Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Flora Ventsislavova Tsvetanova | -- | 3474 | 2022-12-08 11:55:54 | | | |
| 2 | Sirius Huang | Meta information modification | 3474 | 2022-12-09 02:27:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsvetanova, F.; Yankov, D. Valuable Biochemicals from Red Microalgae. Encyclopedia. Available online: https://encyclopedia.pub/entry/38329 (accessed on 07 February 2026).
Tsvetanova F, Yankov D. Valuable Biochemicals from Red Microalgae. Encyclopedia. Available at: https://encyclopedia.pub/entry/38329. Accessed February 07, 2026.
Tsvetanova, Flora, Dragomir Yankov. "Valuable Biochemicals from Red Microalgae" Encyclopedia, https://encyclopedia.pub/entry/38329 (accessed February 07, 2026).
Tsvetanova, F., & Yankov, D. (2022, December 08). Valuable Biochemicals from Red Microalgae. In Encyclopedia. https://encyclopedia.pub/entry/38329
Tsvetanova, Flora and Dragomir Yankov. "Valuable Biochemicals from Red Microalgae." Encyclopedia. Web. 08 December, 2022.
Copy Citation
Red microalgae represent a natural reservoir of beneficial substances with applications in different industrial sectors. They are rich in natural biomolecules known for their antihypertensive, antioxidant, antimicrobial, antiviral, anti-inflammatory, antitumor, and anticoagulant activities. Many red microalgae are a source of vitamins, minerals, photochemicals, polyunsaturated fatty acids, and a wide spectrum of polysaccharides. The content of their valuable compounds and their activities have turned red microalgae into cellular factories of special interest in food, nutraceutical, and pharmaceutical industries.
red microalgae
valuable biochemicals
health benefits
1. General Overview of Red Microalgae
Microalgae are eukaryotic, photoautotrophic, single-cell organisms inhabiting diverse ecosystems, including terrestrial, aquatic, and airborne environments [1][2]. They utilize solar energy, water, and inorganic nutrients to reduce CO2 into complex organic compounds, and some of them are capable of surviving in extreme conditions [3]. One of the markers that divides the microalgae into different classes such as Phaeophyceae, Chlorophyceae, Pyrrophyceae, Bacillariophyceae, Chrysophyceae, and Rhodophyceae (or red microalgae) [4], is the pigment composition of the cells. The red microalgae constitute a major group, which still remains unappreciated and little exploited. This group includes eight genera, the most studied of which are Porphyridium, Rhodella, and Rhodosorus. They are morphologically the simplest of all the red algae. Their size varies between one and one hundred micrometers, depending on the species and stage of growth. In the cytoplasm, many organelles involved in microalgal metabolism are presented. The major part of red microalgae consists of spherical or ovoid unicells [5]. Their color is due to the phycobiliproteins in plastids which serve as light-harvesting pigment–protein complexes in photosynthesis. Among red microalgae, Porhyridium sp. are cultivated for the commercialization of high-value products, including omega-3 long-chain polyunsaturated fatty acids, polysaccharides, antioxidants, and pigments [6][7].
Different from other microalgae, red ones do not contain microfibrillar cellulose. Their cell walls are encapsulated within a matrix of sulfated polysaccharides (PS). During the growth phase, the external part of the capsule dissolves into the medium, imparting an increase in viscosity. Another part remains membrane-bonded [8][9]. In the literature, these polymers are called EPS, meaning either extracellular polymeric substances, extracellular polysaccharides, or exopolysaccharides. The capsules are thinnest in the logarithmic growth phase and thickest in the stationary phase. The size of the capsule depends on the growth conditions. The thickness of the capsule is affected by the rate of production, degree of solubility, and cell surface area [4]. PS are composed of about ten different sugars, among which xylose, glucose, and galactose are present in higher quantities in different quantitative ratios. Rhamnose, ribose, arabinose, mannose, 4-O-methyl galactose, and 3-O-methyl pentose are present in minor quantities [10]. Red microalgae PS are negatively charged due to the presence of glucuronic acid and half-ester groups [11]. It has been found that the extracellular polysaccharide from Porphyridium sp. contains three neutral monosaccharides—xylose, glucose, and galactose, and one uronic acid—glucuronic acid. The uronic degradation with lithium in ethylenediamine resulted in two different oligosaccharides (Figure 1a,b). The chemical determination revealed the presence of D-xylose, D-glucose, D-, and L-galactose [12]:
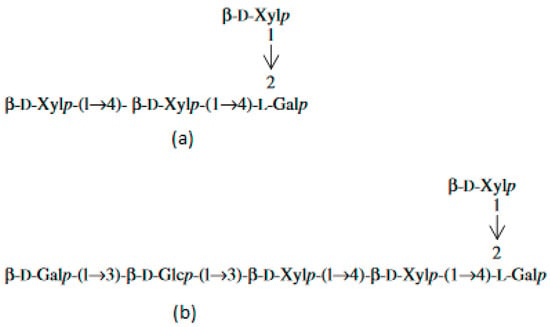
Figure 1. Oligosaccharides released from the extracellular polysaccharide of Porphyridium sp. after the uronic degradation with lithium in ethylenediamine. (a) oligosaccharide 1; (b) oligosaccharide 2.
All the red microalgae show a remarkable adaptation capacity to strong acid media (pH = 0.5–3.5) and high temperatures (38–56 °C) [13][14]. Such extremophile characteristics allow red algae to survive in habitats which other organisms cannot tolerate. They are capable of persisting in different geothermal environments and sulfur springs, boiling mud pools, and hot acidic waters [15]. Moreover, red microalgae tolerate metals; thus, they can survive in rather toxic conditions [16].
