Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nkosingiphile E Zikalala | -- | 6043 | 2022-12-08 11:41:18 | | | |
| 2 | Sirius Huang | -8 word(s) | 6035 | 2022-12-09 02:16:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zikalala, N.E.; Azizi, S.; Zikalala, S.A.; Kamika, I.; Maaza, M.; Zinatizadeh, A.A.; Mokrani, T.; Kaviyarasu, K. Green Synthesis of Zinc Oxide Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/38328 (accessed on 07 February 2026).
Zikalala NE, Azizi S, Zikalala SA, Kamika I, Maaza M, Zinatizadeh AA, et al. Green Synthesis of Zinc Oxide Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/38328. Accessed February 07, 2026.
Zikalala, Nkosingiphile E., Shohreh Azizi, Sithembela A. Zikalala, Ilunga Kamika, Malik Maaza, Ali Akbar Zinatizadeh, Touhami Mokrani, Kasinathan Kaviyarasu. "Green Synthesis of Zinc Oxide Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/38328 (accessed February 07, 2026).
Zikalala, N.E., Azizi, S., Zikalala, S.A., Kamika, I., Maaza, M., Zinatizadeh, A.A., Mokrani, T., & Kaviyarasu, K. (2022, December 08). Green Synthesis of Zinc Oxide Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/38328
Zikalala, Nkosingiphile E., et al. "Green Synthesis of Zinc Oxide Nanoparticles." Encyclopedia. Web. 08 December, 2022.
Copy Citation
Zinc oxide nanoparticles (ZnO NPs) can be used effectively and efficiently for water treatment, along with other nanotechnologies. Owing to rising concerns regarding the environmental unfriendliness and toxicity of nanomaterials, ZnO NPs have been synthesized through biologically available and replenishable sources using a green chemistry or green synthesis protocol. The green-synthesized ZnO NPs are less toxic, more eco-friendly, and more biocompatible than other chemically and physically synthesized materials.
green synthesis
zinc oxide nanoparticles
antimicrobial approaches
photocatalytic activities
phytosynthesis
biosynthesis
1. Introduction
The concurrent occurrence of organic dyes and inorganic toxic pollutants, such as heavy metals (HMs), pharmaceuticals, and pathogenic microorganisms in wastewater is a major worldwide concern [1]. Although the effects of each of the pollutants are well documented, the toxicity that arises from the co-occurrence of, and interactions between, these pollutants remains unknown; therefore, this presents a greater concern than the singular effects of each of the pollutants, with respect to the negative effect on the balance of the ecosystem, plants, and animals [2]. Synthetic dyes cause water to become unaesthetic, and they adversely compromise the health of humans and aquatic organisms by altering the biochemical oxygen demand (BOD), chemical oxygen demand (COD), salt concentration, light penetration, and rate of photosynthesis [3]. HMs are extremely poisonous due to the failure of living systems to remove them from the bodies of animals, thus leading to bioaccumulation. As such, HMs are responsible for neurological complications, carcinogenesis, mutations, and multiorgan failure in different organisms, even at low concentrations [4]. Pathogenic microorganisms comprise another class of public health concerns due to their short lifecycle, high reproductive rate per lifecycle, and ultrafast mutation rates that produce resistant strains. Around 500 waterborne microorganisms have been identified in wastewater treatment plants, including viral, bacterial, parasitic protozoan, and fungal pathogens [5]. These organisms invade a range of animal tissues, and they compromise the immune system through the release of toxins [6]; therefore, such detrimental pollutants must be efficiently eradicated from effluents before they are discharged into surface water bodies for the protection of ecological and biological systems.
Nanomaterials can address bottlenecks in conventional wastewater treatment systems because the nano-dimensions create a high surface area, quantum confinement, an attenuated surface charge, and other peculiar physical and chemical characteristics [7]. As such, various nanostructures have been synthesized for the purification of wastewater. Among the nanomaterials developed are: metallic nanoparticles such as Ag [8], Au [9], and Cu [10], and nanometal oxides such as TiO2 [11], Fe2O3 [12], SnO [13], AgO [14], CuO [15], and ZnO [16].
ZnO is among one of the most used semiconductors because of its ease of synthesis and its photo-driven electronic response. ZnO is a crystalline n-type semiconductor, it is a member of the II–VI group, and it exhibits a wide bandgap (3.37 eV) in the near UV spectrum [17][18]. Moreover, it exhibits high exciton binding energy (60 meV) in standard atmospheric conditions [19]. With respect to its crystallographic structures, ZnO has three polymorphs, namely: zincblende, cubic rock salt, and the hexagonal wurtzite. Of these polymorphs, wurtzite is the most stable, hence its synthesis at room temperature (RT) under pressure. The rock salt polymorph is formed under high pressure which results in the close packing of the Zn and O atoms. The zincblende polymorph ZnO is metastable, and therefore, it cannot exist on its own in ambient conditions [20][21]. With respect to the morphology of ZnO, as seen under transmission electron microscopy (TEM) and scanning electron microscopy (SEM), there is a wide variety of nanoparticulate ZnO morphologies. These include nanorods [22], spheres [23], nanosheets [24], hexagons [25], tripods [26], nanowires [27], nanotubes [28], nanostars [29], nanoflowers [30], and nanocubes [31].
2. Green Synthesis
Over the last few decades, the “green synthesis” approach has received a lot of interest in the field of nanomaterial synthesis. Indeed, the green synthesis technique for nanomaterials is based on twelve green chemistry principles, namely: (i) to improve the reaction efficiency of atoms (atom economy); (ii) to improve the energy efficiency of reaction processes (i.e., avoidance of long synthesis times, high temperatures, and high pressure environments); (iii) to use harmless chemicals in order to reduce the toxicity of the procedures and the synthesized nanomaterials; (iv) to reduce waste throughout the synthesis process; (v) to use easily available renewable precursors; (vi) to design non-toxic biodegradable products; (vii) to follow safer synthesis routes and replace hazardous substances with safe (or at least less toxic) reagents; (viii) to avoid the use of derivatives such as stabilizers; (ix) to avoid the release of toxic waste; (x) to employ eco-friendly solvents, such as water, instead of organic solvents; (xi) to accelerate reactions by including a catalyst, so as to reduce total energy demands in order to improve sustainability and efficiency; and (xii) to limit accidents during and after the synthesis process [32][33][34]. In traditional chemical synthesis, organic capping agents such as oleic acid, linolenic acid, oleylamine, hexadecylamine, and 2-mercaptoethanol need to be used to attain particles with the desired morphology and with a high degree of uniformity [35]. Biological synthesis, on the other hand, encourages the use of readily available resources, such as non-toxic solvents and auxiliaries, for a benign synthesis protocol [36]; therefore, it is in accordance with the principles of green synthesis. Biological synthesis promotes the development of nanoparticles using nature-derived materials such as plants [37], bacteria [38], fungi [39], algae [40], and biological derivatives [41]. These bio-sources (enzymes, polysaccharides, phytochemicals, biomolecules, vitamins, etc.) serve as green capping, stabilizing, or chelating agents [7]. During biosynthesis, a zinc salt such as chloride, acetate, sulfate, or nitrate is generally added to a previously prepared biological extract (from plants, bacteria, fungi, algae, yeast, etc.) to facilitate the complexation of metal ions with the available functional groups in the biological extract (Figure 1). This is achieved by heating and stirring the extract in an alkaline environment. The temperature, pH, and ratio of the precursors determine the growth phase, wherein the nucleated seeds grow either isotropically or anisotropically. Moreover, as is the case in conventional chemical synthesis processes, the synthesis parameters in green synthesis procedures determine the morphology, size, agglomeration, and size distribution of the resultant NPs [42]. After the metal oxide precipitates, it is washed, dried, and/or calcined to produce ZnO NPs, as indicated in Figure 1.
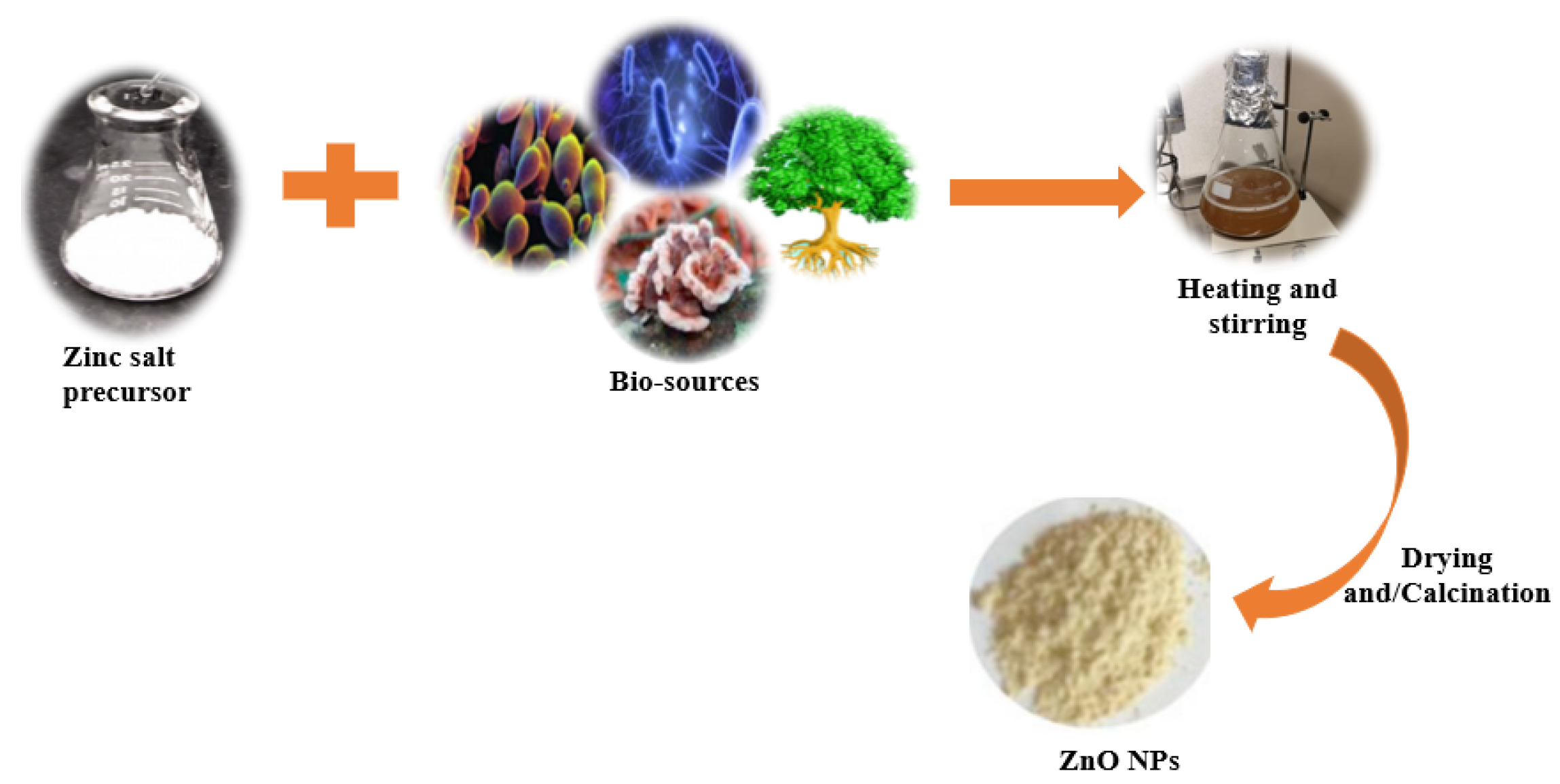
Figure 1. Schematic representation for the green synthesis of ZnO nanoparticles.
