Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nkosingiphile E Zikalala and Version 2 by Sirius Huang.
Zinc oxide nanoparticles (ZnO NPs) can be used effectively and efficiently for water treatment, along with other nanotechnologies. Owing to rising concerns regarding the environmental unfriendliness and toxicity of nanomaterials, ZnO NPs have been synthesized through biologically available and replenishable sources using a green chemistry or green synthesis protocol. The green-synthesized ZnO NPs are less toxic, more eco-friendly, and more biocompatible than other chemically and physically synthesized materials.
- green synthesis
- zinc oxide nanoparticles
- antimicrobial approaches
- photocatalytic activities
- phytosynthesis
- biosynthesis
1. Introduction
The concurrent occurrence of organic dyes and inorganic toxic pollutants, such as heavy metals (HMs), pharmaceuticals, and pathogenic microorganisms in wastewater is a major worldwide concern [1]. Although the effects of each of the pollutants are well documented, the toxicity that arises from the co-occurrence of, and interactions between, these pollutants remains unknown; therefore, this presents a greater concern than the singular effects of each of the pollutants, with respect to the negative effect on the balance of the ecosystem, plants, and animals [2]. Synthetic dyes cause water to become unaesthetic, and they adversely compromise the health of humans and aquatic organisms by altering the biochemical oxygen demand (BOD), chemical oxygen demand (COD), salt concentration, light penetration, and rate of photosynthesis [3]. HMs are extremely poisonous due to the failure of living systems to remove them from the bodies of animals, thus leading to bioaccumulation. As such, HMs are responsible for neurological complications, carcinogenesis, mutations, and multiorgan failure in different organisms, even at low concentrations [4]. Pathogenic microorganisms comprise another class of public health concerns due to their short lifecycle, high reproductive rate per lifecycle, and ultrafast mutation rates that produce resistant strains. Around 500 waterborne microorganisms have been identified in wastewater treatment plants, including viral, bacterial, parasitic protozoan, and fungal pathogens [5]. These organisms invade a range of animal tissues, and they compromise the immune system through the release of toxins [6]; therefore, such detrimental pollutants must be efficiently eradicated from effluents before they are discharged into surface water bodies for the protection of ecological and biological systems.
Nanomaterials can address bottlenecks in conventional wastewater treatment systems because the nano-dimensions create a high surface area, quantum confinement, an attenuated surface charge, and other peculiar physical and chemical characteristics [7]. As such, various nanostructures have been synthesized for the purification of wastewater. Among the nanomaterials developed are: metallic nanoparticles such as Ag [8], Au [9], and Cu [10], and nanometal oxides such as TiO2 [11], Fe2O3 [12], SnO [13], AgO [14], CuO [15], and ZnO [16].
ZnO is among one of the most used semiconductors because of its ease of synthesis and its photo-driven electronic response. ZnO is a crystalline n-type semiconductor, it is a member of the II–VI group, and it exhibits a wide bandgap (3.37 eV) in the near UV spectrum [17][18][17,18]. Moreover, it exhibits high exciton binding energy (60 meV) in standard atmospheric conditions [19]. With respect to its crystallographic structures, ZnO has three polymorphs, namely: zincblende, cubic rock salt, and the hexagonal wurtzite. Of these polymorphs, wurtzite is the most stable, hence its synthesis at room temperature (RT) under pressure. The rock salt polymorph is formed under high pressure which results in the close packing of the Zn and O atoms, as shown in Figure 1. The zincblende polymorph ZnO is metastable, and therefore, it cannot exist on its own in ambient conditions [20][21][20,21]. With respect to the morphology of ZnO, as seen under transmission electron microscopy (TEM) and scanning electron microscopy (SEM), there is a wide variety of nanoparticulate ZnO morphologies. These include nanorods [22], spheres [23], nanosheets [24], hexagons [25], tripods [26], nanowires [27], nanotubes [28], nanostars [29], nanoflowers [30], and nanocubes [31].
