Lower extremity deep vein thrombosis (DVT) leads to significant morbidity including pain, swelling, and difficulty walking in the affected limb. If left untreated, DVT increases the risk of pulmonary embolism (PE), recurrent venous thromboembolism (VTE), and post thrombotic syndrome (PTS). The objective of this overview is to identify catheter-directed interventions for the treatment of lower extremity DVT. Percutaneous mechanical thrombectomy (PMT), catheter-directed thrombolysis (CDT), and pharmacomechanical CDT (PCDT) devices are discussed in detail with a focus on their mechanism of action and indication of use.
- deep vein thrombosis (DVT)
- thrombectomy
- thrombolytic therapy
- anti-thrombotic therapy
- endovascular therapy
1. Systemic Anticoagulation
2. Catheter-Directed Intervention
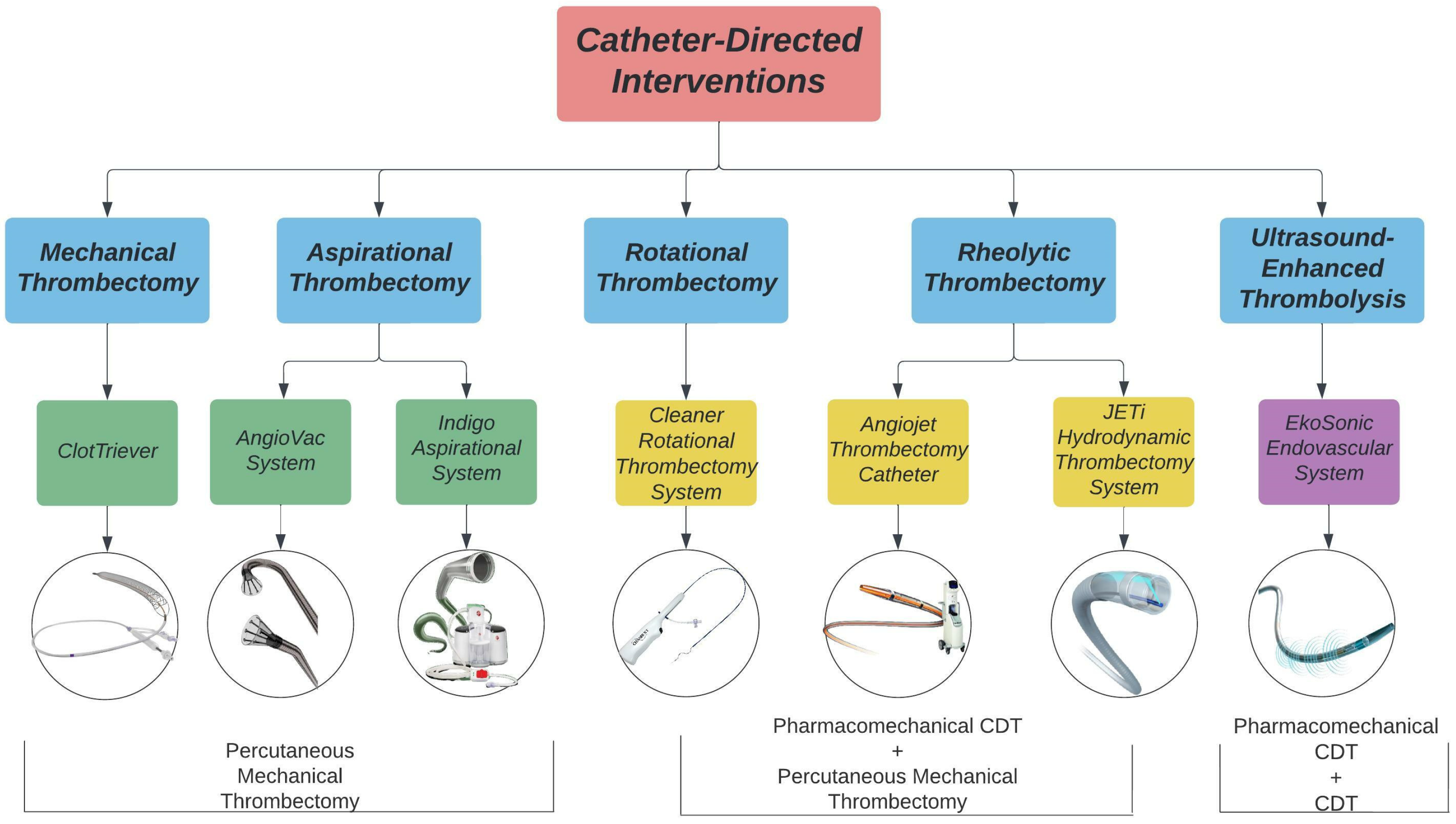
3. Rotational Thrombectomy
Cleaner Rotational Thrombectomy System
4. Aspiration Thrombectomy
4.1. Indigo Aspiration System
4.2. AngioVac System
5. Mechanical Fragmentation
ClotTreiver
6. Ultrasound-Enhanced Thrombolysis
EkoSonic Endovascular System
7. Rheolytic Thrombectomy System
7.1. AngioJet Thrombectomy Catheter
7.2. JETi Hydrodynamic Thrombectomy System
This entry is adapted from the peer-reviewed paper 10.3390/life12121984
References
- Tritschler, T.; Kraaijpoel, N.; Le Gal, G.; Wells, P.S. Venous Thromboembolism: Advances in Diagnosis and Treatment. Jama 2018, 320, 1583–1594.