Several reviews point out the potential of biosynthesized ZnO NPs for various applications. These applications can be broadly grouped into medical, environmental remediation, and agricultural applications [7][36][43][44][45]. The interest in green-synthesized ZnO has increased due to its biocompatibility, photocatalytic capability, antimicrobial effectiveness, physical and chemical stability, and cost effectiveness.
2.1. Plant-Mediated/Phytosynthesis of ZnO NPs
2.1.1. Phytosynthesis Synthesis of Undoped ZnO NPs
In comparison with other ZnO green synthesis techniques, the utilization of plant extracts (phytosynthesis) has dominated scientific research, hence the large number of reports available for review [46][47][48][49]. Researchers prefer using parts of plants over microbes since the use of plants eliminates laborious and delicate procedures such as microbe isolation, growth, and maintenance. Furthermore, phytosynthesis is safer, as it eliminates the health risks associated with the use of hazardous microorganisms [44]. The phytosynthesis approach is preferred for large-scale production, as plant extracts can easily be obtained through the use of eco-friendly solvents such as distilled water or alcohols [50]. Additionally, plant sources are easily accessible, abundant, and hence more sustainable [51]. Since plant extracts naturally possess secondary metabolites, including amino acids, alkaloids, and flavonoids, among others, they are commonly employed as both chelating and reducing agents in the fabrication of nanoparticles [52]. Although the explicit mechanism of their formation cannot yet be explained, Bala et al. and Senthilkumar et al. concluded that phenols, flavonoids, and proteins are the main participants in the development of metallic and metal oxide NPs [53][54].
Various parts of a known plant species, including the leaves, seeds, pericarps, peels, roots, bark, or even the whole plant, have been used to synthesize ZnO NPs. Zinc salts are said to be oxidized by phenols and flavonoids into ZnO NPs with the help of the numerous functional groups that these molecules possess. These functional groups attach onto the surface of Zn2+, and they oxidize the molecule into ZnO NPs [42][54]. Since the coordination chemistry of proteins is specific to that of zinc ions, they are the predominant chelating agents for Zn2+. The H2O molecules of the aqua complexes are exchanged when they bind to the protein ligands [55]. During their interaction with the acidic [Zn(H2O)6]2+, an electron from the −COO− initiates a nucleophilic reaction, which results in the formation of the [Zn(OH) (H2O)5]+ complex [56]. The [Zn(OH) (H2O)5]+ is then transmuted into ZnO NPs [57]. According to Singh et al., the −OH and/or −COO− of the flavonoids react with the ZnO precursor to generate a distinctive Zn flavonoid complex via the electrostatic interaction between negatively charged flavonoids and positively ZnO nuclei. This complex is then later converted to ZnO through thermal treatment [58]. An alkaline pH favors the formation of metal oxide nanoparticles that are stabilized by the phytochemicals in the plant extract [59]; thus, it follows that during the phytosynthesis of metal oxides such as ZnO, a combination of polyphenols, flavonoids, and proteins results in the creation of ZnO NPs [60]. Krol et al. [56] presented a probable reaction mechanism for a plant mediated ZnO.
Table 1 provides an overview of the plant-based synthesis of ZnO NPs. The use of the waste pericarp of Ananas comosus yielded spherical NPs, the sizes of which varied between 10 and 60 nm after sonicating for 1 h at 40 °C [61]. The seed extract of Eriobutria japonica produced hexagonal NPs when a mixture of the seed extract and Zn (CH3COO)2·2H2O was heated at 60 °C for 1 h. Using Fourier-transform infrared spectroscopy (FTIR), the reducing and capping agents were revealed to be phenolic groups, alcohols, and aliphatic amines. Recently, fruit-derived aqueous extracts have been employed to prepare ZnO NPs [22][62][63][64]. Golmohammadi et al. [64] obtained spherical NPs with an average size of 29 nm when they refluxed Zizyphus jujuba fruit and Zn(NO3)2·6H2O with water at 80 °C for 30 min prior to calcination. Recently, Faisal et al. [65] produced semi spherical NPs when synthesizing with the fruit extract of Myristica fragrans.
Biosynthesizing ZnO NPs using flower extracts have also been reported [66][67]. The extract of Hibiscus sabdariffa served as a reducing and stabilizing agent in order to produce wurtzite-phased NPs. The concentration of the employed extract determined the surface structure and size of the NP. The sizes ranged between 8.71 and 88.63 nm [62]. Khara et al. produced spherical and irregular ZnO NPs using Peltophorum pterocarpum, with a mean diameter of 69.45 nm [67]. This flower extract possesses phenolic compounds and flavonoids, among other properties, that potentially reduce and stabilize ZnO NPs. Moreover, Diallo and colleagues obtained very small (1–8.5 nm) quasi-spherical ZnO nanomaterials by applying the flower extract of Aspalathus linearis as a reducing/oxidizing chemical agent [68].
Regarding phytosynthesis, leaves are the most frequently used parts of a plant during extraction. This is likely due to their accessibility, abundance, and ease of grinding. Several authors have reported on the phytosynthesis of various nanomaterials through the use of leaf extracts from various species of plants [69][70][71]. Mostly, the attained NPs were spherical and irregularly shaped. Ekennia and colleagues, however, attained flower-shaped NPs (31 nm) when using an extract of Euphorbia sanguinea and ZnCl2 as the salt precursor [72]. The appearance of the 1638 cm−1 absorption band in the FTIR spectra was ascribed to the N–H bending of amines in alkaloids or C=O stretching in polyphenols, flavonoids, and reducing sugars, which were thought to be responsible for reducing and stabilizing the synthesized NPs. An extract of quince seed mucilage was kept for 4 h in the presence of Zn(NO3)2·6H2O, before it was concurrently heated and stirred at 80 °C for 2 h to produce 25 nm spherical ZnO NPs [73]. Prior to this study, Prasad and colleagues [74] mixed Abelmoschus esculentus mucilage extract with Zn(OOCCH3)2·2(H2O) salt and quince seed mucilage in water, and the mixture was stirred for 4 h, at RT, to obtain spherical ZnO NPs with an average diameter of 29 nm and 70 nm long nanorods. The reducing and capping agents for the NPs were carbohydrates, which are available in excess in plants. The heterogeneous sizes and shapes were ascribed to the method employed and the high calcination temperature (700 °C for 2 h); this poses a challenge with regard to controlling the morphology of the prepared particles.
There have also been reports on the utilization of the aqueous extracts in roots, regarding the biogenic preparation of ZnO NPs with various morphologies [75]. A methanolic extract was employed by Verma et al. [47] to obtain hexagonal and rod-shaped NPs after stirring Zn(CH3COO)2·2H2O in the presence of Salvadora persica root extract for 3 h at 80 °C; it was then heated at 400 °C for an appropriate length of time. Matinise et al. prepared ZnO NPs by capping the NPs with an aqueous solution of Moringa oleifera leaf. The extract completely dissolved the zinc salts at an ambient temperature; this was evidenced by the fact that the solution changed color, from clear to brown, after 18 h without any precipitation. Spherically shaped ZnO NPs were formed after drying the concentrated solution at 100 °C for 1 h [76]. Highly crystalline single phase zincite ZnO NPs were produced by adding an aqueous solution of Agathosma betulina, in order to reduce the presence of zinc nitrate hexahydrate salt; in this scenario, the temperature was set at 100 °C for 2 h. The resultant precipitate was further annealed at 500 °C for 2 h to obtain (15.8 nm) quasi-spherical NPs [77]. When Ngom et al. prepared the same nanoparticles by employing Moringa oleifera leaves as the chelating agent, wurtzite phased ZnO nanomaterials, with an average diameter of 10.81 nm, were produced [78].
Table 1. Plant-based synthesis of ZnO nanoparticles.
| Serial No. | Plant Name | Plant Part | Extraction Solvent Temp (°C) Time |
Zinc Precursor | Biosynthesis Time (h)/Temp (°C) |
Calcination Temp (°C)/Time (h) |
Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pithecellobium dulce | Peel | Water, 70 °C for 50 min | Zn(CH₃CO₂)₂ | 400/2 | Spherical | 30 | [62] | |
| 2 | Ananas comosus | Pericarp | Water, 80 °C for 20 min | Zn(CH3COO)2·2H2O, | Sonicated 1/40 | − | Spherical | 10–60 | [61] |
| 3 | Punica granatum | Fruit peel | Water, 65 °C for 1 h | Zn(NO3)2·6H2O | –/90 | 600/1 | Spherical and hexagonal | 38.98 | [63] |
| 4 | Zizyphus jujuba | Fruit | Refluxed with water, 80 °C for 30 min | Zn(NO3)2·6H2O | 4/80 | 550/3 | Spherical | 29 | [64] |
| 5 | Myristica fragrans | Fruit | Water, 150 °C for 20 min | Zn(NO3)2·2H2O | 2/60 | 500/2 | Semispherical | 41.23 | [65] |
| 6 | Hibiscus sabdariffa | Flower | Water, 60 °C, for 1 h after 2 h stirring at RT | Zn(NO3)2 | 1/RT. | 400/1 | Semi spherical | 8.7–88.6 | [66] |
| 7 | Peltophorum pterocarpum | Flower | Water, 80 °C for 1 h | Zn(NO3)2 | –/80 | 400/2 | Spherical and irregular | 69.45 | [67] |
| 8 | Aspalathus linearis | Flower | Water, 25 °C for 48 h | Zn(NO3)2·6H2O | −/− | 300/2 | Quasi-spherical | 1–8.5 | [68] |
| 9 | Eucalyptus spp. | Leaves | Water, 90 °C for 90 min | Zn(NO3)2·6H2O | 2/110 | 250/6 | Irregular shapes | 32.71 | [69] |
| 10 | Capparis zeylanica | Leaves | Water, 60 °C for 20 min | Zn(OOCCH3)2·2(H2O), | 2/80 | 400/2 | Spherical | 20–40 | [70] |
| 11 | Ocimum tenuiflorum | Leaves | Powder | ZnSO4 | 0.5/RT | 600/0.5 | Spherical and granular | 50–63 | [71] |
| 12 | Euphorbia sanguinea | Leaves | Water, 80 °C for 45 min | ZnCl2 | 2/90 | – | Flower-like | 34 | [72] |
| 13 | Moringa oleifera | Leaves | Water, 50 °C for 105 min | Zn(NO3)2·6H2O | 18/RT then 100 | 500/1 | Spherical | 16–31.9 | [76] |
| Gathosma betulina | Leaves | Water, 100 °C for 60 min | Zn(NO3)2·6H2O | 2/100 | 500/2 | Quasi-spherical | 15.8 | [77] | |
| 14 | Quince seeds | Mucilage | Water, 60 °C for 4 h | Zn(NO3)2·6H2O | 2/80 after keeping for 4 h at RT | 600/2 | Spherical | 25 | [73] |
| 15 | Abelmoschus esculentus | Mucilage | Soaked for 24 h at RT | Zn(OOCCH3)2·2(H2O), | 4/RT | 700/2 | Spherical Rods |
29 70 |
[74] |
| 16 | Panax spp. | Roots and stems | Water, 85 °C for 8 h | Zn(NO3)2 | 2.5/85 | 500/2 | Leafy flower | 480 | [75] |
| 17 | Salvadora persica | Roots | Water and methanol, 80 °C for 3 h | Zn(CH3COO)2·2H2O | 2–3/80 | 400/2 | Hexagonal and rods | 24–25 | [47] |
| 18 | Rubus fairholmianus | Roots | Acetone using Soxhlet apparatus | Zn(NO3)2 | 48/80 | − | Spherical | 1–100 clusters | [79] |
2.1.2. Phytosynthesis of Doped ZnO Nanoparticles
Recently, the number of biogenically doped ZnO NPs has been increasing, which speaks to the efficiency of plant metabolites in nanomaterial synthesis. Doping occurs as a result of the wide band gap of ZnO, which causes ZnO to only absorb light that falls within the UV spectrum. Doping is a process wherein a point defect in a nanomaterial is formed by inserting a specific ion into the crystal lattice of a nanomaterial to modify its electronic band structure. The band structure is modified by the formation of shallow or deep energy level, known as mid-gap states, that extend the absorption range from shorter to longer wavelengths; this can even extend to the visible range of the electromagnetic spectrum [80][81]. The physicochemical properties of doped ZnO significantly depend on the dopant type and its concentration. p-type dopants tend to withdraw electrons from the ZnO, whereas n-type dopants increase the number of electrons. As the dopant concentration increases, the energy band gap Eg of ZnO decreases until an optimal dopant concentration is reached, beyond which, the dopant ions act as charge recombination centers and reduce the efficiency of ZnO in photocatalytic processes. The dopants also become active sites that are different from the ZnO active sites, thereby increasing the mass transfer of pollutants from the solution to the ZnO surface. This phenomenon is not only useful for increasing the adsorption efficiency in cases where ZnO is used as an adsorbent, but it also helps to increase the level of light that penetrates the polluted water, thereby increasing the photocatalytic efficiency [82]. As well as induced defects, ZnO exhibits intrinsic defects, which include zinc vacancy (VZn), oxygen vacancy (VO), zinc interstitial (Zni), oxygen interstitial (Oi), or antisite oxygen (OZn) defects [83]. As with dopants, it is generally accepted that surface defects (i.e., surface VO, VZn, Oi, and Zni, especially the anionic Vo) are beneficial to photocatalytic activity because they can serve as active sites for photocatalysis. These defects create mid-gap states that alter the electronic band structure of photocatalysts to facilitate charge separation with longer lifetimes [80]. Conversely, deep level defects (VO and VZn), and other nonradiative defects, have negative impacts on photocatalysis because they create recombination centers for photogenerated charges; this causes electronic delocalization and low photocatalytic efficiency. These defects are repaired via annealing at appropriate temperatures [84].