2. Green Synthesis
Over the last few decades, the “green synthesis” approach has received a lot of interest in the field of nanomaterial synthesis. Indeed, the green synthesis technique for nanomaterials is based on twelve green chemistry principles, namely: (i) to improve the reaction efficiency of atoms (atom economy); (ii) to improve the energy efficiency of reaction processes (i.e., avoidance of long synthesis times, high temperatures, and high pressure environments); (iii) to use harmless chemicals in order to reduce the toxicity of the procedures and the synthesized nanomaterials; (iv) to reduce waste throughout the synthesis process; (v) to use easily available renewable precursors; (vi) to design non-toxic biodegradable products; (vii) to follow safer synthesis routes and replace hazardous substances with safe (or at least less toxic) reagents; (viii) to avoid the use of derivatives such as stabilizers; (ix) to avoid the release of toxic waste; (x) to employ eco-friendly solvents, such as water, instead of organic solvents; (xi) to accelerate reactions by including a catalyst, so as to reduce total energy demands in order to improve sustainability and efficiency; and (xii) to limit accidents during and after the synthesis process [32][33][34][53,54,55]. In traditional chemical synthesis, organic capping agents such as oleic acid, linolenic acid, oleylamine, hexadecylamine, and 2-mercaptoethanol need to be used to attain particles with the desired morphology and with a high degree of uniformity [35][56]. Biological synthesis, on the other hand, encourages the use of readily available resources, such as non-toxic solvents and auxiliaries, for a benign synthesis protocol [36][57]; therefore, it is in accordance with the principles of green synthesis. Biological synthesis promotes the development of nanoparticles using nature-derived materials such as plants [37][58], bacteria [38][59], fungi [39][60], algae [40][61], and biological derivatives [41][62]. These bio-sources (enzymes, polysaccharides, phytochemicals, biomolecules, vitamins, etc.) serve as green capping, stabilizing, or chelating agents [7]. During biosynthesis, a zinc salt such as chloride, acetate, sulfate, or nitrate is generally added to a previously prepared biological extract (from plants, bacteria, fungi, algae, yeast, etc.) to facilitate the complexation of metal ions with the available functional groups in the biological extract (Figure 13). This is achieved by heating and stirring the extract in an alkaline environment. The temperature, pH, and ratio of the precursors determine the growth phase, wherein the nucleated seeds grow either isotropically or anisotropically. Moreover, as is the case in conventional chemical synthesis processes, the synthesis parameters in green synthesis procedures determine the morphology, size, agglomeration, and size distribution of the resultant NPs [42][63]. After the metal oxide precipitates, it is washed, dried, and/or calcined to produce ZnO NPs, as indicated in Figure 13.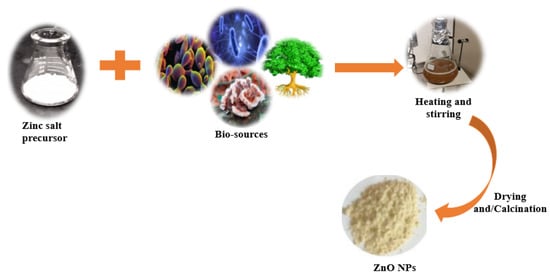
Figure 13.
Schematic representation for the green synthesis of ZnO nanoparticles.