Microalgae are an important part of the food and nutraceutical industries due to their rich protein content. The microalgal protein content is higher than that of some vegetable sources such as rice, wheat, and legumes but poorer than the protein content of animal sources (meat and milk) [17]. Protein hydrolyzation results in releasing amino acids required for the growth and regeneration of the body and the maintenance of good health conditions. The microalgal protein content is affected by a variety of factors such as light intensity and spectrum, temperature, pH, and the nutrition media content [18]. Microalgal biomass is incorporated as a supplement in a wide range of health-promoting products such as tablets, gel capsules, and liquids. It is usually added to foods as protein supplementation or as a colorant in the form of dried powder—in pasta, snack foods, noodles, biscuits, ice cream, candies, and beverages [19]. In addition to their nutritional value, microalgal proteins play the role of precursors for the synthesis of antibodies, enzymes, and cytokines. Moreover, they can be used as oral vaccines, intestinal bioactive agents, and complex antitumor agents [20]. Algal proteins have already been used as food additives, dietary supplements, products of pharmaceutical value, and cosmetics. The prospect of the global algal protein market is to reach USD 0.84 billion by 2023, from USD 0.6 billion in 2018 [21].
2. Valuable Biochemicals with Therapeutic and Nutritional Potential Produced by Red Microalgae
Red microalgae are of significant biotechnological importance as they are a rich source of biomolecules with health and nutritional value. Polysaccharides, pigments, polyunsaturated fatty acids, and microelements are the highlighted beneficial compounds from red microalgae.
2.1. Polysaccharides
Thanks to their physical and chemical properties such as a high viscosity, high molecular weight, monosugar content, flexibility of the macromolecular chain, and level of sulfation, the exopolysaccharides of red microalgae have gained significant attention in recent decades [22]. The functions of PS are in accordance with the organism necessities in the environment it inhabits. For example, in the case of the red microalga Porphyridium sp. isolated from marine sand where the climate conditions are fluctuating, and illumination is strong, sulfated PS provide the required moisture [23]. Sulfated PS also act as a free radical scavenger which protects the cells from high solar irradiation [24]. It was also supposed that PS play the role of a buffer layer, preventing exposure from extreme pH values and temperatures [10]. PS are relatively stable in a wide range of temperatures (30–160 °C), pH (2–9), and salinity. PS can also provide a barrier against bacteria, viruses, and fungi [9].
Another important feature of the red microalgal PS for their industrial value is their dynamic fluid behavior which means that high solution viscosity is reached at a relatively low concentration of PS. This peculiarity lends microalgal PS similar qualities to those of industrial PS (for example, xanthan gum). In addition, microalgal solutions are stable at high temperatures, pH, and ionic strengths [9]. The EPS from Porphyridium are sustainable under environmental changes and against hyaluronidase-degrading enzymes. These qualities make them suitable to be employed as biolubricants. Liberman and co-workers found that EPS and their acid-hydrolysate fractions exhibit even higher antioxidant activity than carrageenan. They achieved ~70 and ~35% inhibition against Escherichia coli and Bacillus subtilis, respectively, with a 0.1% w/v of the EPS solution [25].
The biological activities of EPS are promoted by the presence of uronic acid and other bonded chemicals such as trace metals and proteins [26][27]. Comparing the physico-chemical characteristics of the polysaccharides of Dixoniella grisea and Porphyridium aerugineum, the group of Liberman concluded that the polysaccharides from brackish and fresh water species, such as Dixoniella grisea, are characterized by a smaller number of charged groups but demonstrate higher viscosity in comparison with Porphyridium sp. [26].
The typical sulfated structure of PS is responsible for various biological activities such as immunomodulatory, anti-inflammatory, hypocholesterolemic, antimicrobial, antiviral [28], antioxidant [29], and antihyperglycemic [30]. The sulfate content ranges between 1 and 4% (w/w) [31]. According to the bibliographic study, two families of PS are characteristic of red microalgae—the intracellular storage PS (IPS) and the extracellular structural PS (EPS). The basic IPS of microalgae are starch and starch-like PS. The IPS of red microalgae are floridean starch (floridean glycogen), an α–polyglucan structure similar to starch, lacking amylose [32]. On the other hand, some microalgae are known to contain amylose—P. aerugineum, P. purpureum, P. sordidum, Rhodosorus marinus, Rhodella violaceae, Flintiella sanguinaria [33]. Floridean starch is a storage polysaccharide present in the cellular component (cytosol) [34]. Sulfated PS are normally located as intracellular-bonded to the cytoplasmic membrane, as well as extracellular (exopolysaccharides) [8]. The EPS of Porphyridium are non-toxic, and their main sugar content includes xylose, galactose, and glucose, as well as glucuronic acid and sulfate groups [9]. The exopolysaccharides from P. marinum were reported to exhibit antimicrobial and anticancer activities. With the addition of only 31.3 μg/mL, the biofilm formation of Candida albicans was reduced by about 90%. The viability of the breast cancer cells was reduced by 55% [35].
Recently, a group of scientists assumed that the sulfated PS from Porphyridium could be used as a coating material on sanitary materials for COVID-19 prevention on the basis of their proven activity against respiratory viruses from the coronavirus family [36]. Huang and co-workers (2001) established that microalgal PS exhibit anti-hepatitis B activity [37]. Moreover, all of the red microalgal polysaccharide extracts demonstrate strong activity against the V. stomatitis virus, and this activity is even higher than the activity of all of the chemicals tested so far [38]. Huleihel and collaborators established that sulfur-containing polysaccharides in Porphyridium showed enhanced antiviral activity by blocking the adsorption of virions against HSV-1 and HSV-2 [39].
In recent years, lots of studies have demonstrated that microalgal PS can enhance antioxidant enzyme activity, eliminate free radicals, and inhibit lipid peroxidation [39]. Antihyperglycemic activity is important for diabetes therapy. This is a condition occurring in the case of enhanced blood glucose concentrations (hyperglycemia) and decreased insulin secretion [40]. Conventional drugs often lead to intestinal disorders. For that reason, the antihyperglycemic activity of external PS is one possible opportunity for the prevention of these undesirable effects. In a recent study, the antihyperglycemic effect of P. cruentum’s PS was demonstrated in vivo [30]. The antihyperlipidemic activities of red microalgae were proved by Dvir and colleagues in experiments with rats. The rats’ diet was supplemented with Porphyridium biomass, which resulted in a reduction in the levels of cholesterol, triglycerides, and very low-density lipoproteins and improved the hepatic cholesterol levels. These beneficial effects were observed due to the presence of dietary fibers and PS in Porphyridium [27].