The most used doping agents in the biogenetic synthesis of ZnO include Ag, Cu, Mg, Ce, Fe, and Co, though Ag doping is the most common [37][85][86]. Saeed et al. synthesized AgNO3-ZnO NPs at an ambient temperature using different plant species and parts. Owing to its strong reduction potential, the extract of Calotropis gigantea resulted in the formation of rod-shaped NPs [85]. Moringa olifera seed extract yielded flower-like NPs [37], whereas Ocimum tenuiflorum seed extract produced hexagonal and spherical NPs ranging between 50 and 60 nm in diameter [86]. Mn-ZnO NPs were prepared by mixing a salt precursor with Melastoma malabathricum. The mixture was heated at 60 °C until a paste formed, and it was further heated at 400 °C to obtain spherical NPs with an average diameter of 222 nm [87]. Fe-ZnO NPs were produced by Jan et al. This was achieved by reducing zinc ions with the flavonoids, phenolics, terpenoids, triterpenes, tannins, and fatty acids present in the leaf extract of Myrtus communis [88]. The NPs were produced after 3 h of stirring and heating at 60 °C, after which they were calcinated at 400 °C for 3 h. Their diameters were small, at 17 nm on average, with a pseudo-spherical shape. Okeke et al. prepared Mg-ZnO NPs by reacting Zn(NO3)2 and Mg(NO3)2 with Piper guineense leaf extract, which was then stirred and heated for 15 min at 80 °C before calcining the paste at 350 °C for 2 h [89]. A dual doping of ZnO NPs with Cu and Mg was successfully completed by Rahman et al. [90], who utilized a Ziziphus mauritiana solution to reduce Zn(NO3)2·6H2O. The synthesized NPs were nearly spherical, but relatively large in size (0.1–1 µum), depending on the Cu/Mg dopant concentration. The FTIR suggests that the reduction of ions was controlled by tannins, flavonoids, saponins, and phenols. An eco-friendly fabrication of Nb-ZnO using Vernonia amygdalina leaf extract was reported by Nguyen et al., and the obtained Nb-ZnO NPs showed an improved photocatalytic degradation of tetracycline under natural light in comparison with pristine ZnO. The composite reached a 93.2% degradation efficiency after 3 h [91]. A summary of the synthetic procedures and the results of the doped-ZnO NPs are given in Table 2.
Table 2. Phytosynthesis of the doped ZnO nanoparticles.
| Serial No. | Plant Name | Dopant | Extraction Solvent, Temp and Time | Precursors | Biosynthesis Time (h) and Temp (°C) | Calcination Temp (°C)/Time (h) |
Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Calotropis gigantea (leaves) | Ag | Refluxing | Zn(NO3)2·6H2O AgNO3 |
3/RT | 400/3 | Rods | None | [85] |
| 2 | Moringa oleifera (seeds) | Ag | Aqueous powder, RT | Zn(OOCCH3)2 AgNO3 |
50 min/RT | - | Flower-like | 54.1 | [37] |
| 3 | Ocimum tenuiflorum (seeds) | Ag | Water, boiled | Zn(NO3)2·6H2O AgNO3 |
2/RT | 450/3 | Hexagonal and spherical | 50–60 | [86] |
| 4 | Melastoma malabathricum (leaves) | Mn | Water, 60 °C | Zn(NO3)2·6H2O Mn(CH3CO2)2·4H2O |
–/60 | 400/2 | Spherical | 222 | [87] |
| 5 | Myrtus communis L. | Fe | Water, 40 °C for 1 h | Zn(NO3)2·6H2O Fe(NO)3·9H2O |
3/60 | 400/3 | Pseudo spherical | 17 | [88] |
| 6 | Piper guineense (leaves) | Mg | Water, 60 °C for 20 min | Zn(NO3)2 Mg(NO3)2 |
0.25/80 | 350/2 | Spherical | 364 | [89] |
| 7 | Ziziphus mauritiana Lam. (leaves) | Mg and Cu | Water, RT for 1 h | Zn(NO3)2·6H2O Cu(NO3)2·3H2O Mg(NO3)2 |
–/60 | 400/2 | Hexagonal disc shaped | 100–1000 | [90] |
| 8 | Vernonia amygdalina | Nb | Not available | Not mentioned | 1/60 | 400/1 | Spherical | 1000 | [91] |
2.2. Microbial Synthesis of ZnO Nanoparticles
Microorganisms, or microbes comprising bacteria, fungus, and algae, have also been exploited for the biological synthesis of metal oxide NPs. Although many metal oxides have been synthesized biologically using microorganisms, the microbial production of ZnO has not been thoroughly investigated. An advantage of the microbial synthesis of NPs over phytosynthesis is the ease with which microorganisms can be reproduced, as they are easily grown in a laboratory. During microbial synthesis, metal reduction is made possible with the available enzymes, carbohydrates, proteins, and other metabolites that are intrinsically present in the microorganisms. The biomolecules that are secreted in the growth medium influence the shape, size, and dispersity of the produced NPs [92]. Under stressful conditions, or in the presence of heavy metals, microbes reduce these ions to metals in order to enable their own survival; thus, they act as natural nano-factories [93].
2.2.1. Bacterial Synthesis
Prokaryotic microorganisms, such as bacteria and algae, have been extensively studied because they are easy to handle and genetically modify [94]. Bacteria offer the advantage of being able to quickly multiply, thus creating a readily available source of secondary metabolites that are involved in the fabrication of ZnO. The most used microbes in the synthesis of NPs comprise Actinobacter sp., Corynebacterium sp., Klebsiella pneumonia, Lactobacillus sp., and Pseudomonas sp. [95]. The biological preparation of metal, and metal oxide nanomaterials, using bacterial cultures may occur in the intra- or extracellular environment [96]. The mechanism of formation during intracellular synthesis is challenging due to the intricacy of the bacterial cell make-up and its metabolic processes. The mechanism for the intracellular generation of ZnO in microbes is illustrated in Figure 2a. The cell walls of microbes have different types of polysaccharides and proteins that provide active sites for the binding of metal oxides through electrostatic interactions [97]. Then, reductases (NADH and NADPH) that are either present in the cell wall or secreted as soluble enzymes [98] reduce the trapped ions into elemental atoms [99]. Finally, the nuclei develop and assemble in the cytoplasm or cell wall, while the peptides and amino acids stabilize the NPs [100]. Bacteria-mediated nanoparticle synthesis creates less hazardous metal oxide nanoparticles, such as TiO2, CuO, and ZnO; however, the downside to this is that it involves isolating, screening, and culturing potential microbes, which are time consuming processes. In addition, the process may involve the use of expensive chemicals, such as growth media [99]. The size distribution, shape, and crystallinity of microbe-synthesized NPs are not easy to monitor [101]. Moreover, the intracellularly formed nanomaterials can only be released by lysing the bacterial cells; thus, time consuming and laborious steps are unavoidable [102]. As a result, research on bacterial synthesis has, to date, focused on extracellular synthesis. Regarding extracellular synthesis, the enzymes that are released by microorganisms into the growth culture are responsible for the bio-reduction of metal ions into NPs. The bio-reduction of zinc ions occurs when an electron is transferred from NADH with NADH reductase [103]. The extracellular synthesis pathways are depicted diagrammatically in Figure 2b.
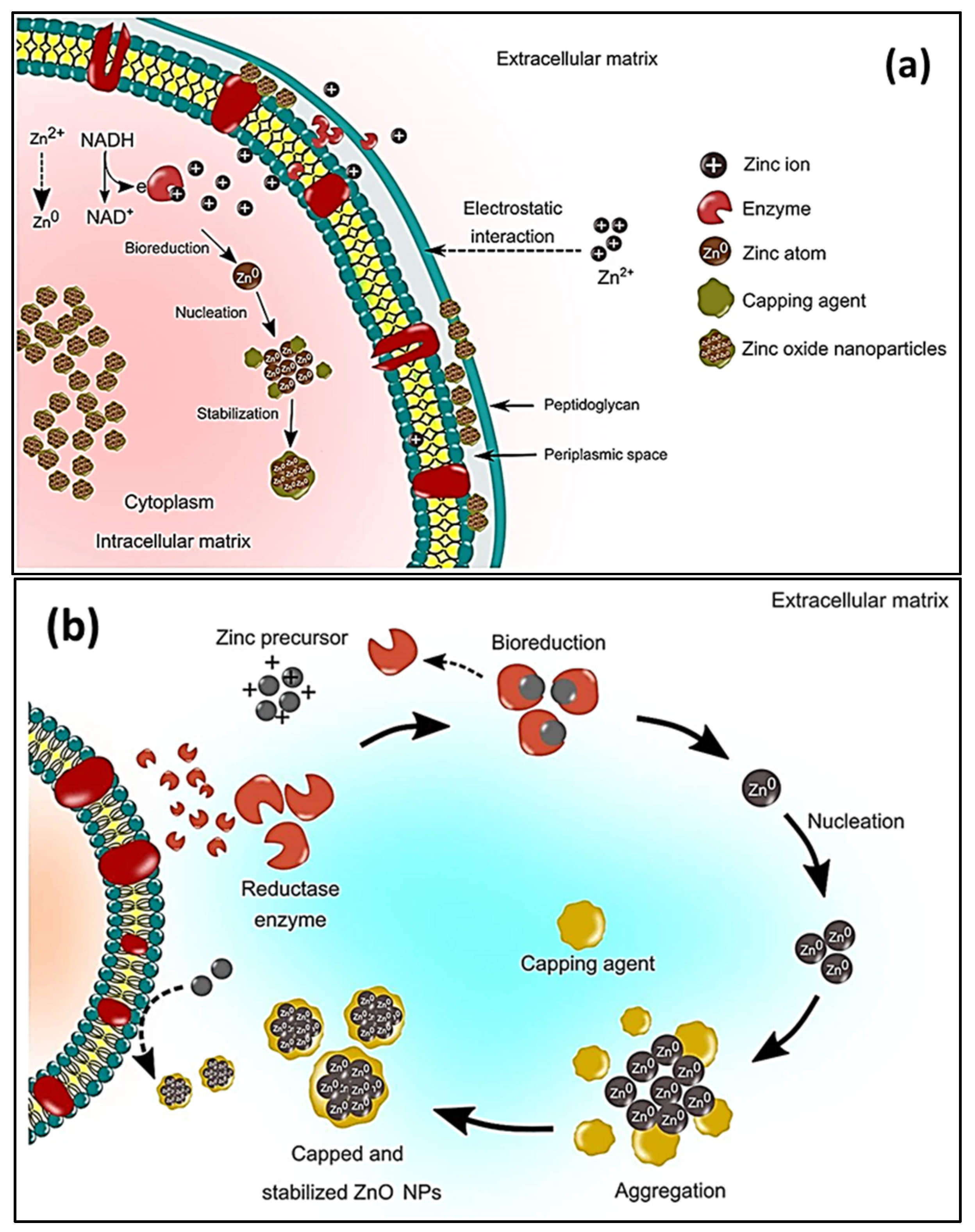
Figure 2. Schematic representation of the (a) microbial intracellular synthesis and (b) microbial extracellular synthesis of ZnO NPs [99].