2.1. Plant-Mediated/Phytosynthesis of ZnO NPs
2.1.1. Phytosynthesis Synthesis of Undoped ZnO NPs
In comparison with other ZnO green synthesis techniques, the utilization of plant extracts (phytosynthesis) has dominated scientific research, hence the large number of reports available for review [46][47][48][49][67,68,69,70]. Researchers prefer using parts of plants over microbes since the use of plants eliminates laborious and delicate procedures such as microbe isolation, growth, and maintenance. Furthermore, phytosynthesis is safer, as it eliminates the health risks associated with the use of hazardous microorganisms [44][65]. The phytosynthesis approach is preferred for large-scale production, as plant extracts can easily be obtained through the use of eco-friendly solvents such as distilled water or alcohols [50][71]. Additionally, plant sources are easily accessible, abundant, and hence more sustainable [51][72]. Since plant extracts naturally possess secondary metabolites, including amino acids, alkaloids, and flavonoids, among others, they are commonly employed as both chelating and reducing agents in the fabrication of nanoparticles [52][73]. Although the explicit mechanism of their formation cannot yet be explained, Bala et al. and Senthilkumar et al. concluded that phenols, flavonoids, and proteins are the main participants in the development of metallic and metal oxide NPs [53][54][74,75]. Various parts of a known plant species, including the leaves, seeds, pericarps, peels, roots, bark, or even the whole plant, have been used to synthesize ZnO NPs. Zinc salts are said to be oxidized by phenols and flavonoids into ZnO NPs with the help of the numerous functional groups that these molecules possess. These functional groups attach onto the surface of Zn2+, and they oxidize the molecule into ZnO NPs [42][54][63,75]. Since the coordination chemistry of proteins is specific to that of zinc ions, they are the predominant chelating agents for Zn2+. The H2O molecules of the aqua complexes are exchanged when they bind to the protein ligands [55][76]. During their interaction with the acidic [Zn(H2O)6]2+, an electron from the −COO− initiates a nucleophilic reaction, which results in the formation of the [Zn(OH) (H2O)5]+ complex [56][77]. The [Zn(OH) (H2O)5]+ is then transmuted into ZnO NPs [57][78]. According to Singh et al., the −OH and/or −COO− of the flavonoids react with the ZnO precursor to generate a distinctive Zn flavonoid complex via the electrostatic interaction between negatively charged flavonoids and positively ZnO nuclei. This complex is then later converted to ZnO through thermal treatment [58][79]. An alkaline pH favors the formation of metal oxide nanoparticles that are stabilized by the phytochemicals in the plant extract [59][80]; thus, it follows that during the phytosynthesis of metal oxides such as ZnO, a combination of polyphenols, flavonoids, and proteins results in the creation of ZnO NPs [60][81]. Krol et al. [56][77] presented a probable reaction mechanism for a plant mediated ZnO. Table 1 provides an overview of the plant-based synthesis of ZnO NPs. The use of the waste pericarp of Ananas comosus yielded spherical NPs, the sizes of which varied between 10 and 60 nm after sonicating for 1 h at 40 °C [61][82]. The seed extract of Eriobutria japonica produced hexagonal NPs when a mixture of the seed extract and Zn (CH3COO)2·2H2O was heated at 60 °C for 1 h. Using Fourier-transform infrared spectroscopy (FTIR), the reducing and capping agents were revealed to be phenolic groups, alcohols, and aliphatic amines. Recently, fruit-derived aqueous extracts have been employed to prepare ZnO NPs [22][62][63][64][22,83,84,85]. Golmohammadi et al. [64][85] obtained spherical NPs with an average size of 29 nm when they refluxed Zizyphus jujuba fruit and Zn(NO3)2·6H2O with water at 80 °C for 30 min prior to calcination. Recently, Faisal et al. [65][86] produced semi spherical NPs when synthesizing with the fruit extract of Myristica fragrans. Biosynthesizing ZnO NPs using flower extracts have also been reported [66][67][87,88]. The extract of Hibiscus sabdariffa served as a reducing and stabilizing agent in order to produce wurtzite-phased NPs. The concentration of the employed extract determined the surface structure and size of the NP. The sizes ranged between 8.71 and 88.63 nm [62][83]. Khara et al. produced spherical and irregular ZnO NPs using Peltophorum pterocarpum, with a mean diameter of 69.45 nm [67][88]. This flower extract possesses phenolic compounds and flavonoids, among other properties, that potentially reduce and stabilize ZnO NPs. Moreover, Diallo