Carrageenans, which belong to the group of sulfated PS, are known to have antiviral activity. Periera (2018) stated that carrageenans selectively inhibit the binding of many enveloped and nonenveloped viruses [41]. In a study by Grassauer and Grassauer (2011), they established that 400 µg/mL of carrageenan from red algae resulted in the inhibition of cell death caused by the coronavirus infection. They found out that if the cells were pretreated with carrageenans, they were protected against infection with coronavirus as well [42].
All of the above-listed features attach high value to red microalgae PS for their employment in pharmaceutic, cosmetic, and nutritional fields. PS from the red microalga Porphyridium are incorporated into nutraceutical products with antioxidant activity produced by the Solazyme company. This formula contributes to the reduction in inflammation and oxidative stress in mammalian tissues [43]. Becker and co-workers (2007) pointed out that the nutritional potential of red microalgae biomass is comparable to that of vegetables. Their advantage is the absence of rigid cell walls, thus making their proteins more accessible [44].
Microalgal PS have the potential to be added to cosmetic products, as they implement the function of strengthening the skin barrier and hydrating agent. A scientific group examined the sulfated PS of P. cruentum for their capacity to be incorporated in cosmetic and pharmaceutical products for the skin. They tested their activity on three enzymes with features contributing to good skin conditions. The results obtained confirm that PS are capable of reducing the activity of hyaluronidase and elastase [45]. This activity was patented by the Solazyme company in 2014 [46].
The three main activities of PS are patented—the stimulation of collagen, elastane synthesis, and collagenase inhibition. Another patent of PS in the cosmetic field concerns the improvement of the barrier function and/or skin hydration. This is the patent of the L’Oréal Company [47] and refers to a mixture of PS of the Porphyridium sp., sulfated PS from marine bacteria, and ulvan, associated with D-glucosidase. L’Oréal has patented sulfated PS for their antidandruff activity [48]. A patent from 2009 comprises red microalgae PS and heavy metals [49]. The deficiency of metals has raised a problem as more and more people suffer from them. PS, such as hyaluronate, can be used as a chelate in pharmaceutical products carrying the deficient metal [50]. This is the ground of Arad’s patent US20110070159A1 [49]. According to another patent of the Arad’s group (WO1997000689A1), the PS from red microalgae protect against virus infections, especially from the Varicella Zoster virus [51]. The company Frutarom in Israel specializes in the cultivation of Porphyridium sp. for the production of sulfated PS [52]. Greensea (Méze, France) and AlgoSolis (GEPEA, Université de Nantes, CNRS, France) commercially cultivate Porphyridium cruentum [36]. The exopolysaccharide of Porphyridium cruentum is claimed to be able to replace carrageenans in many applications [5].
As a whole, the synthesis of exopolysaccharides is induced by nutrient limitation. The decrease in nitrogen leads to a stop in the microalgal growth and the beginning of the stationary phase. However, the microalgae proceed photosynthesizing and carbon fixation. Carbon is employed in the forming of energy reserves such as starch and lipids or is excreted in the form of exopolysaccharides [53].
2.2. Pigments
2.2.1. Phycobiliproteins
The pigments documented to be produced by red microalgae are phycobiliproteins, chlorophyll, and carotenoids. Phycobiliproteins (PB) are a class of water-soluble colored proteins, located in phycobilisomes on the outer surface of the thylakoid membrane and are typical for three types of algae, featuring Rhodophyta, Cyanophyta, and Cryptophyta. They absorb light in the spectrum range of 450–650 nm [54]. Among phycobiliproteins, phycoerythrin (PE), phycocyanin (PC) and allophycocyanin are found in the Rhodophyta genus [55]. Their structures are presented in Figure 2, Figure 3 and Figure 4. Phycocyanin and allophycocyanin are approved as food colorants by the European Food Safety Authority. In Porphyridium sp., the main pigment is phycoerythrin (PE), constituting about 60–80% of the total soluble protein [56]. The pink/red color of Porphyridium is due to the content of phycoerythrin. The commercial PE used as a fluorescent dye is mainly obtained from large red algae but in low content (<0.1% of dry weight). The purification process is complicated and expensive [57]. In contrast, Porphyridium contains a high concentration of PE (about 8% of dry weight) and can be cultivated on a large scale [58]. PE demonstrates antioxidant, anti-inflammatory, and hepatoprotective properties [59]. The health impact of the antioxidant properties of phycobiliproteins is in reducing the rate of diseases such as cancer, diabetes, inflammation, and neurodegenerative disorders [6][39]. Richa et al. (2011) reported the potential for the commercial application of PB in biomedicine as antioxidant, anti-inflammatory, neuroprotective, hepatoprotective agents, and in fluorescence-based assays [60]. The effect of B-phycoerythrin (B-PE) from P. cruentum on the proliferation of Graffy myeloid tumor cells was studied in vitro. The results demonstrated an approximate 50 and 63% suppression of cellular growth when 50 and 100 µg of B-phycoerythrin, respectively, were added [61]. PE extracted from Porphyra haitanensis was shown to exhibit anticancer activities [62].

Figure 2. Structure of phycoerythrin.
