; Lu, Z.F.; et al. Assessment of histological characteristics, imaging markers, and rt-PA susceptibility of ex vivo venous thrombi. Sci. Rep. 2021, 11, 22805.
- Oklu, R.; Wicky, S. Catheter-directed thrombolysis of deep venous thrombosis. Semin. Thromb. Hemost. 2013, 39, 446–451.
- Prandoni, P.; Lensing, A.W.; Prins, M.H.; Bernardi, E.; Marchiori, A.; Bagatella, P.; Frulla, M.; Mosena, L.; Tormene, D.; Piccioli, A.; et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann. Intern. Med. 2002, 137, 955–960.
- Fahrni, J.; Husmann, M.; Gretener, S.B.; Keo, H.H. Assessing the risk of recurrent venous thromboembolism—A practical approach. Vasc. Health Risk Manag. 2015, 11, 451–459.
- Fleck, D.; Albadawi, H.; Shamoun, F.; Knuttinen, G.; Naidu, S.; Oklu, R. Catheter-directed thrombolysis of deep vein thrombosis: Literature review and practice considerations. Cardiovasc. Diagn. Ther. 2017, 7, S228–S237.
- Vedantham, S. Valvular dysfunction and venous obstruction in the post-thrombotic syndrome. Thromb. Res. 2009, 123, S62–S65.
- Kahn, S.R.; Comerota, A.J.; Cushman, M.; Evans, N.S.; Ginsberg, J.S.; Goldenberg, N.A.; Gupta, D.K.; Prandoni, P.; Vedantham, S.; Walsh, M.E.; et al. The Postthrombotic Syndrome: Evidence-Based Prevention, Diagnosis, and Treatment Strategies. Circulation 2014, 130, 1636–1661.
- Henke, P.K.; Comerota, A.J. An update on etiology, prevention, and therapy of postthrombotic syndrome. J. Vasc. Surg. 2011, 53, 500–509.
- Kahn, S.R.; Shrier, I.; Julian, J.A.; Ducruet, T.; Arsenault, L.; Miron, M.J.; Roussin, A.; Desmarais, S.; Joyal, F.; Kassis, J.; et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann. Intern. Med. 2008, 149, 698–707.
- Kearon, C.; Akl, E.A. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014, 123, 1794–1801.
- Al-Samkari, H.; Connors, J.M. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019, 3, 3770–3779.
- Ribic, C.; Crowther, M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 188–195.
- Robert-Ebadi, H.; Le Gal, G.; Righini, M. Use of anticoagulants in elderly patients: Practical recommendations. Clin. Interv. Aging 2009, 4, 165–177.
- Alotaibi, G.S.; Almodaimegh, H.; McMurtry, M.S.; Wu, C. Do women bleed more than men when prescribed novel oral anticoagulants for venous thromboembolism? A sex-based meta-analysis. Thromb. Res. 2013, 132, 185–189.
- Barrios, D.; Morillo, R.; Guerassimova, I.; Barbero, E.; Escobar-Morreale, H.; Cohen, A.T.; Becattini, C.; Tapson, V.; Yusen, R.; Jimenez, D. Sex differences in the characteristics and short-term prognosis of patients presenting with acute symptomatic pulmonary embolism. PLoS ONE 2017, 12, e0187648.
- Wadhera, R.K.; Russell, C.E.; Piazza, G. Cardiology patient page. Warfarin versus novel oral anticoagulants: How to choose? Circulation 2014, 130, e191–e193.
- Gross, P.L.; Weitz, J.I. New Anticoagulants for Treatment of Venous Thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 380–386.
- Burgazli, K.M.; Atmaca, N.; Mericliler, M.; Parahuleva, M.; Erdogan, A.; Daebritz, S.H. Deep vein thrombosis and novel oral anticoagulants: A clinical review. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3123–3131.
- Nathan, A.S.; Giri, J. Reexamining the Open-Vein Hypothesis for Acute Deep Venous Thrombosis. Circulation 2019, 139, 1174–1176.
- Patterson, B.O.; Hinchliffe, R.; Loftus, I.M.; Thompson, M.M.; Holt, P.J.E. Indications for Catheter-Directed Thrombolysis in the Management of Acute Proximal Deep Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 669–674.
- Goldhaber, S.Z.; Magnuson, E.A.; Chinnakondepalli, K.M.; Cohen, D.J.; Vedantham, S. Catheter-directed thrombolysis for deep vein thrombosis: 2021 update. Vasc. Med. 2021, 26, 662–669.
More
Information
Subjects:
Peripheral Vascular Disease
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
12 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