Table 3 presents the current research on ZnO NP microbial synthesis. A variety of bacterial species are used in the green synthesis of ZnO NPs, including Cyanobacterium Nostoc sp. [29], Aeromonas hydrophila [38], Serratia nematodiphila [84], Bacillus subtillis [104], Bacillus haynesii [105], and Alkalibacillus sp. [106], to name but a few. Jayabalan et al. [107] reduced Zn (NO3)2 salt by incubating a mixture of the salt with a Pseudomonas putida cell culture solution at 37 °C for 24 h before calcining the resultant paste at 400 °C for 2 h. The resultant NPs were spherical agglomerates with diameters ranging between 25 and 45 nm. A green Taguchi method was employed to obtain optimum conditions in the extracellular bacterial-based production of ZnO NPs, using a Halomonas elongate extract, in order to reduce the presence of ZnCl2 salt. Spherical NPs, with an average diameter of 18 nm, were obtained [108]. To produce ZnO NPs, a biomass of zinc-tolerant Lactobacillus plantarum TA4 was used as a nanofactory. The presence of protein, carboxyl, and hydroxyl on the surfaces of the resulting ZnO NPs was verified using FTIR analysis. Furthermore, TEM analysis of the generated NPs revealed a flower-like morphology for ZnO NP (supernatant) samples, and an irregular shape for ZnO NP (cell biomass) samples, with particle sizes of 291.1 and 191.8 nm, respectively. The presence of protein, carboxyl, and hydroxyl on the surfaces of the resulting ZnO NPs were verified using FTIR analysis. Furthermore, TEM analysis of the generated NPs revealed a flower-like morphology for ZnO NP (supernatant) samples, and an irregular shape for ZnO NP (cell biomass) samples, with particle sizes of 291.1 and 191.8 nm, respectively [109]. The presence of protein, carboxyl, and hydroxyl on the surfaces of the resulting ZnO NPs was verified using FTIR analysis. In addition, TEM analysis of the generated NPs revealed a flower-like morphology for ZnO NP (supernatant) samples and an irregular shape for ZnO NP (cell biomass) samples, with particle sizes of 291.1 nm and 191.8 nm, respectively [109]. The biosynthesis of ZnO NPs was carried out using Lactococcus lactis NCDO1281(T) and Bacillus sp. PTCC 1538. A pure hexagonal crystalline structure of the particles was confirmed using XRD spectra. The SEM showed that ZnO NPs produced by Bacillus sp. were nanorods with an average size of 99 nm, whereas L. lactis produced NPs that were spherical, with diameter sizes ranging between 55 and 60.5 nm. The presence of amino and carbonyl groups in biosynthesized ZnO was confirmed using FTIR, and it was most likely related to residues of cell membrane proteins involved in NP biosynthesis [30].
Table 3. Microbial synthesis of ZnO nanoparticles.
| Serial No. | Microbe Name | Extraction Method | Salt | Biosynthesis Time (h)/Temp (°C) |
Calcination Temp (°C) Time (h) |
Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||
| 1 | Cyanobacterium Nostoc sp. | Water, 60 °C for 15 min | Zn(CH3COO)2·2H2O | 2/RT | − | Star-shaped | 60 | [29] |
| 2 | Aeromonas hydrophila | Cell culture grew for 24 h and was diluted with water | ZnO | 24/30 | − | [38] | ||
| 3 | Serratia nematodiphila. | Inoculated in Luria-Bertani (LB) broth | ZnSO4 | 0.17/80 Then stored for 24 h |
− | Near spherical | 15–30 | [84] |
| 4 | Bacillus subtillis | Cell culture in ammonium carbonate | Zn(CH3COO)2 | 3/80 | 250/3 | Spheres with hair-like appendages | 10–15 and 1.2–1.3 um aggregates |
[104] |
| 5 | Bacillus haynesii | Cell culture grown on agar | ZnSO4 | 24/55 | 700/5 | Spherical | 20–200 | [105] |
| 6 | Alkalibacillus sp. | Cell-free supernatant collected by centrifugation | ZnSO4·7H2O | 48/35 | − | Quasi-spherical | 1–30 | [106] |
| 7 | Pseudomonas putida | Cell culture solution | Zn (NO3)2 | 24/37 | 400/2 | Spherical agglomerates | 25–45 | [107] |
| 8 | Halomonas elongata | Centrifugation | ZnCl2 | Taguchi | − | Multiform shapes | 18.11 | [108] |
| 9 | Lactobacillus plantarum | Cell biomass collected by centrifugation | Zn(NO3)2·6H2O, | 24/37 | − | Flower-like | 152.8–613.5 | [109] |
| 10 | Streptomyces spp. | Broth dilution then centrifugation | ZnSO4 | 0.25/40 | 400/8 | Needle-like | 12–35 | [110] |
| Fungi | ||||||||
| 11 | Aspergillus aeneus | Cell-free filtrate | Zn (NO3)2 | 48/28 | − | Spherical | 100–140 | [39] |
| 12 | Aspergillus fumigatus | Plating the inoculum on Martin Rose-Bengal agar medium | Zn (NO3)2 | 72/28 | − | Oblate spherical | 1.2–6.8 | [111] |
| 13 | Aspergillus fumigatus | Cell biomass | ZnSO4 | 72/32 | − | Spherical | 60–80 | [112] |
| 14 | Aspergillus niger. | Culture filtrate | Zn(NO3)2 | 48/32 | − | Near spherical | 53–69 | [113] |
| 15 | Agaricus bisporus | Purchased | Zn(NO3)2·6H2O | 2/90 | 400/4 | Spheroids | 32 | [114] |
| 16 | Xylaria acuta | Cultured then centrifuged | Zn(NO3)2·6H2O | –/400 | 700/2 | Hexagonal | 34–55 | [115] |
| 17 | Pichia kudriavzevii | Mycelia separated from the culture | Zn(CH3COO)2·2H2O | 36/35 | − | Hexagonal | 10–61 (TEM, XRD) | [116] |
| 18 | Cochliobolus geniculatus | Mycelial-free filtrate | Zn(CH3COO)2 | 72/28 | − | Quasi-spherical | 2–6 (TEM) | [117] |
| 19 | Saccharomyces cerevisiae | Cell-free filtrate | Zn(CH3COO)2·2H2O | 24/30 | − | Spherical | 20–30 | [118] |
| 20 | Cladosporium tenuissimum FCBGr | Extracellular culture | Zn(NO3)2 | 36/RT | − | Bouquet-like | 57 | [119] |
| Algae | ||||||||
| 21 | Chlorella | Soaking method | Zn(CH3COO)2·2H2O | 1/58 | − | Hexagonal | 20 | [120] |
| 22 | Arthrospira platensis | Biomass mixed with supernatant | Zn(CH3COO)2·2H2O | 24/30 | − | Spherical | 30–55 | [121] |
| 23 | Padina gymnospora | Millipore water stirred for 2 h at 100 °C | Zn(NO3)2 AgNO3 |
2/60 | − | Spherical | 20–40 | [122] |
| 24 | Sargassum species | Ball-milled | Zn (NO3)2·6H2O Co(NO3)2·6H2O |
2/RT | − | Nearly spherical | 5.4–6.8 | [123] |
2.2.2. Fungal Synthesis
Fungi can release higher concentrations of secondary metabolites than bacteria. In addition, they exhibit a higher tolerance to metal concentrations, stronger binding capabilities, and better metal bioaccumulation than bacteria [124]. It is therefore possible that fungi have more potential than bacteria with regard to the upscaling of green-produced NPs. Moreover, fungal cells are more tolerant to intermediate products during the synthesis process; hence, they are more suitable for large-scale synthesis [125]. The mechanisms for generating metal and metal oxide NPs using fungal biomass or cultures are similar to the one discussed for green synthesis using bacteria. Different Aspergillus species have already been employed in the green synthesis of ZnO NPs, such as Aspergillus aeneus [39], Aspergillus fumigatus [111], Aspergillus fumigatus [112], and Aspergillus niger [113], as shown in Table 3. The ability to reduce and cap the NPs is conferred by the alcohols, phenols, and the aromatic and primary amines that are present in the fungal extracts of the various Aspergillus species [112]. During extracellular synthesis, fungi secrete enzymes that produce pure and monodispersed NPs. These particles are free from the cellular components that are associated with organisms [111]. The biomass of A. fumigatus reduced ZnSO4 and chelated the formed ZnO NPs after 72 h at 32 °C. The formed NPs were spherical with diameters ranging between 60 and 80 nm. The attained ZnO exhibited antimicrobial activities against Klebsiella pneumonia, P. aeruginosa, E. coli, S. aureus, and B. subtilis, respectively, as indicated in Figure 3. The synthesis of ZnO NPs, using the aqueous extract of Agaricus bisporus, was carried out in [114]. According to the FTIR results, the phenol groups contained the prominent ingredients for reducing and capping ZnO NPs. The zeta potential (−20.5 mV) validated the NPs’ stability, whereas the SEM and TEM revealed that the NPs were spheroids. ZNO NPs were produced with Zn(NO3)2·6H2O and Xylaria acuta fungal filtrate using the combustion method at 400 °C. The resultant sample was then calcined for 2 h at 700 °C to obtain hexagonal shaped NPs with diameters ranging between 34 and 55 nm [115].