and colleagues obtained very small (1–8.5 nm) quasi-spherical ZnO nanomaterials by applying the flower extract of Aspalathus linearis as a reducing/oxidizing chemical agent [68][89]. Regarding phytosynthesis, leaves are the most frequently used parts of a plant during extraction. This is likely due to their accessibility, abundance, and ease of grinding. Several authors have reported on the phytosynthesis of various nanomaterials through the use of leaf extracts from various species of plants [69][70][71][90,91,92]. Mostly, the attained NPs were spherical and irregularly shaped. Ekennia and colleagues, however, attained flower-shaped NPs (31 nm) when using an extract of Euphorbia sanguinea and ZnCl2 as the salt precursor [72][93]. The appearance of the 1638 cm−1 absorption band in the FTIR spectra was ascribed to the N–H bending of amines in alkaloids or C=O stretching in polyphenols, flavonoids, and reducing sugars, which were thought to be responsible for reducing and stabilizing the synthesized NPs. An extract of quince seed mucilage was kept for 4 h in the presence of Zn(NO3)2·6H2O, before it was concurrently heated and stirred at 80 °C for 2 h to produce 25 nm spherical ZnO NPs [73][94]. Prior to this study, Prasad and colleagues [74][95] mixed Abelmoschus esculentus mucilage extract with Zn(OOCCH3)2·2(H2O) salt and quince seed mucilage in water, and the mixture was stirred for 4 h, at RT, to obtain spherical ZnO NPs with an average diameter of 29 nm and 70 nm long nanorods. The reducing and capping agents for the NPs were carbohydrates, which are available in excess in plants. The heterogeneous sizes and shapes were ascribed to the method employed and the high calcination temperature (700 °C for 2 h); this poses a challenge with regard to controlling the morphology of the prepared particles. There have also been reports on the utilization of the aqueous extracts in roots, regarding the biogenic preparation of ZnO NPs with various morphologies [75][96]. A methanolic extract was employed by Verma et al. [47][68] to obtain hexagonal and rod-shaped NPs after stirring Zn(CH3COO)2·2H2O in the presence of Salvadora persica root extract for 3 h at 80 °C; it was then heated at 400 °C for an appropriate length of time. Matinise et al. prepared ZnO NPs by capping the NPs with an aqueous solution of Moringa oleifera leaf. The extract completely dissolved the zinc salts at an ambient temperature; this was evidenced by the fact that the solution changed color, from clear to brown, after 18 h without any precipitation. Spherically shaped ZnO NPs were formed after drying the concentrated solution at 100 °C for 1 h [76][97]. Highly crystalline single phase zincite ZnO NPs were produced by adding an aqueous solution of Agathosma betulina, in order to reduce the presence of zinc nitrate hexahydrate salt; in this scenario, the temperature was set at 100 °C for 2 h. The resultant precipitate was further annealed at 500 °C for 2 h to obtain (15.8 nm) quasi-spherical NPs [77][98]. When Ngom et al. prepared the same nanoparticles by employing Moringa oleifera leaves as the chelating agent, wurtzite phased ZnO nanomaterials, with an average diameter of 10.81 nm, were produced [78][99].Table 1.
Plant-based synthesis of ZnO nanoparticles.
| Serial No. | Plant Name | Plant Part | Extraction Solvent Temp (°C) Time |
Zinc Precursor | Biosynthesis Time (h)/Temp (°C) |
Calcination Temp (°C)/Time (h) |
Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pithecellobium dulce | Peel | Water, 70 °C for 50 min | Zn(CH₃CO₂)₂ | 400/2 | Spherical | 30 | [62][83] | |
| 2 | Ananas comosus | Pericarp | Water, 80 °C for 20 min | Zn(CH3COO)2·2H2O, | Sonicated 1/40 | − | Spherical | 10–60 | [61][82] |
| 3 | Punica granatum | Fruit peel | Water, 65 °C for 1 h | Zn(NO3)2·6H2O | –/90 | 600/1 | Spherical and hexagonal | 38.98 | [63][84] |
| 4 | Zizyphus jujuba | Fruit | Refluxed with water, 80 °C for 30 min | Zn(NO3)2·6H2O | 4/80 | 550/3 | Spherical | 29 | [64][85] |
| 5 | Myristica fragrans | Fruit | Water, 150 °C for 20 min | Zn(NO3)2·2H2O | 2/60 | 500/2 | Semispherical | ||
| 18 | |||||||||
| Rubus fairholmianus | |||||||||
| Roots | |||||||||
| Acetone using Soxhlet apparatus | |||||||||
| Zn(NO | |||||||||
| 3 | |||||||||
| ) | |||||||||
| 2 | |||||||||
| 48/80 | − | Spherical | 1–100 clusters | [ | 79 | ] | [ | 100 | ] |
2.1.2. Phytosynthesis of Doped ZnO Nanoparticles