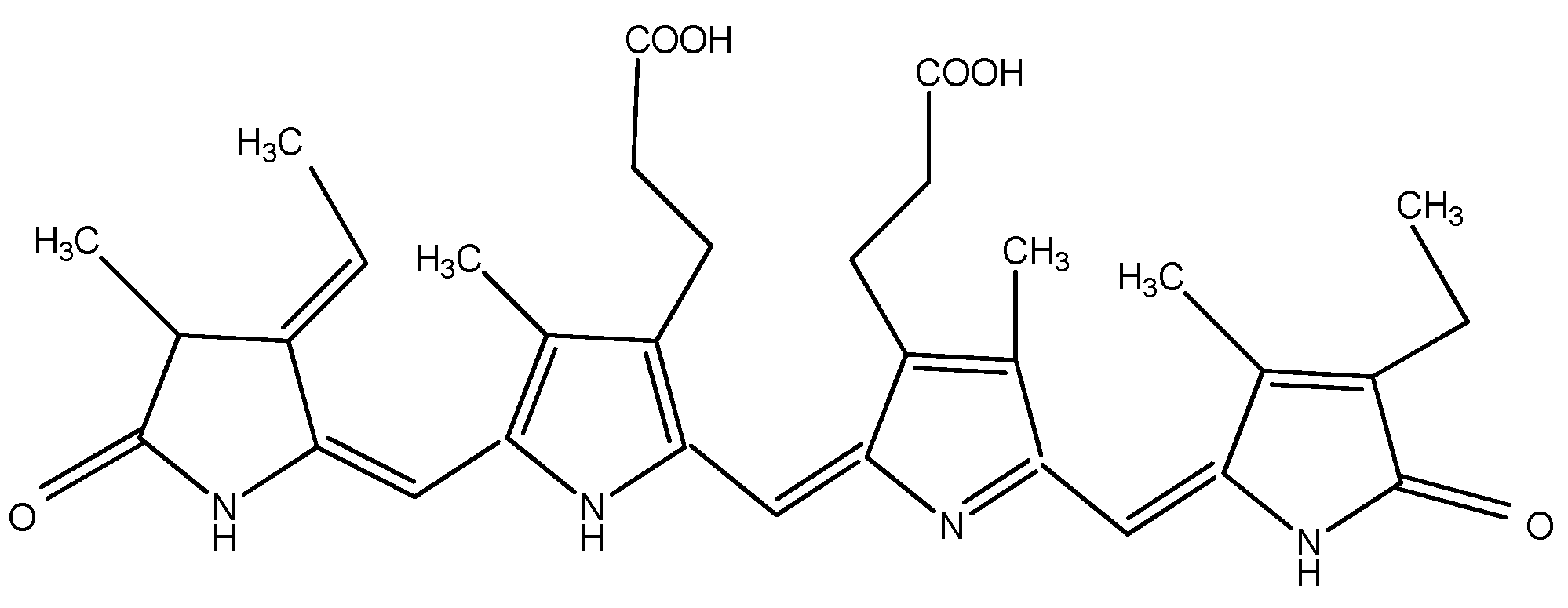
Figure 3. Structure of phycocyanin.
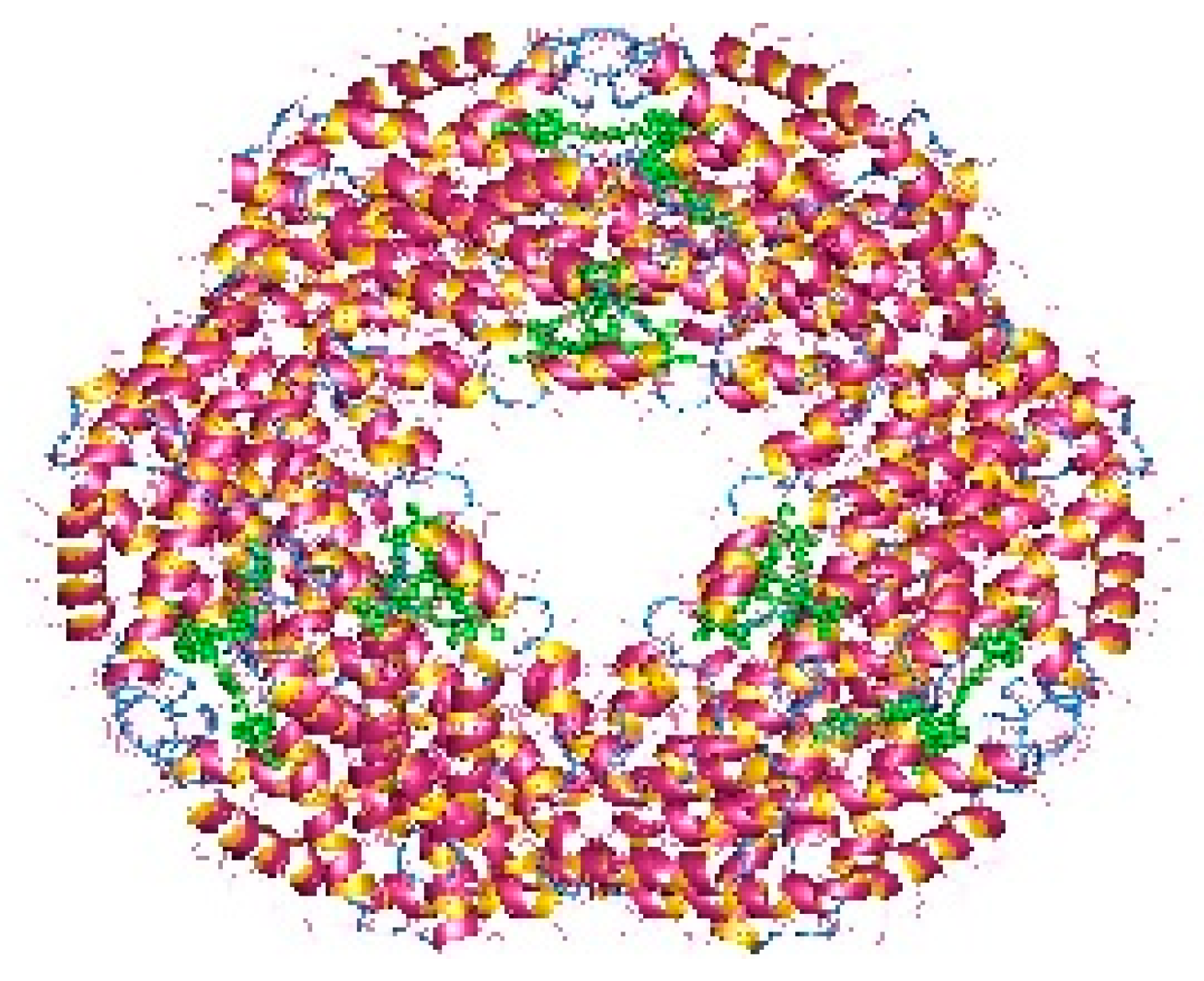
Figure 4. Allophycocyanin dodecamer (available online on https://en.wikipedia.org/wiki/Allophycocyanin, accessed on 14 November 2022).