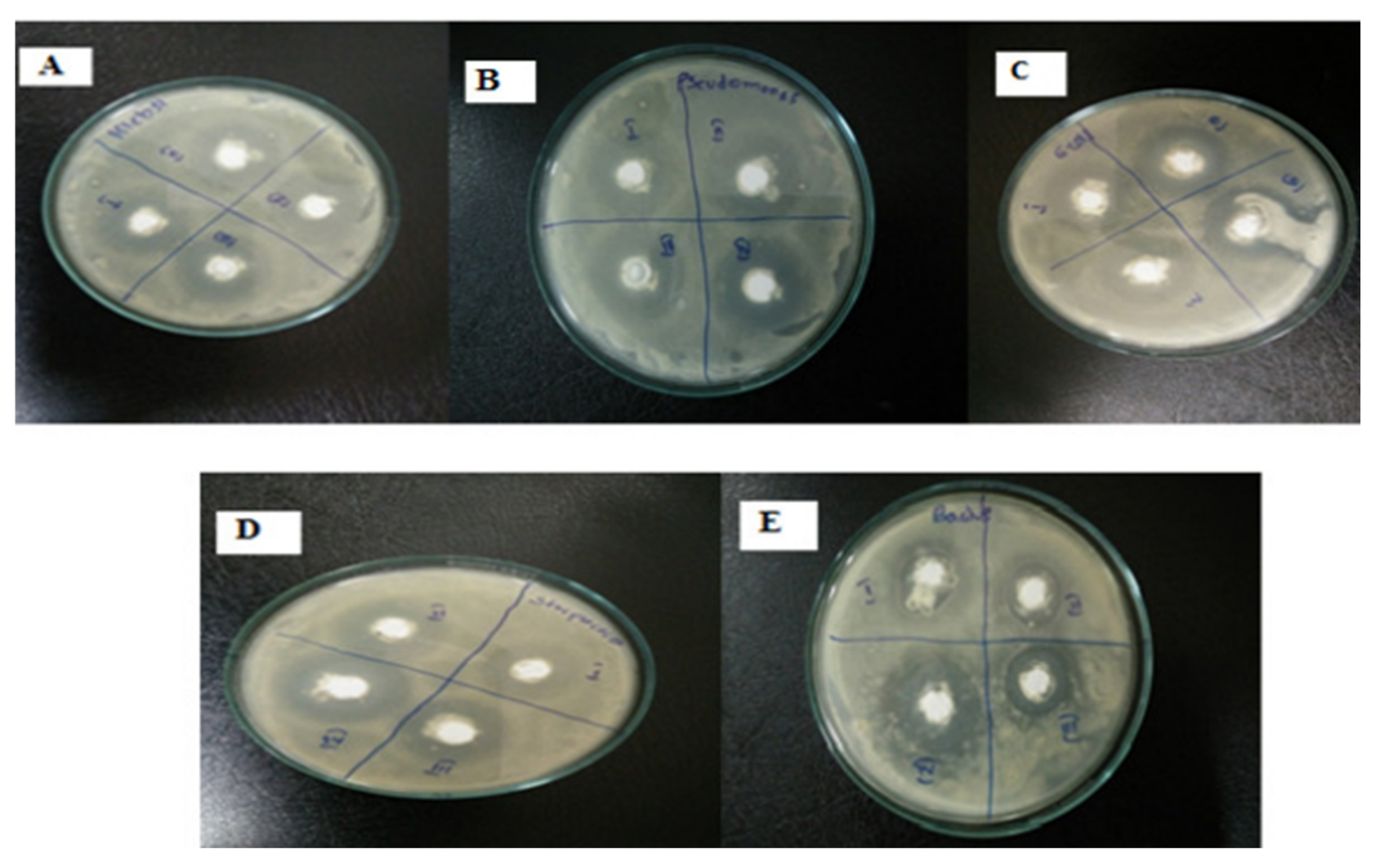
Figure 3. Antimicrobial activity of Aspergillus fumigatus synthesized ZnO NPs [112] working against (A) Klebsiella pneumonia, (B) P. aeruginosa, (C) E. coli, (D) S. aureus, and (E) B. subtilis at different concentrations.
Moghaddam et al. [116] utilized Pichia kudriavzevii in the mycogenic synthesis of ZnO. The reaction proceeded for 36 h at 35 °C, the resultant NPs were hexagonal, and they exhibited diameters that ranged between 10 and 61 nm. Small, quasi-spherical particles (2–6 nm) were prepared in [117] using Cochliobolus geniculatus. Fluorescence spectroscopy confirmed the presence of proteins on the prepared ZnO in the form of tyrosine moieties, as per the emission peak at 350 nm. The FTIR absorption peaks confirmed the role of aromatic, aliphatic amines (amide I and amide II) in the fungal extract, and potentially in the stabilization of the ZnO NPs as well. Motazedi et al. [118] prepared 20 nm to 30 nm spherical ZnO NPs using a cell-free extract of Saccharomyces cerevisiae. The FTIR results suggested that the presence of alcohols, proteins, the phenolic group, fatty acids, and carbohydrates in the extracellular excretions of S. cerevisiae were potentially responsible for reducing the zinc ions so that ZnO NPs could be obtained. Mani et al. [119] prepared ZnO NPs at a pH of 7.2 using an endophytic fungus, Cladosporium tenuissimum FCBGr, which was isolated from a Vilva tree; bouquet-like NPs were obtained after 36 h of continuous stirring at RT.
2.2.3. Algal Synthesis
Algae are mono and multicellular aquatic photosynthesizing organisms, but unlike plants, algae are without roots and leaves [95]; however, similarly to plants, macroalgae have active metabolites such as alkaloids, peptides, polysaccharides, proteins, tannins, quinones, lipids, and glycerol [126]. These metabolites have –OH and –COOH functional groups that can chelate and stabilize ZnO NPs [126]; therefore, the chemical formation route of ZnO NPs during algal synthesis is similar to that of plants [36]. Khalafi et al. [120] propose a mechanism of ZnO NP formation using a Chlorella extract (Figure 4). Based on the assumption that chlorella contains a significant amount of carbohydrates (20%), it was presumed that carbohydrates predominantly served as Zn2+ reducing agents and as stabilizers of the prepared ZnO NPs. Moreover, the biosynthesis of green ZnO NPs was assumed to occur through a donor–acceptor mechanism that occurred between the oxygen atoms of functional moieties in chlorella and Zn2+ wherein the −OH groups on the carbohydrates transfers an electron to the electrotrophilic Zn species, thus resulting in the oxidation of the −OH group and Zn2+ reduction to Zn atoms.
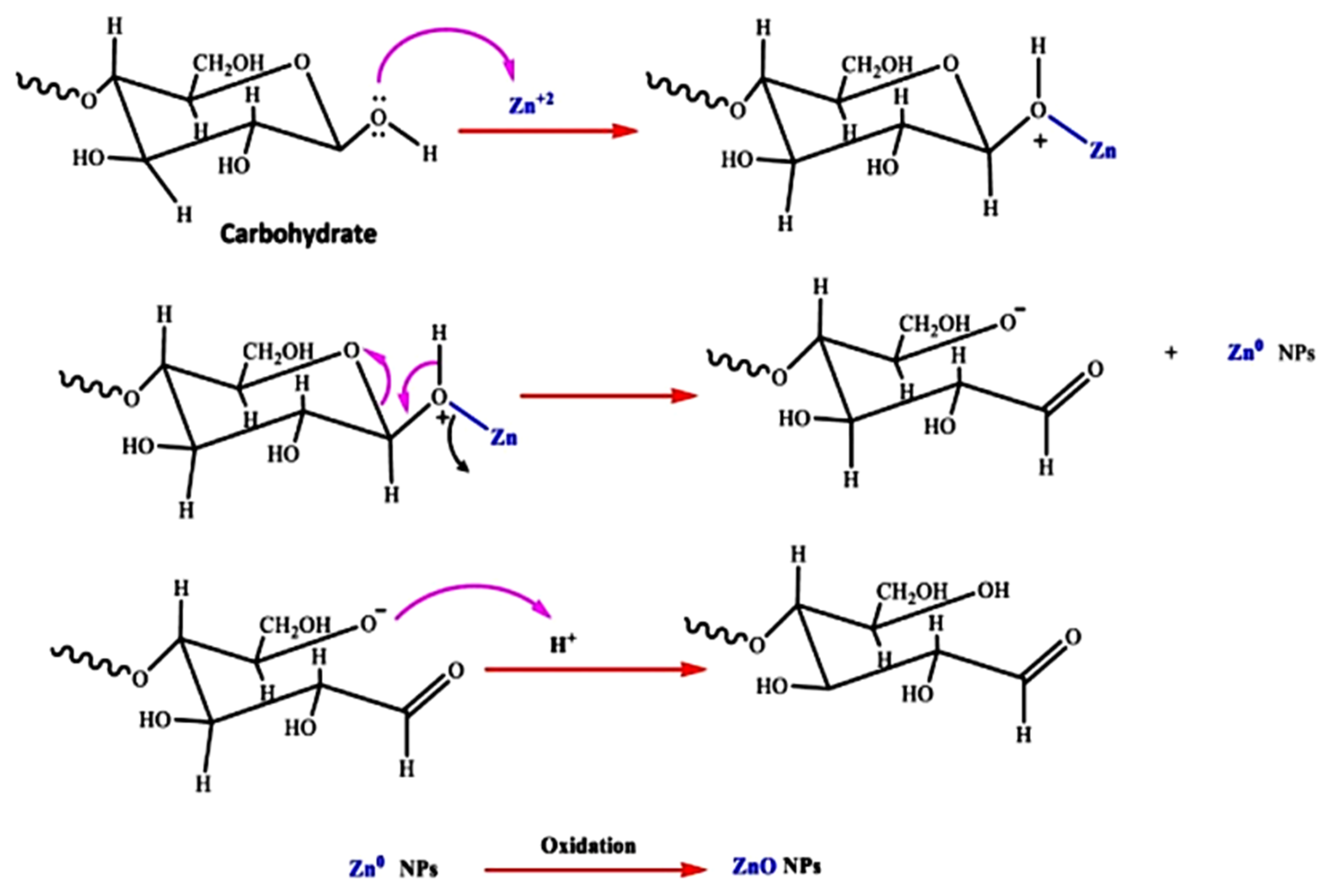
Figure 4. The probable method for the algal production of ZnO NPs, using aqueous Chlorella extract, is depicted schematically [120].
Algal species have been used to prepare ZnO NPs by reacting the extract with various zinc salts to obtain spheres, flower-like rods, and hexagonal shapes (Table 3). Recently, [121] produced 30 to 55 nm spherical NPs by reducing Zn(CH3COO)2·2H2O with Arthrospira platensis extract at 30 °C for 24 h. The emergence of Zn–O characteristics, peaking at 503 cm−1 according to the FTIR results, confirmed the presence of ZnO particles. Algal extracts that were investigated for their ability to be synthesized with doped ZnO NPs have been successfully produced (Table 3). For instance, Rajaboopathi and Thambidurai prepared Ag-ZnO NPs by mixing separate solutions of AgNO3 and Zn(NO3)2 salts with the leaf extract of Padina gymnospora. The solution was stirred at 60 °C for 30 min which led to a brown–white precipitate emerging from the light brown solution. The stretching and bending vibrations of water molecules (−OH) occurred at 3435 and 1638 cm−1, respectively, whereas the stretching and bending vibrational bands of carbonate ions appeared at 1448 and 860 cm−1, respectively. For the pristine ZnO NPs, the Zn-O band appeared at 420 cm−1, whereas for Ag-ZnO, the Zn-O stretching vibrational bands appeared at 417 cm−1 [122]. Sargassum species extract was used to reduce the Zn (NO3)2·6H2O and Co(NO3)2·6H2O salts, and to stabilize the resultant Co-ZnO NPs with the aid of microwave radiation at pH 10.5. The obtained NPs were nearly spherical (5.4–6.8 nm). The interaction between Zn2+, Co2+, and the algae was ascertained as a result of the variation in the intensity of the FTIR spectra that corresponded with the Metal–O bond [123].