Recently, the number of biogenically doped ZnO NPs has been increasing, which speaks to the efficiency of plant metabolites in nanomaterial synthesis. Doping occurs as a result of the wide band gap of ZnO, which causes ZnO to only absorb light that falls within the UV spectrum. Doping is a process wherein a point defect in a nanomaterial is formed by inserting a specific ion into the crystal lattice of a nanomaterial to modify its electronic band structure. The band structure is modified by the formation of shallow or deep energy level, known as mid-gap states, that extend the absorption range from shorter to longer wavelengths; this can even extend to the visible range of the electromagnetic spectrum [80][81][101,102]. The physicochemical properties of doped ZnO significantly depend on the dopant type and its concentration. p-type dopants tend to withdraw electrons from the ZnO, whereas n-type dopants increase the number of electrons. As the dopant concentration increases, the energy band gap Eg of ZnO decreases until an optimal dopant concentration is reached, beyond which, the dopant ions act as charge recombination centers and reduce the efficiency of ZnO in photocatalytic processes. The dopants also become active sites that are different from the ZnO active sites, thereby increasing the mass transfer of pollutants from the solution to the ZnO surface. This phenomenon is not only useful for increasing the adsorption efficiency in cases where ZnO is used as an adsorbent, but it also helps to increase the level of light that penetrates the polluted water, thereby increasing the photocatalytic efficiency [82][103]. As well as induced defects, ZnO exhibits intrinsic defects, which include zinc vacancy (VZn), oxygen vacancy (VO), zinc interstitial (Zni), oxygen interstitial (Oi), or antisite oxygen (OZn) defects [83][104]. As with dopants, it is generally accepted that surface defects (i.e., surface VO, VZn, Oi, and Zni, especially the anionic Vo) are beneficial to photocatalytic activity because they can serve as active sites for photocatalysis. These defects create mid-gap states that alter the electronic band structure of photocatalysts to facilitate charge separation with longer lifetimes [80][101]. Conversely, deep level defects (VO and VZn), and other nonradiative defects, have negative impacts on photocatalysis because they create recombination centers for photogenerated charges; this causes electronic delocalization and low photocatalytic efficiency. These defects are repaired via annealing at appropriate temperatures [84][105]. The most used doping agents in the biogenetic synthesis of ZnO include Ag, Cu, Mg, Ce, Fe, and Co, though Ag doping is the most common [37][85][86][33,58,106]. Saeed et al. synthesized AgNO3-ZnO NPs at an ambient temperature using different plant species and parts. Owing to its strong reduction potential, the extract of Calotropis gigantea resulted in the formation of rod-shaped NPs [85][33]. Moringa olifera seed extract yielded flower-like NPs [37][58], whereas Ocimum tenuiflorum seed extract produced hexagonal and spherical NPs ranging between 50 and 60 nm in diameter [86][106]. Mn-ZnO NPs were prepared by mixing a salt precursor with Melastoma malabathricum. The mixture was heated at 60 °C until a paste formed, and it was further heated at 400 °C to obtain spherical NPs with an average diameter of 222 nm [87][107]. Fe-ZnO NPs were produced by Jan et al. This was achieved by reducing zinc ions with the flavonoids, phenolics, terpenoids, triterpenes, tannins, and fatty acids present in the leaf extract of Myrtus communis [88][108]. The NPs were produced after 3 h of stirring and heating at 60 °C, after which they were calcinated at 400 °C for 3 h. Their diameters were small, at 17 nm on average, with a pseudo-spherical shape. Okeke et al. prepared Mg-ZnO NPs by reacting Zn(NO3)2 and Mg(NO3)2 with Piper guineense leaf extract, which was then stirred and heated for 15 min at 80 °C before calcining the paste at 350 °C for 2 h [89][109]. A dual doping of ZnO NPs with Cu and Mg was successfully completed by Rahman et al. [90][110], who utilized a Ziziphus mauritiana solution to reduce Zn(NO3)2·6H2O, as depicted in Figure 5. The synthesized NPs were nearly spherical, but relatively large in size (0.1–1 µum), depending on the Cu/Mg dopant concentration. The FTIR suggests that the reduction of ions was controlled by tannins, flavonoids, saponins, and phenols. An eco-friendly fabrication of Nb-ZnO using Vernonia amygdalina leaf extract was reported by Nguyen et al., and the obtained Nb-ZnO NPs showed an improved photocatalytic degradation of tetracycline under natural light in comparison with pristine ZnO. The composite reached a 93.2% degradation efficiency after 3 h [91][111]. A summary of the synthetic procedures and the results of the doped-ZnO NPs are given in Table 2.Table 2.