As natural pigments, phycobiliproteins are normally added to foods, cosmetics, and edible dyes. B-PE has become commercially valuable as a coloring agent. As it is not toxic, it is ready to be added to foods, drinks, and cosmetics [63]. The pigments synthesized by Rhodophyta are applicable in the pharmaceutical field for their fluorescence. PB are employed as fluorescent agents in medical diagnostics, immunochemistry, and bioengineering research [64]. They are shown to be good substitutes for radioimmunological tracers, with the same sensitivity of detection. Among PB produced by red microalgae, B-PE from Porphyridium is commercially available as a fluorescent agent for flow cytometry and immunofluorescent staining (by Invitrogen, Waltham, Massachusetts, USA; Colombia Bio-sciences, Maryland, USA; AnaSpec, Fremont, CA, USA) [65].
Phycocyanin (PC) is the major pigment in Galdieria sulphuraria. In general, other microalgae produce PC in minor concentrations which means that its commercial production is unprofitable. In contrast, Galdieria, as mentioned above, accumulates an enormous quantity of biomass of up to 100 g/L dry weight [66]; therefore, higher concentrations of PC are reached, even in a dark process [67]. PC concentrations produced by Galdieria sulphuraria are even higher than those produced by Arthrospira (Spirulina) platensis which is employed for commercial PC production and is dependent on the presence of light [67]. Phycocyanin and allophycocyanin find applications in various fields of industry: the food/feed market, pharmaceuticals, nutraceuticals, and cosmetics. In Japan, the company “Dainippon Ink and Chemicals Ink” imparts natural edible blue dye containing about 17% of phycocyanin. There are lots of Japanese patents concerning food coloring [4]. The market for 2025 is scheduled to reach USD 19.0 million for phycocyanin and 6.3 million for phycoerythrin [68].
2.2.2. Chlorophyll and Carotenoids
Chlorophyll a exists in all photosynthetic organisms. Chlorophyll c and d are registered to be synthesized by red microalgae from the Rhodophyta genus, as well as other pigments such as R—phycocyanin and α/β—carotenes [69]. Carotenoids are of special interest as they offer protection from solar radiation. The basic carotenoids in Porphyridium sp. are β-carotene (2.6% of the total carotenoid content) and zeaxanthin (97.4% of the total carotenoid content) [70], a small amount of fucoxanthin, violaxanthin, diadinoxanthin, and lutein [71]. Carotenoids are a common supplement as a source of vitamin A precursor in foods and as natural colorants in chewing gums [72]. They possess antioxidant activity, therefore, preventing diseases and protecting the skin [73]. Carotenoids have a definite capacity for degenerative disease prevention (macular degeneration and eye cataracts) as well [74]. Owing to the antioxidant properties of β-carotene (Figure 5), it was proposed for the prevention of cancer and chronic illnesses, for neurodegenerative diseases as a potential life-extender, and as an ulcer, heart attack, and coronary artery disease inhibitor. It also effectively controls blood cholesterol [18][75][76][77][78].
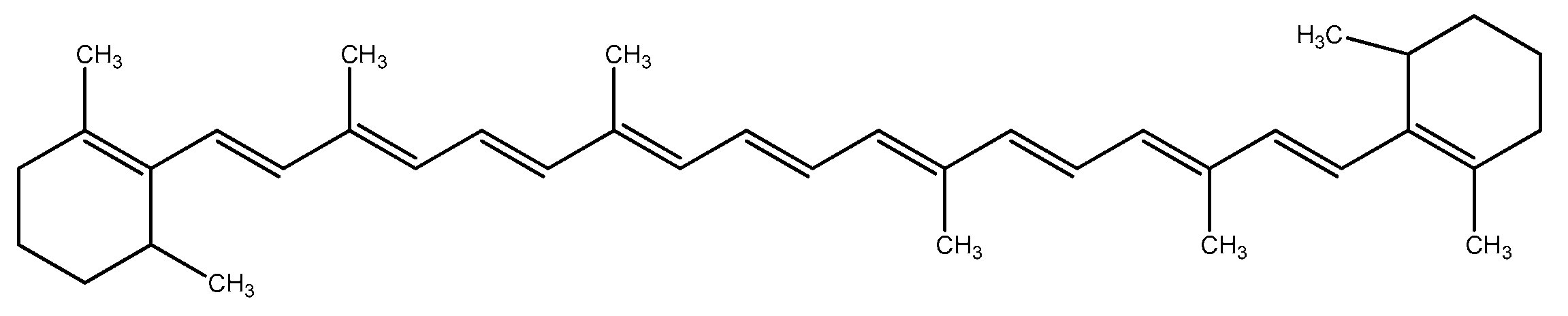
Figure 5. Structure of beta-carotene.
For zeaxanthin from Porphyridium purpureum, it was established that it induces apoptosis in human melanoma cells which suggests a capacity for adjuvant therapy [71].
2.3. Polyunsaturated Fatty Acids
Polyunsaturated fatty acids (PUFAs) are associated with very important physiological functions in human organisms. However, the human body is not capable of producing them. The common sources of fatty acids—marine fish oil and animal tissues—are diminishing [79]. Porphyridium is reported to synthesize a wide range of PUFAs, including palmitic, palmitoleic, stearic, oleic, linoleic, arachidonic (ARA) and eicosapentaenoic acid (EPA), as ARA and EPA comprise more than 40% of the total fatty acids. Their structures are shown in Figure 6. P. purpureum is claimed to be a promising accumulator of ARA, which is the most important PUFA, required for normal brain function, as an immune suppressant, a natural antifreeze, and an important intermediate for key physiological functions such as the metabolism of lipoproteins, blood flow, and white blood cell function [80]. Jiao and colleagues (2018) reported high levels of ARA in Porphyridium at 211.47 mg/L [81]. In the human body, EPA affects important systems and functions, including the cardiovascular system; it cleanses the arteries, treats atherosclerosis, diabetes, and high blood pressure, suppresses the inflammatory systems, and has a therapeutic effect on some types of cancer. Both EPA and ARA can be added as a supplement in functional foods, milk, and eggs. ARA, obtained from Porphyridium, is incorporated in infant formulas and in foods as a nutritional supplement [82]. The low cholesterol content and the fact that they are odorless make the microalgal PUFAs appropriate to be added to foods and nutraceuticals [83]. However, the production cost is not economically reasonable in comparison with other PUFA sources [4]. P. cruentum is biotechnologically employed for ARA, pigments (phycocyanin, phycoerythrin), and extracellular polysaccharide production.