2.2.4. Preparation of ZnO NPs Using Biological Derivatives Synthesis
Apart from macro- and microorganisms, several biological derivatives have been used as precursors to synthesize NPs; however, only a handful reports discuss the use of biological derivatives for the green fabrication of ZnO, as shown in Table 4. Moreover, the polysaccharide pullulan was stirred together with Zn(NO3)2·6H2O for 5 h at RT. This was followed by a 1 h heat treatment at 400 °C to yield spherical and hexagonal ZnO NPs with an average diameter of 58.13 nm [127]. El-Saied and Ibrahim, 2020, used chitosan to synthesize ZnO NPs, with average size of 55 to 70 nm [128]. Vijayakumar et al. [129] prepared egg albumen wrapped ZnO NPs, with diameters ranging between 20 and 60 nm, and with spherical and diagonal platelets. Additionally, artemia eggshells were used to hydrothermally produce ellipsoidal ZnO NPs. The prepared NPs had a 50 nm diameter on average, according to TEM [130]. Highly crystalline crustin-capped ZnO NPs were produced after the coprecipitation of the salt with an extract of crustin at RT for 2 h. The average size of the resultant NPs were 50 nm [131]. Amino acid-capped NPs were produced via a wet chemical synthesis, prior to calcining at 550 °C for 3.5 h. The produced sheet-like NPs had diameters ranging between 22.46 and 40.29 nm [132]. Smaller ZnO NPs were produced with the aid of tannic acid, as both the size influencer and capping agent were treated at 70 °C for 1.5 h without further heat treatment. The particles had a spherical morphology, with diameters ranging between 26 and 34 nm [133]. A separate study reported that honey and cow urine, respectively, yielded spherical and hexagonal leaf-like ZnO NPs [134].
Table 4. ZnO nanoparticles prepared from biological derivatives.
| Serial No. | Bio-Product | Precursors | Biosynthesis Temp (°C)/Duration |
Thermal Treatment Temp (°C)/Time (h) |
Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Alginate | Purchased ZnO | − | Irregular spheres | 20–90 | [135] | |
| 2 | Starch | Zn(NO3)2·6H2O HAuCl4·3H2O |
Stirred at 90–100 °C | Oven dried at 80 °C Calcined at 600 °C for 4 h |
Spherical | 18.9 | [136] |
| 3 | Lignin | Zn(NO3)2·6H2O | Oven at 120 °C for 4 h | Oven dried at 120 °C for 4 h | Rods | 5–10 | [137] |
| 4 | Pullulan | Zn(NO3)2·6H2O, | Stirred at RT for 5 h | Oven dried at 60 °C for 24 h Calcined at 400 °C for 1 h |
Spherical and hexagonal | 58.13 | [110] |
| 5 | Chitosan | Zn(NO3)2·6H2O | Stirred at 80 °C for 2 h | Oven dried at 80 °C for 24 h | Rods | 50–70 | [128] |
| 6 | Egg albumen | Zn(CH3COO)2 | Hydrothermal, at 85 °C for 3 h | − | Spherical and diagonal platelet | 20–60 | [129] |
| 7 | Artemia eggshell | Zn(CH3COO)2 | Hydrothermal, 180 °C for 20 h | − | Ellipsoidal | 50 | [130] |
| 8 | Crustin | Zn(CH3COO)2 | Co-precipitation, magnetically stirred for 2 h | Vacuum dried at 30 °C | 50 | [131] | |
| 9 | Amino acids | Zn(NO3)2·6H2O | Stirred at 22 °C for 30 min prior to increasing the temperature to 150 °C | Calcined at 550 °C for 3 h 20 min |
Sheet-like particles | 22.46–40.29 | [132] |
| 10 | Tannic acid | Zn powder | Stirred at 70 °C for 1 h and 30 min | − | Spherical | 26–34 | [133] |
| 11 | Honey and cow urine | Zn(NO3)2·6H2O | Combustion method, further stirred at 100 °C | − | Hexagonal, leaf-like structure (cow urine) Spherical (honey) |
27 44 |
[134] |
References
- Herrmann, S.; De Matteis, L.; de la Fuente, J.M.; Mitchell, S.G.; Streb, C. Removal of Multiple Contaminants from Water by Polyoxometalate Supported Ionic Liquid Phases (POM-SILPs). Angew. Chem.-Int. Ed. 2017, 56, 1667–1670.
- Sun, H.; Cao, L.; Lu, L. Magnetite/Reduced Graphene Oxide Nanocomposites: One Step Solvothermal Synthesis and Use as a Novel Platform for Removal of Dye Pollutants. Nano Res. 2011, 4, 550–562.
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Hai, N.D.; Che Hassan, C.R. Adsorption of Dyes Using Poly(Vinyl Alcohol) (PVA) and PVA-Based Polymer Composite Adsorbents: A Review. J. Polym. Environ. 2020, 28, 775–793.
- Dhiman, V.; Kondal, N. ZnO Nanoadsorbents: A Potent Material for Removal of Heavy Metal Ions from Wastewater. Colloids Interface Sci. Commun. 2021, 41, 100380.
- Ashbolt, N.J. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Heal. Rep. 2015, 2, 95–106.
- Roberts, K.; Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Walter, P.; Edition, F.; Roberts, K.; Alberts, B.; Johnson, A.; et al. Molecular Biology of the Cell, Fourth Edition Description, 6th ed.; W.W. Norton & Company: Boca Raton, FL, USA, 2017; ISBN 9781315735368.
- Prasad, A.R.; Williams, L.; Garvasis, J.; Shamsheera, K.O.; Basheer, S.M.; Kuruvilla, M.; Joseph, A. Applications of Phytogenic ZnO Nanoparticles: A Review on Recent Advancements. J. Mol. Liq. 2021, 331, 115805.
- Oluwafemi, O.S.; Anyik, J.L.; Zikalala, N.E.; Sakho, E.H.M. Biosynthesis of Silver Nanoparticles from Water Hyacinth Plant Leaves Extract for Colourimetric Sensing of Heavy Metals. Nano-Struct. Nano-Objects 2019, 20, 7–9.
- Singh, A.K.; Srivastava, O.N. One-Step Green Synthesis of Gold Nanoparticles Using Black Cardamom and Effect of pH on Its Synthesis. Nanoscale Res. Lett. 2015, 10, 1–12.
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508.
- Zikalala, S.A.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. The Effect of Synthetic Routes on the Physicochemical Properties and Optical Response of N-Doped Titania–Oxidized Carbon Nanotube Nanohybrids. Mater. Today Chem. 2018, 10, 1–18.
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285.
- Kuriganova, A.B.; Vlaic, C.A.; Ivanov, S.; Leontyeva, D.V.; Bund, A.; Smirnova, N.V. Electrochemical Dispersion Method for the Synthesis of SnO2 as Anode Material for Lithium Ion Batteries. J. Appl. Electrochem. 2016, 46, 527–538.
- Sobhani-Nasab, A.; Behpour, M. Synthesis and Characterization of AgO Nanostructures by Precipitation Method and Its Photocatalyst Application. J. Mater. Sci. Mater. Electron. 2016, 27, 1191–1196.
- Siddiqui, H.; Qureshi, M.S.; Haque, F.Z. Surfactant Assisted Wet Chemical Synthesis of Copper Oxide (CuO) Nanostructures and Their Spectroscopic Analysis. Optik 2016, 127, 2740–2747.
- Sahu, K.; Choudhary, S.; Singh, J.; Kuriakose, S.; Singhal, R.; Mohapatra, S. Facile Wet Chemical Synthesis of ZnO Nanosheets: Effects of Counter Ions on the Morphological, Structural, Optical and Photocatalytic Properties. Ceram. Int. 2018, 44, 23094–23101.
- Champi, A.; Marques, F.C. Structural Changes in Amorphous Carbon Nitride Films Due to Bias Voltage. Thin Solid Film. 2006, 501, 362–365.
- Aminuzzaman, M.; Ying, L.P.; Goh, W.S.; Watanabe, A. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Pericarp and Their Photocatalytic Activity. Bull. Mater. Sci. 2018, 41, 1–10.
- Janotti, A.; Van De Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 12–15.
- Xu, S.; Weintraub, B.; Wang, Z.L. Zinc Oxide Nanowire Arrays on Flexible Substrates. Wet Chemical Growth and Applications in Energy Conversion, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; ISBN 9781437778236.
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of Defects for Enhancing Their Technological Potentials. Nanotechnol. Rev. 2022, 11, 575–619.
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C. ZnO Nanoparticles Mediated by Aqueous Extracts of Dovyalis Caffra Fruits and the Photocatalytic Evaluations. Mater. Res. Express 2019, 6, 11–14.
- Narendra Kumar, H.K.; Chandra Mohana, N.; Nuthan, B.R.; Ramesha, K.P.; Rakshith, D.; Geetha, N.; Satish, S. Phyto-Mediated Synthesis of Zinc Oxide Nanoparticles Using Aqueous Plant Extract of Ocimum americanum and Evaluation of Its Bioactivity. SN Appl. Sci. 2019, 1, 1–9.
- Awwad, A.M.; Albiss, B.; Ahmad, A.L. Green Synthesis, Characterization and Optical Properties of Zinc Oxide Nanosheets Using Olea europea Leaf Extract. Adv. Mater. Lett. 2014, 5, 520–524.
- Shabaani, M.; Rahaiee, S.; Zare, M.; Jafari, S.M. Green Synthesis of ZnO Nanoparticles Using Loquat Seed Extract; Biological Functions and Photocatalytic Degradation Properties. LWT 2020, 134, 110133.
- Rajabairavi, N.; Raju, C.S.; Karthikeyan, C.; Varutharaju, K.; Nethaji, S.; Hameed, A.S.H.; Shajahan, A. Biosynthesis of Novel Zinc Oxide Nanoparticles (ZnO NPs) Using Endophytic Bacteria Sphingobacterium thalpophilum. Springer Proc. Phys. 2017, 189, 245–254.
- Wu, J.; Yin, C.; Zhou, J.; Li, H.; Liu, Y.; Shen, Y.; Garner, S.; Fu, Y.; Duan, H. Ultrathin Glass-Based Flexible, Transparent, and Ultrasensitive Surface Acoustic Wave Humidity Sensor with ZnO Nanowires and Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 39817–39825.
- Timerkaev, B.A.; Felsinger, V.S.; Kaleeva, A.A.; Erlingayte, E.A.; Uktamov, J.A.; Nuriddinov, H.S. Plasma-Chemical Synthesis of Zinc Oxide Nanotubes. J. Phys. Conf. Ser. 2021, 1870, 012003.
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Shafiei, M.; Zahiri, H.S.; Noghabi, K.A. A Bio-Inspired Strategy for the Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) Using the Cell Extract of Cyanobacterium: Nostoc Sp. EA03: From Biological Function to Toxicity Evaluation. RSC Adv. 2019, 9, 23508–23525.
- Mahdi, Z.S.; Talebnia Roshan, F.; Nikzad, M.; Ezoji, H. Biosynthesis of Zinc Oxide Nanoparticles Using Bacteria: A Study on the Characterization and Application for Electrochemical Determination of Bisphenol A. Inorg. Nano-Met. Chem. 2021, 51, 1249–1257.
- Kiani, A.; Dastafkan, K. Zinc Oxide Nanocubes as a Destructive Nanoadsorbent for the Neutralization Chemistry of 2-Chloroethyl Phenyl Sulfide: A Sulfur Mustard Simulant. J. Colloid Interface Sci. 2016, 478, 271–279.
- Jose Varghese, R.; Zikalala, N.; Sakho, E.H.M.; Oluwafemi, O.S. Green Synthesis Protocol on Metal Oxide Nanoparticles Using Plant Extracts. In Colloidal Metal Oxide Nanoparticles-Synthesis, Characterization and Applications; Thomas, S., Sani, A.T., Velayudhan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–82. ISBN 978-0-12-813357-6.
- Jessop, P.G.; Trakhtenberg, S.; Warner, J. The Twelve Principles of Green Chemistry. ACS Symp. Ser. 2009, 1000, 401–436.