Phytosynthesis of the doped ZnO nanoparticles.
| Serial No. | Plant Name | Dopant | Extraction Solvent, Temp and Time | Precursors | Biosynthesis Time (h) and Temp (°C) | Calcination Temp (°C)/Time (h) |
Shape | Size (nm) | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Calotropis gigantea (leaves) | Ag | Refluxing | Zn(NO3)2·6H2O AgNO3 |
3/RT | 400/3 | Rods | None | [85][33] | ||||
| 2 | Moringa oleifera (seeds) | Ag | Aqueous powder, RT | Zn(OOCCH3)2 AgNO3 |
50 min/RT | - | Flower-like | 54.1 | [37][58] | ||||
| 3 | Ocimum tenuiflorum (seeds) | Ag | Water, boiled | Zn(NO3)2·6H2O AgNO3 |
2/RT | 450/3 | Hexagonal and spherical | 50–60 | [86][106] | ||||
| 4 | Melastoma malabathricum (leaves) | Mn | Water, 60 °C | Zn(NO3)2·6H2O Mn(CH3CO2)2·4H2O |
–/60 | 400/2 | Spherical | 222 | [87][107] | ||||
| 5 | Myrtus communis L. | Fe | Water, 40 °C for 1 h | Zn(NO3)2·6H2O Fe(NO)3·9H2O |
3/60 | 41.23 | [ | 65 | ][86] | ||||
| 400/3 | Pseudo spherical | 17 | [ | 88 | ] | [ | 108 | ] | 6 | Hibiscus sabdariffa | Flower | Water, 60 °C, for 1 h after 2 h stirring at RT | Zn(NO |
| 6 | Piper guineense (leaves) | 3 | ) | 2 | Mg | Water, 60 °C for 20 min | Zn(NO1/RT. | 3)2400/1 | Mg(NO3Semi spherical |
)8.7–88.6 | 2 | 0.25/80 | 350/2[66][87] |
| Spherical | 364 | [ | 89 | ] | [ | 109 | ] | 7 | Peltophorum pterocarpum | Flower | Water, 80 °C for 1 h | Zn(NO3) | |
| 7 | Ziziphus mauritiana Lam. (leaves) | Mg and Cu | 2 | –/80 | 400/2 | Water, RT for 1 h | Zn(NOSpherical and irregular | 3)2·6H2O Cu(NO3)2·3H2O Mg(NO3)2 |
–/60 | 400/2 | Hexagonal disc shaped | 100–1000 | [90][110] |
| 8 | Vernonia amygdalina | Nb | Not available | Not mentioned | 1/60 | 400/1 | Spherical | 1000 | [91][111] |
2.2. Microbial Synthesis of ZnO Nanoparticles
Microorganisms, or microbes comprising bacteria, fungus, and algae, have also been exploited for the biological synthesis of metal oxide NPs. Although many metal oxides have been synthesized biologically using microorganisms, the microbial production of ZnO has not been thoroughly investigated. An advantage of the microbial synthesis of NPs over phytosynthesis is the ease with which microorganisms can be reproduced, as they are easily grown in a laboratory. During microbial synthesis, metal reduction is made possible with the available enzymes, carbohydrates, proteins, and other metabolites that are intrinsically present in the microorganisms. The biomolecules that are secreted in the growth medium influence the shape, size, and dispersity of the produced NPs [92][112]. Under stressful conditions, or in the presence of heavy metals, microbes reduce these ions to metals in order to enable their own survival; thus, they act as natural nano-factories [93][113].2.2.1. Bacterial Synthesis
Prokaryotic microorganisms, such as bacteria and algae, have been extensively studied because they are easy to handle and genetically modify [94][114]. Bacteria offer the advantage of being able to quickly multiply, thus creating a readily available source of secondary metabolites that are involved in the fabrication of ZnO. The most used microbes in the synthesis of NPs comprise Actinobacter sp., Corynebacterium sp., Klebsiella pneumonia, Lactobacillus sp., and Pseudomonas sp. [95][115]. The biological preparation of metal, and metal oxide nanomaterials, using bacterial cultures may occur in the