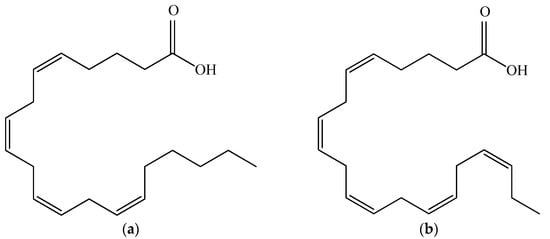
Figure 6. Structural formulas of arachidonic acid (a) and eicosapentaenoic acid (b).
The accumulation of lipids and the fatty acid composition are dependent on a variety of factors, including light intensity, temperature and nutrition in the culture medium, and the biomass productivity of the culture. For that reason, the light and temperature are controlled in order to achieve the maximal ratios of ARA and EPA [4].
2.4. Micronutrients
Red microalgae are known to produce a variety of health-boosting vitamins such as A, B1, B2, B6, B12, C, E, nicotinate, biotin, folic acid, and pantothenic acid [44]. The vitamin content of microalgae is of great importance for their implementation as food additives. Porhyridium is capable of accumulating large quantities of tocopherols (vitamin E). The tocopherols are lipid-soluble antioxidants that protect the membrane lipids from oxidative stress [84]. Vitamin E, extracted from P. cruentum, is a key factor in the prevention of many diseases such as atherosclerosis, heart disease, and multiple sclerosis [85][86]. Durmaz and colleagues reported the accumulation of 55.2 µg/g of dry weight α-tocopherol and 51.3 µg/g of dry weight γ-tocopherol [87]. Galdieria sulfuraria was also reported to synthesize vitamin E in different quantities, depending on the operating conditions—9 and 15 mg kg−1 of dry weight for autotrophic and heterotrophic culture, respectively. With respect to water-soluble vitamins, the same microalga was demonstrated in the heterotrophic growth accumulation of vitamin B2 (30 mg kg−1) and B3 (32 mg kg−1). In the autotrophic growth, the content of vitamin B3 was only 20 mg kg−1, and no vitamin B2 was produced [88].
The red microalgae produce a great variety of biochemicals with beneficial effects on human health. Considering the fast-growing human population, the importance of red microalgae as a source of biochemicals with nutritional and medicinal applications will increase to meet human needs.
References
- Rajvanshi, S.; Sharma, M.P. Microalgae: A potential source of biodiesel. J. Sustain. Bioenergy Syst. 2012, 2, 49–59.
- Tesson, S.V.M.; Skjøth, C.A.; Šanti-Temkiv, T.; Löndahl, J. Airborne microalgae: Insights, opportunities, and challenges. Appl. Environ. Microbiol. 2016, 82, 1978–1991.
- Pushkareva, E.; Johansen, J.R.; Elster, J. A review of the ecology, ecophysiology and biodiversity of microalgae in Arctic soil crusts. Polar Biol. 2016, 39, 2227–2240.
- Arad, S.M.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Technol. 1992, 3, 92–97.
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendry, I.; Abdelkafi, S.; Micgaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222.
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685.
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-S.; Lee, D.-J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67.
- Yang, H.; Jin, X.; Lam, C.W.K.; Yan, S.-K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782.
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotech. 2010, 21, 358–364.
- Geresh, S.; Arad, S. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991, 38, 195–201.
- Arad, S. Production of sulfated polysaccharides from red unicellular algae. In Algal Biotechnology; Stadler, T., Mollion, J., Verduset, M.C., Eds.; Elsevier Applied Science: London, UK, 1988; pp. 65–87.
- Castenholz, R.W.; McDermott, T.R. The Cyanidiales: Ecology, biodiversity, and biogeography. In Red Algae Genomic Age; Springer: Dordrecht, The Netherlands, 2010; pp. 357–371. ISBN 978-90-481-3794-7.
- Gloaguen, V.; Ruiz, G.; Morvan, H.; Mouradi-Givernaud, A.; Maes, E.; Krausz, P.; Strecker, G. The extracellular polysaccharide of Porphyridium sp.: An NMR study of lithium-resistant oligosaccharidic fragments. Carbohydr. Res. 2004, 339, 97–103.
- Sakurai, T.; Aoki, M.; Ju, X.; Ueda, T.; Nakamura, Y.; Fujiwara, S.; Umemura, T.; Tsuzuki, M.; Minoda, A. Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresour. Technol. 2016, 200, 861–866.
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372.
- Mobin, S.M.A.; Firoz, A. A review of microalgal biofuels, challenges and future directions. In Application of Thermo-Fluid Processes in Energy Systems: Key Issues and Recent Developments for a Sustainable Future, Singapore; Khan, M., Chowdhury, A., Hassan, N., Eds.; Springer Nature: Singapore, 2018; pp. 83–108. ISBN 978-981-10-0695-1.
- Chugh, M.; Kumar, L.; Shah, M.; Bharadvaja, N. Algal Bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129.
- Navarra, T. The Encyclopedia of Vitamins, Minerals and Supplements, 2nd ed.; Facts on File, Inc.: New York, NY, USA, 2004; ISBN 978-081604998.
- Metsoviti, M.N.; Katsoulas, N.; Karapanagiotidis, I.T.; Papapolymerou, G. Effect of nitrogen concentration, two-stage and prolonged cultivation on growth rate, lipid and protein content of Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2019, 94, 1466–1473.