- Rubab, L.; Anum, A.; Al-hussain, S.A.; Irfan, A.; Ahmad, S.; Ullah, S.; Al-mutairi, A.A.; Zaki, M.E.A. Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1, 2, 4-Thiadiazoles. Catalysts 2022, 12, 1329.
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476.
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223.
- Swati; Verma, R.; Chauhan, A.; Shandilya, M.; Li, X.; Kumar, R.; Kulshrestha, S. Antimicrobial Potential of Ag-Doped ZnO Nanostructure Synthesized by the Green Method Using Moringa oleifera Extract. J. Environ. Chem. Eng. 2020, 8, 103730.
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.V.B. Novel Microbial Route to Synthesize ZnO Nanoparticles Using Aeromonas hydrophila and Their Activity against Pathogenic Bacteria and Fungi. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84.
- Jain, N.; Bhargava, A.; Tarafdar, J.C.; Singh, S.K.; Panwar, J. A Biomimetic Approach towards Synthesis of Zinc Oxide Nanoparticles. Appl. Microbiol. Biotechnol. 2013, 97, 859–869.
- Rao, M.D.; Gautam, D.P. Nanoflowers Using Chlamydomonas reinhardtii: A Green Approach. Environ. Prog. Sustain. Energy 2016, 35, 1020–1026.
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer Gelatin-Coated Zinc Oxide Nanoparticles Showed High Antibacterial, Antibiofilm and Anti-Angiogenic Activity. J. Photochem. Photobiol. B Biol. 2018, 178, 211–218.
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis Protocols for Colloidal Metal Oxide Nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299.
- Vijayaraghavan, K.; Ashokkumar, T. Plant-Mediated Biosynthesis of Metallic Nanoparticles: A Review of Literature, Factors Affecting Synthesis, Characterization Techniques and Applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883.
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284.
- Akbar, S.; Tauseef, I.; Subhan, F.; Sultana, N.; Khan, I.; Ahmed, U.; Haleem, K.S. An Overview of the Plant-Mediated Synthesis of Zinc Oxide Nanoparticles and Their Antimicrobial Potential. Inorg. Nano-Met. Chem. 2020, 50, 257–271.
- Prasad, A.R.; Anagha, M.; Shamsheera, K.O.; Joseph, A. Bio-Fabricated ZnO Nanoparticles: Direct Sunlight-Driven Selective Photodegradation, Antibacterial Activity, and Thermoluminescence-Emission Characteristics. New J. Chem. 2020, 44, 8273–8279.
- Verma, P.R.; Khan, F.; Banerjee, S. Salvadora persica Root Extract-Mediated Fabrication of ZnO Nanoparticles and Characterization. Inorg. Nano-Met. Chem. 2020, 51, 427–433.
- Fagier, M.A. Plant-Mediated Biosynthesis and Photocatalysis Activities of Zinc Oxide Nanoparticles: A Prospect towards Dyes Mineralization. J. Nanotechnol. 2021, 2021.
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Adaikala Raj, G. Bio-Approach: Plant Mediated Synthesis of ZnO Nanoparticles and Their Catalytic Reduction of Methylene Blue and Antimicrobial Activity. Adv. Powder Technol. 2015, 26, 1639–1651.
- Gebre, S.H.; Sendeku, M.G. New Frontiers in the Biosynthesis of Metal Oxide Nanoparticles and Their Environmental Applications: An Overview. SN Appl. Sci. 2019, 1, 1–28.
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-Synthesized Nanocatalysts and Nanomaterials for Water Treatment: Current Challenges and Future Perspectives. J. Hazard. Mater. 2021, 401, 123401.
- Sharma, A.; Nagraik, R.; Sharma, S.; Sharma, G.; Pandey, S.; Azizov, S.; Chauhan, P.K.; Kumar, D. Green Synthesis of ZnO Nanoparticles Using Ficus palmata: Antioxidant, Antibacterial and Antidiabetic Studies. Results Chem. 2022, 4, 100509.
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus subdariffa Leaf Extract: Effect of Temperature on Synthesis, Anti-Bacterial Activity and Anti-Diabetic Activity. RSC Adv. 2015, 5, 4993–5003.
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, D.; Vimalan, M.; Vetha Potheher, I. Synthesis of ZnO Nanoparticles Using Leaf Extract of Tectona grandis (L.) and Their Anti-Bacterial, Anti-Arthritic, Anti-Oxidant and in Vitro Cytotoxicity Activities. New J. Chem. 2017, 41, 10347–10356.
- Bowles, D.; Lim, E.K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of Lipophilic Small Molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597.
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Phytochemical Investigation of Medicago sativa L. Extract and Its Potential as a Safe Source for the Synthesis of ZnO Nanoparticles: The Proposed Mechanism of Formation and Antimicrobial Activity. Phytochem. Lett. 2019, 31, 170–180.
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19.
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green Approach for Fabrication and Applications of Zinc Oxide Nanoparticles. Bioinorg. Chem. Appl. 2014, 2014.
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599.
- De Souza, R.F.V.; Giovani, W.F. De Synthesis, Spectral and Electrochemical Properties of Al (III) and Zn (II) Complexes with Flavonoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1985–1990.
- Mirgane, N.A.; Shivankar, V.S.; Kotwal, S.B.; Wadhawa, G.C.; Sonawale, M.C. Waste Pericarp of Ananas comosus in Green Synthesis Zinc Oxide Nanoparticles and Their Application in Waste Water Treatment. Mater. Today Proc. 2020, 37, 886–889.
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green Synthesis, Characterization and Antifungal and Photocatalytic Activity of Pithecellobium dulce Peel–Mediated ZnO Nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51.
- Mohamad Sukri, S.N.A.; Shameli, K.; Mei-Theng Wong, M.; Teow, S.Y.; Chew, J.; Ismail, N.A. Cytotoxicity and Antibacterial Activities of Plant-Mediated Synthesized Zinc Oxide (ZnO) Nanoparticles Using Punica granatum (Pomegranate) Fruit Peels Extract. J. Mol. Struct. 2019, 1189, 57–65.
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A Green Approach to Synthesis of ZnO Nanoparticles Using Jujube Fruit Extract and Their Application in Photocatalytic Degradation of Organic Dyes. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 229, 117961.
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722.
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the Effect of the Concentration of Hibiscus sabdariffa Extract on the Green Synthesis of ZnO Nanoparticles. Results Phys. 2019, 15, 102807.
- Khara, G.; Padalia, H.; Moteriya, P.; Chanda, S. Peltophorum pterocarpum Flower-Mediated Synthesis, Characterization, Antimicrobial and Cytotoxic Activities of ZnO Nanoparticles. Arab. J. Sci. Eng. 2018, 43, 3393–3401.
- Diallo, A.; Ngom, B.D.; Park, E.; Maaza, M. Green Synthesis of ZnO Nanoparticles by Aspalathus linearis: Structural & Optical Properties. J. Alloys Compd. 2015, 646, 425–430.
- Chauhan, A.K.; Kataria, N.; Garg, V.K. Green Fabrication of ZnO Nanoparticles Using Eucalyptus Spp. Leaves Extract and Their Application in Wastewater Remediation. Chemosphere 2020, 247, 125803.
- Nilavukkarasi, M.; Vijayakumar, S.; Prathipkumar, S. Capparis Zeylanica Mediated Bio-Synthesized ZnO Nanoparticles as Antimicrobial, Photocatalytic and Anti-Cancer Applications. Mater. Sci. Energy Technol. 2020, 3, 335–343.
- Bala Chennaiah, M.; Kumar, K.D.; Kumar, B.S.; Tanneeru, S.R. Characterisation of Zinc Oxide Nanoparticles–Herbal Synthesised Coated with Ocimum tenuiflorum. Adv. Mater. Process. Technol. 2021, 1–12.
- Ekennia, A.C.; Uduagwu, D.N.; Nwaji, N.N.; Oje, O.O.; Emma-Uba, C.O.; Mgbii, S.I.; Olowo, O.J.; Nwanji, O.L. Green Synthesis of Biogenic Zinc Oxide Nanoflower as Dual Agent for Photodegradation of an Organic Dye and Tyrosinase Inhibitor. J. Inorg. Organomet. Polym. Mater. 2021, 31, 886–897.
- Tabrizi Hafez Moghaddas, S.M.; Elahi, B.; Javanbakht, V. Biosynthesis of Pure Zinc Oxide Nanoparticles Using Quince Seed Mucilage for Photocatalytic Dye Degradation. J. Alloys Compd. 2020, 821, 153519.
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Bio-Inspired Green Synthesis of Zinc Oxide Nanoparticles Using Abelmoschus esculentus Mucilage and Selective Degradation of Cationic Dye Pollutants. J. Phys. Chem. Solids 2019, 127, 265–274.
- Kaliraj, L.; Ahn, J.C.; Rupa, E.J.; Abid, S.; Lu, J.; Yang, D.C. Synthesis of Panos Extract Mediated ZnO Nano-Flowers as Photocatalyst for Industrial Dye Degradation by UV Illumination. J. Photochem. Photobiol. B Biol. 2019, 199, 111588.
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO Nanoparticles via Moringa Oleifera Green Synthesis: Physical Properties & Mechanism of Formation. Appl. Surf. Sci. 2017, 406, 339–347.
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma betulina Natural Extract. Mater. Lett. 2015, 161, 124–127.
- Ngom, I.; Ndiayea, N.M.; Bakayoko, A.F.M.; Ngom, B.D.; Maaza, M. On the Use of Moringa oleifera Leaves Extract for the Biosynthesis of NiO and ZnO Nanoparticles. MRS Adv. 2020, 5, 1145–1155.
- Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of Zinc Oxide Nanoparticles Using Rubus Fairholmianus Root Extract and Their Activity against Pathogenic Bacteria. Molecules 2021, 26, 3029.
- Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Recent Development in Defects Engineered Photocatalysts: An Overview of the Experimental and Theoretical Strategies. Energy Environ. Mater. 2022, 5, 68–114.
- Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A.; Kejzlar, P. Photocatalytic Behaviour of Zinc Oxide Nanostructures on Surface Activation of Polymeric Fibres. Polymers 2021, 13, 1227.
- Shelar, S.G.; Mahajan, V.K.; Patil, S.P.; Sonawane, G.H. Effect of Doping Parameters on Photocatalytic Degradation of Methylene Blue Using Ag Doped ZnO Nanocatalyst. SN Appl. Sci. 2020, 2, 1–10.
- Srivastava, A.K.; Gupta, V.K.; Lal, B.; Roy, S.; Yadav, S.K.; Gurjar, M.S.; Bag, T.K.; Pandey, N.K.; Singh, B.P. Assessment of the Level of Knowledge and Training Needs of Potato Growing Tribal Farmers of Meghalaya. Int. J. Agric. Environ. Biotechnol. 2012, 5, 483–487.
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial Fabrication of Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Photocatalytic Properties. Front. Chem. 2020, 8, 778.
- Saeed, M.; Siddique, M.; Ibrahim, M.; Akram, N.; Usman, M.; Aleem, M.A.; Baig, A. Calotropis Gigantea Leaves Assisted Biosynthesis of ZnO and Catalysts for Degradation of Rhodamine B Dye in Aqueous Medium. Environ. Prog. Sustain. Energy 2020, 39, e13408.
- Panchal, P.; Paul, D.R.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S.P. Biogenic Mediated Ag/ZnO Nanocomposites for Photocatalytic and Antibacterial Activities towards Disinfection of Water. J. Colloid Interface Sci. 2020, 563, 370–380.