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738.
- Hirsh, J.; Hoak, J. Management of Deep Vein Thrombosis and Pulmonary Embolism. Circulation 1996, 93, 2212–2245.
- Hendley, S.A.; Dimov, A.; Bhargava, A.; Snoddy, E.; Mansour, D.; Afifi, R.O.; Wool, G.D.; Zha, Y.; Sammet, S.; Lu, Z.F.; et al. Assessment of histological characteristics, imaging markers, and rt-PA susceptibility of ex vivo venous thrombi. Sci. Rep. 2021, 11, 22805.
- Oklu, R.; Wicky, S. Catheter-directed thrombolysis of deep venous thrombosis. Semin. Thromb. Hemost. 2013, 39, 446–451.
- Prandoni, P.; Lensing, A.W.; Prins, M.H.; Bernardi, E.; Marchiori, A.; Bagatella, P.; Frulla, M.; Mosena, L.; Tormene, D.; Piccioli, A.; et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann. Intern. Med. 2002, 137, 955–960.
- Fahrni, J.; Husmann, M.; Gretener, S.B.; Keo, H.H. Assessing the risk of recurrent venous thromboembolism—A practical approach. Vasc. Health Risk Manag. 2015, 11, 451–459.
- Fleck, D.; Albadawi, H.; Shamoun, F.; Knuttinen, G.; Naidu, S.; Oklu, R. Catheter-directed thrombolysis of deep vein thrombosis: Literature review and practice considerations. Cardiovasc. Diagn. Ther. 2017, 7, S228–S237.
- Vedantham, S. Valvular dysfunction and venous obstruction in the post-thrombotic syndrome. Thromb. Res. 2009, 123, S62–S65.
- Kahn, S.R.; Comerota, A.J.; Cushman, M.; Evans, N.S.; Ginsberg, J.S.; Goldenberg, N.A.; Gupta, D.K.; Prandoni, P.; Vedantham, S.; Walsh, M.E.; et al. The Postthrombotic Syndrome: Evidence-Based Prevention, Diagnosis, and Treatment Strategies. Circulation 2014, 130, 1636–1661.
- Henke, P.K.; Comerota, A.J. An update on etiology, prevention, and therapy of postthrombotic syndrome. J. Vasc. Surg. 2011, 53, 500–509.
- Kahn, S.R.; Shrier, I.; Julian, J.A.; Ducruet, T.; Arsenault, L.; Miron, M.J.; Roussin, A.; Desmarais, S.; Joyal, F.; Kassis, J.; et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann. Intern. Med. 2008, 149, 698–707.
- Kearon, C.; Akl, E.A. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014, 123, 1794–1801.
- Al-Samkari, H.; Connors, J.M. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv. 2019, 3, 3770–3779.
- Ribic, C.; Crowther, M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 188–195.
- Robert-Ebadi, H.; Le Gal, G.; Righini, M. Use of anticoagulants in elderly patients: Practical recommendations. Clin. Interv. Aging 2009, 4, 165–177.
- Alotaibi, G.S.; Almodaimegh, H.; McMurtry, M.S.; Wu, C. Do women bleed more than men when prescribed novel oral anticoagulants for venous thromboembolism? A sex-based meta-analysis. Thromb. Res. 2013, 132, 185–189.
- Barrios, D.; Morillo, R.; Guerassimova, I.; Barbero, E.; Escobar-Morreale, H.; Cohen, A.T.; Becattini, C.; Tapson, V.; Yusen, R.; Jimenez, D. Sex differences in the characteristics and short-term prognosis of patients presenting with acute symptomatic pulmonary embolism. PLoS ONE 2017, 12, e0187648.
- Wadhera, R.K.; Russell, C.E.; Piazza, G. Cardiology patient page. Warfarin versus novel oral anticoagulants: How to choose? Circulation 2014, 130, e191–e193.
- Gross, P.L.; Weitz, J.I. New Anticoagulants for Treatment of Venous Thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 380–386.
- Burgazli, K.M.; Atmaca, N.; Mericliler, M.; Parahuleva, M.; Erdogan, A.; Daebritz, S.H. Deep vein thrombosis and novel oral anticoagulants: A clinical review. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3123–3131.
- Nathan, A.S.; Giri, J. Reexamining the Open-Vein Hypothesis for Acute Deep Venous Thrombosis. Circulation 2019, 139, 1174–1176.
- Patterson, B.O.; Hinchliffe, R.; Loftus, I.M.; Thompson, M.M.; Holt, P.J.E. Indications for Catheter-Directed Thrombolysis in the Management of Acute Proximal Deep Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 669–674.
- Goldhaber, S.Z.; Magnuson, E.A.; Chinnakondepalli, K.M.; Cohen, D.J.; Vedantham, S. Catheter-directed thrombolysis for deep vein thrombosis: 2021 update. Vasc. Med. 2021, 26, 662–669.