- Mobin, S.M.A.; Firoz, A. Some promising microalgal species for commercial applications: A review. Energy Procedia 2017, 110, 510–517.
- Rasala, B.A.; Mayfield, S.P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth. Res. 2015, 123, 227–239.
- Barkallah, M.; Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Ayadi, M.-A.; Michaud, P.; Abdelkafi, S. Effect of Spirulina platensis biomass with high polysaccharides content on quality attributes of common carp (Cyprinus carpio) and common barbel (Barbus barbus) fish burgers. Appl. Sci. 2019, 9, 2197.
- Painter, T.J. Carbohydrate polymers in desert reclamation: The potential of microalgal biofertilizers. Carbohydr. Polym. 1993, 20, 77–86.
- Arad, S.; Richmond, A. Industrial production of microalgal cell-mass and secondary products-species of high potential: Porphyridium sp. In Handbook of Microalgal Culture: Biotechnology & Applied Phycology; Richmond, A., Ed.; Blackwell Science: Carlton, Australia, 2004; pp. 289–297.
- Liberman, G.N.; Ochbaum, G.; Arad, S.M.; Bitton, R. The sulfated polysaccharide from a marine red microalga as a platform for the incorporation of zinc ions. Carbohydr. Polym. 2016, 152, 658–664.
- Liberman, G.N.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Ronit Bitton, R.; Arad, S.M. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179.
- Wang, W.-N.; Li, Y.; Zhang, Y.; Xiang, W.; Li, A.F.; Li, T. Comparison on characterization and antioxidant activity of exopolysaccharides from two Porphyridium strains. J. Appl. Phycol. 2021, 33, 2983–2994.
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients 2009, 1, 156–167.
- Tannin-Spitz, T.; Bergman, M.; van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222.
- Setyaningsih, I.; Prasetyo, H.; Agungpriyono, D.R.; Tarman, K. Antihyperglycemic activity of Porphyridium cruentum biomass and extra-cellular polysaccharide in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2020, 156, 1381–1386.
- De Jesus Raposo, M.F.; de Morais, R.M.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486.
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217.
- Shimonaga, T.; Fujiwara, S.; Kaneko, M.; Izumo, A.; Nihei, S.; Francisco, P.B.; Satoh, A.; Fujita, N.; Nakamura, Y.; Tsuzuki, M. Variation in storage a-polyglucans of red algae: Amylose and semi-amylopectin types in Porphyridium and glycogen type in Cyanidium. Mar. Biotechnol. 2007, 9, 192–202.
- Viola, R.; Nyvall, P.; Pedersen, M. The unique features of starch metabolism in red algae. Proc. R Soc. Lond. B 2001, 268, 1417–1422.
- Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton, L.; Abdelkafi, S.; Fendri, I.; et al. Potential of exopolysaccharide from Porphyridium marinum to contend with bacterial proliferation, biofilm formation, and breast cancer. Mar. Drugs 2021, 19, 66.
- Nagle, V.; Gaikwad, M.; Pawar, Y.; Dasgupta, S. Marine red alga Porphyridium sp. as a source of sulfated polysaccharides (SPs) for combating against COVID-19. biology, 2020; Preprints.
- Huang, J.; Chen, B.; You, W. Studies on separation of extracellular polysaccharide from Porphyridium cruentum and its anti-HBV activity in vitro. Chin. J. Drugs 2001, 6.
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63.
- Huleihel, M.; Ishamu, V.; Tal, J.; Arad, S.M. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134.
- Li, T.; Xu, J.; Wang, W.; Chen, Z.; Li, C.; Wu, H.; Wu, H.; Xiang, W. A novel three-step extraction strategy for high-value products from red algae Porphyridium purpureum. Foods 2021, 10, 2164.
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68.
- Grassauer, A.; Prieschl-Grassauer, E. Antiviral Composition Comprising a Sulfated Polysaccharide. U.S. Patent No. 10,342,820, 9 July 2019.
- Dillon, H.F.; Somanchi, A.; Rao, K. Methods and Compositions for Cholesterol Reduction in Mammals. U.S. Patent No. US 20070167398A1, 19 January 2006.
- Becker, E. Microalgae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210.
- Yanhui, C. Cosmetic Composition with Functions of Repairing and Reinforcing Skin Barrier and Application Thereof. CN Patent No. CN 107412042, 17 June 2015.
- Bayona, K.C.D.; Gallon, S.M.N.; Estrada, A.L.; Rios, J.C.; Garces, L.A.; Martinez, A.M. Activity of sulfated polysaccharides from microalga Porphyridium cruentum over degenerative mechanisms of the skin. Int. J. Sci. Adv. Technol. 2012, 2, 85–92.
- Potter, A.; Ghibaudo, M.; Baltenneck, C. Association of Sulfated Polysaccharides and C-Glycoside and the Uses Thereof. WO Patent No. WO2014174188A1, 26 April 2013.
- Potter, A.; Thibaut, S.; Ribaut, C. Use of Sulphated Polysaccharides as Antidandruff Agent. WO Patent No. WO2013093307A1, 20 December 2011.
- Arad, S. Compositions Comprising Red Microalgae Polysaccharides and Metals. U.S. Patent No. US20110070159A1, 11 February 2014.
- Nimrod, A.; Greenman, B. Heavy Metal Salts of Hyaluronic Acid and Their Use as Antimicrobial Agents. U.S. Patent No. US4746504A, 24 May 1988.
- Arad, S.; Huliheil, M.; Tal, J. Antiviral Agents. WO Patent No. WO1997000689A1, 9 January 1997.
- Coragliotti, A.; Franklin, S.; Day, A.G.; Decker, S.M. Microalgal Polysaccharide. U.S. Patent No. US8927522B2, 6 January 2015.
- Pannard, A.; Pédrono, J.; Bormans, M.; Briand, E.; Claquin, P.; Lagadeue, Y. Production of exopolymers (EPS) by cyanobacteria: Impact on the carbon-to-nutrient ratio of the particulate organic matter. Aquat. Ecol. 2016, 50, 29–44.