- Khan, M.M.; Harunsani, M.H.; Tan, A.L.; Hojamberdiev, M.; Azamay, S.; Ahmad, N. Antibacterial Activities of Zinc Oxide and Mn-Doped Zinc Oxide Synthesized Using Melastoma malabathricum (L.) Leaf Extract. Bioprocess Biosyst. Eng. 2020, 43, 1499–1508.
- Akbar Jan, F.; Wajidullah; Ullah, R.; Ullah, N.; Salman; Usman, M. Exploring the Environmental and Potential Therapeutic Applications of Myrtus communis L. Assisted Synthesized Zinc Oxide (ZnO) and Iron Doped Zinc Oxide (Fe-ZnO) Nanoparticles. J. Saudi Chem. Soc. 2021, 25, 101278.
- Okeke, I.S.; Agwu, K.K.; Ubachukwu, A.A.; Madiba, I.G.; Maaza, M.; Whyte, G.M.; Ezema, F.I. Impact of Particle Size and Surface Defects on Antibacterial and Photocatalytic Activities of Undoped and Mg-Doped ZnO Nanoparticles, Biosynthesized Using One-Step Simple Process. Vacuum 2021, 187, 110110.
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Ahmad, N.; Min, B.K.; Khan, M.M. Influence of Mg and Cu Dual-Doping on Phytogenic Synthesized ZnO for Light Induced Antibacterial and Radical Scavenging Activities. Mater. Sci. Semicond. Process. 2021, 128, 105761.
- Nguyen, T.H.A.; Le, V.T.; Doan, V.D.; Tran, A.V.; Nguyen, V.C.; Nguyen, A.T.; Vasseghian, Y. Green Synthesis of Nb-Doped ZnO Nanocomposite for Photocatalytic Degradation of Tetracycline Antibiotic under Visible Light. Mater. Lett. 2022, 308, 131129.
- Kitching, M.; Ramani, M.; Marsili, E. Fungal Biosynthesis of Gold Nanoparticles: Mechanism and Scale Up. Microb. Biotechnol. 2015, 8, 904–917.
- Salvadori, M.R.; Ando, R.A.; Oller Do Nascimento, C.A.; Corrêa, B. Intracellular Biosynthesis and Removal of Copper Nanoparticles by Dead Biomass of Yeast Isolated from the Wastewater of a Mine in the Brazilian Amazonia. PLoS ONE 2014, 9, e87968.
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262.
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-Inspired Green Nanoparticles: Synthesis, Mechanism, and Antibacterial Application. Toxicol. Res. 2016, 32, 95–102.
- Tripathi, R.M.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B.R. ZnO Nanoflowers: Novel Biogenic Synthesis and Enhanced Photocatalytic Activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295.
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 1–20.
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent Trends and Methodologies in Gold Nanoparticle Synthesis – A Prospective Review on Drug Delivery Aspect. OpenNano 2017, 2, 37–46.
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 1–22.
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial Synthesis of Gold Nanoparticles: Current Status and Future Prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48.
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67.
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szucs, R.; et al. Green Synthesis of Gold Nanoparticles by Thermophilic Filamentous Fungi. Sci. Rep. 2018, 8, 1–12.
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of Nanoparticles Using Microbes-A Review. Colloids Surf. B Biointerfaces 2014, 121, 474–483.
- Dhandapani, P.; Prakash, A.A.; AlSalhi, M.S.; Maruthamuthu, S.; Devanesan, S.; Rajasekar, A. Ureolytic Bacteria Mediated Synthesis of Hairy ZnO Nanostructure as Photocatalyst for Decolorization of Dyes. Mater. Chem. Phys. 2020, 243, 122619.
- Rehman, S.; Jermy, B.R.; Akhtar, S.; Borgio, J.F.; Abdul Azeez, S.; Ravinayagam, V.; Al Jindan, R.; Alsalem, Z.H.; Buhameid, A.; Gani, A. Isolation and Characterization of a Novel Thermophile; Bacillus haynesii, Applied for the Green Synthesis of ZnO Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2072–2082.
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical Optimization of Experimental Parameters for Extracellular Synthesis of Zinc Oxide Nanoparticles by a Novel Haloalaliphilic alkalibacillus sp. W7. Sci. Rep. 2021, 11, 1–14.
- Jayabalan, J.; Mani, G.; Krishnan, N.; Pernabas, J.; Devadoss, J.M.; Jang, H.T. Green Biogenic Synthesis of Zinc Oxide Nanoparticles Using Pseudomonas putida Culture and Its In Vitro Antibacterial and Anti-Biofilm Activity. Biocatal. Agric. Biotechnol. 2019, 21, 101327.
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO Nanoparticles by Halomonas Elongata IBRC-M 10214 in Different Conditions of Medium. BioImpacts 2018, 8, 81–89.
- Mohd Yusof, H.; Abdul Rahman, N.A.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of Zinc Oxide Nanoparticles by Cell-Biomass and Supernatant of Lactobacillus plantarum TA4 and Its Antibacterial and Biocompatibility Properties. Sci. Rep. 2020, 10, 1–13.
- Rajivgandhi, G.; Mythili Gnanamangai, B.; Heela Prabha, T.; Poornima, S.; Maruthupandy, M.; Alharbi, N.S.; Kadaikunnan, S.; Li, W.-J. Biosynthesized Zinc Oxide Nanoparticles (ZnO NPs) Using Actinomycetes Enhance the Anti-Bacterial Efficacy against K. pneumoniae. J. King Saud Univ.-Sci. 2022, 34, 101731.
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57.
- Rajan, A.; Cherian, E.; Baskar, G. Biosynthesis of Zinc Oxide Nanoparticles Using Aspergillus fumigatus JCF and Its Antibacterial Activity. Int. J. Mod. Sci. Technol. 2016, 1, 52–57.
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Devi Rajeswari, V. Biosynthesis of Zinc Oxide Nanoparticles Using Culture Filtrates of Aspergillus niger: Antimicrobial Textiles and Dye Degradation Studies. OpenNano 2018, 3, 48–55.
- Mohana, S.; Sumathi, S. Synthesis of Zinc Oxide Using Agaricus Bisporus and Its In-Vitro Biological Activities. J. Environ. Chem. Eng. 2020, 8, 104192.
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536.
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872.
- Kadam, V.V.; Ettiyappan, J.P.; Mohan Balakrishnan, R. Mechanistic Insight into the Endophytic Fungus Mediated Synthesis of Protein Capped ZnO Nanoparticles. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 243, 214–221.
- Motazedi, R.; Rahaiee, S.; Zare, M. Efficient Biogenesis of ZnO Nanoparticles Using Extracellular Extract of Saccharomyces cerevisiae: Evaluation of Photocatalytic, Cytotoxic and Other Biological Activities. Bioorg. Chem. 2020, 101, 103998.
- Mani, V.M.; Nivetha, S.; Sabarathinam, S.; Barath, S.; Das, M.P.A.; Basha, S.; Elfasakhany, A.; Pugazhendhi, A. Multifunctionalities of Mycosynthesized Zinc Oxide Nanoparticles (ZnONPs) from Cladosporium tenuissimum FCBGr: Antimicrobial Additives for Paints Coating, Functionalized Fabrics and Biomedical Properties. Prog. Org. Coat. 2022, 163, 106650.
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 1–10.
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (Zno-Nps) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of Their Biomedical Activities. Nanomaterials 2021, 11, 95.
- Rajaboopathi, S.; Thambidurai, S. Enhanced Photocatalytic Activity of Ag-ZnO Nanoparticles Synthesized by Using Padina gymnospora Seaweed Extract. J. Mol. Liq. 2018, 262, 148–160.
- Rabie, A.M.; Abukhadra, M.R.; Rady, A.M.; Ahmed, S.A.; Labena, A.; Mohamed, H.S.H.; Betiha, M.A.; Shim, J.J. Instantaneous Photocatalytic Degradation of Malachite Green Dye under Visible Light Using Novel Green Co–ZnO/Algae Composites. Res. Chem. Intermed. 2020, 46, 1955–1973.
- Syed, A.; Ahmad, A. Extracellular Biosynthesis of Platinum Nanoparticles Using the Fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2012, 97, 27–31.
- Zielonka, A.; Klimek-Ochab, M. Fungal Synthesis of Size-Defined Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 043001.
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Brown Marine Macroalga Sargassum muticum Aqueous Extract. Mater. Lett. 2014, 116, 275–277.
- Mohamed Isa, E.D.; Shameli, K.; Ch’ng, H.J.; Che Jusoh, N.W.; Hazan, R. Photocatalytic Degradation of Selected Pharmaceuticals Using Green Fabricated Zinc Oxide Nanoparticles. Adv. Powder Technol. 2021, 32, 2398–2409.
- El-Saied, H.A.A.; Ibrahim, A.M. Effective Fabrication and Characterization of Eco-Friendly Nano Chitosan Capped Zinc Oxide Nanoparticles for Effective Marine Fouling Inhibition. J. Environ. Chem. Eng. 2020, 8, 103949.
- Vijayakumar, T.S.; Mahboob, S.; Bupesh, G.; Vasanth, S.; Al-Ghanim, K.A.; Al-Misned, F.; Govindarajan, M. Facile Synthesis and Biophysical Characterization of Egg Albumen-Wrapped Zinc Oxide Nanoparticles: A Potential Drug Delivery Vehicles for Anticancer Therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 102015.
- Qian, C.; Yin, J.; Zhao, J.; Li, X.; Wang, S.; Bai, Z.; Jiao, T. Facile Preparation and Highly Efficient Photodegradation Performances of Self-Assembled Artemia Eggshell-ZnO Nanocomposites for Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125752.
- Rekha, R.; Mahboob, S.; Ramya, A.K.; Kerthekeyan, S.; Govindarajan, M.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Vaseeharan, B. Synthesis and Bio-Physical Characterization of Crustin Capped Zinc Oxide Nanoparticles, and Their Photocatalytic, Antibacterial, Antifungal and Antibiofilm Activity. J. Clust. Sci. 2021, 32, 843–855.
- Almehizia, A.A.; Al-Omar, M.A.; Naglah, A.M.; Bhat, M.A.; Al-Shakliah, N.S. Facile Synthesis and Characterization of ZnO Nanoparticles for Studying Their Biological Activities and Photocatalytic Degradation Properties toward Methylene Blue Dye. Alex. Eng. J. 2021, 61, 2386–2395.
- Kamaruzaman, A.; Lah, N.A.C. Formation of ZnO Nanoparticles in the Presence of Tannic Acid. Mater. Today Proc. 2020, 41, 61–64.
- Ranjithkumar, B.; Ramalingam, H.B.; Kumar, E.R.; Srinivas, C.; Magesh, G.; Rahale, C.S.; El-Metwaly, N.M.; Shekar, B.C. Natural Fuels (Honey and Cow Urine) Assisted Combustion Synthesis of Zinc Oxide Nanoparticles for Antimicrobial Activities. Ceram. Int. 2021, 47, 14475–14481.
- Motshekga, S.C.; Sinha Ray, S.; Maity, A. Synthesis and Characterization of Alginate Beads Encapsulated Zinc Oxide Nanoparticles for Bacteria Disinfection in Water. J. Colloid Interface Sci. 2018, 512, 686–692.
- Peychev, B.; Vasileva, P. Novel Starch-Mediated Synthesis of Au/ZnO Nanocrystals and Their Photocatalytic Properties. Heliyon 2021, 7, e07402.
- Wang, Y.; Wang, H.; Li, Z.; Yang, D.; Qiu, X.; Liu, Y.; Yan, M.; Li, Q. Fabrication of Litchi-like Lignin/Zinc Oxide Composites with Enhanced Antibacterial Activity and Their Application in Polyurethane Films. J. Colloid Interface Sci. 2021, 594, 316–325.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
13 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