- Bermejo, R.R.; Alvarez-Pez, J.M.; Acien Fernandez, F.G.; Molina Grima, E. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85.
- Wright, S.W.; Jeffrey, S.W. Pigment markers for phytoplankton production. In Marine Organic Matter: Biomarkers, Isotopes and DNA; The Handbook of Environmental Chemistry; Volkman, J.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 2N, ISBN 978-3-540-28401-7.
- Gantt, E.; Lipschultz, C.A. Phycobilisomes of Porphyridium cruentum. Pigment analysis. Biochemistry 1974, 13, 2960–2966.
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136.
- Dvir, I.; Moppes, D.; Arad, S. Foodomics: To discover the health potential of microalgae. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Oxford, UK, 2021; pp. 658–671.
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600.
- Richa, V.K.K.; Minu, K.I.G.; Rajeshwar, P.S. Biotechnological potentials of phycobiliproteins. Int. J. Pharma Bio Sci. 2011, 2, 1–9.
- Minkova, K.; Toshkova, R.; Gardeva, E.; Tchorbadjieva, M.; Ivanova, N.J.; Yossifova, L.; Gigova, L. Antitumor activity of B-phycoerythrin from Porphyridium cruentum. J. Pharm. Res. 2011, 4, 1480–1482.
- Pan, Q.; Chen, M.; Li, J.; Wu, Y.; Zhen, C.; Liang, B. Antitumor function and mechanism of phycoerythrin from Porphyra haitanensis. Biol. Res. 2013, 46, 87–95.
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112.
- Thoisen, C.; Hansen, B.W.; Nielsen, S.L. A simple and fast method for extraction and quantification of cryptophyte phycoerythrin. MethodsX 2017, 4, 209–213.
- Ibanez-Gonzáleza, J.M.; Mazzuca-Sobczuka, T.; Redondo-Mirandaa, R.M.; Molina-Grimaa, E.; Cooney, C.L. A novel vortex flow reactor for the purification of B-phycoerythrin from Porphyridium cruentum. Chem. Eng. Res. Des. 2016, 111, 24–33.
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Characterization and comparison of Porphyridium sordidum and Porphyridium purpureum concerning growth characteristics and polysaccharide production. Algal Res. 2020, 49, 101931.
- Gross, W.; Schnarrenberger, C. Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol. 1995, 36, 633–638.
- Global Forecast to 2025, and Geography MRFB-104360; Meticulous Market Research Pvt. Ltd.: Maharashtra, India, 2020; p. 150.
- Kim, S.M.; Kang, S.W.; Kwon, O.N.; Chung, D.; Pan, C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. App. Biol. Chem. 2012, 55, 477–483.
- Schubert, N.; Garcia-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216.
- Juin, C.; de Oliveira Junior, R.G.; Fleury, A.; Oudinet, C.; Pytowski, L.; Bérard, J.B.; Nicolau, E.; Thiéry, V.; Lanneluc, I.; Beaugeard, L.; et al. Zeaxanthin from Porphyridium purpureum induces apoptosis in human melanoma cells expressing the oncogenic BRAF V600E mutation and sensitizes them to the BRAF inhibitor vemurafenib. Braz. J. Pharmacogn. 2018, 28, 457–467.
- Toker, O.S. Porphyridium cruentum as a natural colorant in chewing gum. Food Sci. Technol. 2019, 39, 195–201.
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2018, 31, 309–318.
- Gallego, R.; Martínez, M.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Development of a green downstream process for the valorization of Porphyridium cruentum biomass. Molecules 2019, 24, 1564.
- Morais, M.G.; Vaz, B.S.; Morais, E.G.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. BioMed Res. Int. 2015, 2015, 835761.
- Herrero, M.; Mendiola, J.A.; Plaza, M.; Ibañez, E. Screening for bioactive compounds from algae. In Advanced Biofuels and Bioproducts; Lee, J., Ed.; Springer: New York, NY, USA, 2013; pp. 833–872.
- Fu, W.; David, R.N.; Zhiqian, Y.; Maonian, X.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. In Studies in Natural Products Chemistry; Elsevier B.V.: Cambridge, MA, USA, 2017; pp. 199–225.
- Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Carotenoids from marine microalgae, a valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 14, 5128–5155.
- Gill, I.; Valivety, R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997, 15, 401–409.
- Su, G.M.; Jiao, K.L.; Chang, J.Y.; Li, Z.; Guo, X.Y.; Sun, Y.; Zeng, X.H.; Lu, Y.H.; Lin, L. Enhancing total fatty acids and arachidonic acid production by the red microalgae Porphyridium purpureum. Bioresour. Bioproc. 2016, 3, 33.
- Jiao, K.L.; Xiao, W.P.; Xu, Y.C.; Zeng, X.H.; Ho, S.-H.; Laws, E.A.; Lu, Y.; Ling, X.P.; Shi, T.; Sun, Y. Using a trait-based approach to optimize mixotrophic growth of the red microalga Porphyridium purpureum towards fatty acid production. Biotechnol. Biofuel. 2018, 11, 273.
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13.
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684.
- Sato, N.; Moriyama, T.; Mori, N.; Toyoshima, M. Lipid metabolism and potentials of biofuel and high added-value oil production in red algae. World J. Microbiol. Biotechnol. 2017, 33, 74.
- Vismara, R.; Vestir, S.; Kusmic, C.; Barsanti, L.; Gualtieri, P. Natural vitamin E enrichment of Artemia salina red freshwater and marine microalgae. J. Appl. Phycol. 2003, 15, 75–80.
- Jialal, I.; Traber, M.; Devaraj, S. Is there a vitamin E paradox? Curr. Opin. Lipidol. 2001, 12, 49–53.
- Durmaz, Y.; Monteiro, M.; Bandarra, N.; Gökpinar, S.; Işik, O. The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J. Appl. Phycol. 2007, 19, 223–227.
- Vítová, M.; Goecke, F.; Sigler, K.; Řezanka, T. Lipidomic analysis of the extremophilic red alga Galdieria sulphuraria in response to changes in pH. Algal Res. 2016, 13, 218–226.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
09 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