intra- or extracellular environment [96][116]. The mechanism of formation during intracellular synthesis is challenging due to the intricacy of the bacterial cell make-up and its metabolic processes. The mechanism for the intracellular generation of ZnO in microbes is illustrated in Figure 26a. The cell walls of microbes have different types of polysaccharides and proteins that provide active sites for the binding of metal oxides through electrostatic interactions [97][117]. Then, reductases (NADH and NADPH) that are either present in the cell wall or secreted as soluble enzymes [98][118] reduce the trapped ions into elemental atoms [99][119]. Finally, the nuclei develop and assemble in the cytoplasm or cell wall, while the peptides and amino acids stabilize the NPs [100][120]. Bacteria-mediated nanoparticle synthesis creates less hazardous metal oxide nanoparticles, such as TiO2, CuO, and ZnO; however, the downside to this is that it involves isolating, screening, and culturing potential microbes, which are time consuming processes. In addition, the process may involve the use of expensive chemicals, such as growth media [99][119]. The size distribution, shape, and crystallinity of microbe-synthesized NPs are not easy to monitor [101][121]. Moreover, the intracellularly formed nanomaterials can only be released by lysing the bacterial cells; thus, time consuming and laborious steps are unavoidable [102][122]. As a result, research on bacterial synthesis has, to date, focused on extracellular synthesis. Regarding extracellular synthesis, the enzymes that are released by microorganisms into the growth culture are responsible for the bio-reduction of metal ions into NPs. The bio-reduction of zinc ions occurs when an electron is transferred from NADH with NADH reductase [103][123]. The extracellular synthesis pathways are depicted diagrammatically in Figure 26b.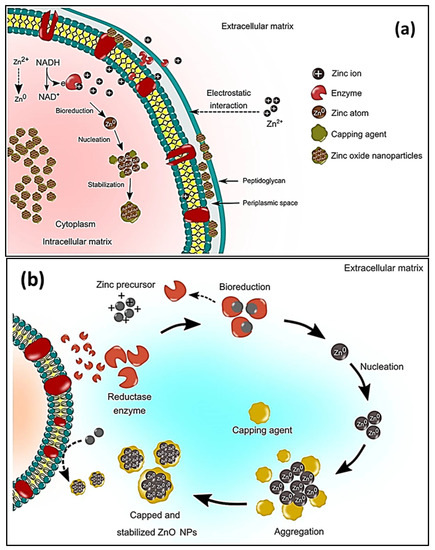
Figure 26.
Schematic representation of the (
a
) microbial intracellular synthesis and (
Table 3.
Microbial synthesis of ZnO nanoparticles.
| Serial No. | Microbe Name | Extraction Method | Salt | Biosynthesis Time (h)/Temp (°C) |
Calcination Temp (°C) Time (h) |
Shape | Size (nm) | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||||||||||||
| 1 | Cyanobacterium Nostoc sp. | Water, 60 °C for 15 min | Zn(CH3COO)2·2H2O | 2/RT | − | Star-shaped | 60 | [29] | ||||||||||
| 2 | Aeromonas hydrophila | Cell culture grew for 24 h and was diluted with water | ZnO | 24/30 | − | [38][59] | ||||||||||||
| 3 | Serratia nematodiphila. | Inoculated in Luria-Bertani (LB) broth | ZnSO4 | 0.17/80 Then stored for 24 h |
− | Near spherical | 15–30 | [84][105] | ||||||||||
| 4 | Bacillus subtillis | Cell culture in ammonium carbonate | Zn(CH3COO)2 | 3/80 | 250/3 | Spheres with hair-like appendages | 10–15 and 1.2–1.3 um aggregates |
[104][124] | ||||||||||
| 5 | Bacillus haynesii | Cell culture grown on agar | ZnSO4 | 24/55 | 700/5 | Spherical | 20–200 | [105][125] | ||||||||||
| 6 | Alkalibacillus sp. | Cell-free supernatant collected by centrifugation | ZnSO4·7H2O | 48/35 | − | Quasi-spherical | 69.45 | 1–30 | [106][126][67][88] | |||||||||
| 8 | Aspalathus linearis | Flower | ||||||||||||||||
| 7 | Water, 25 °C for 48 h | Pseudomonas putida | Cell culture solution | Zn (NO3)Zn(NO3)2·6H2O | −/− | 300/2 | 2Quasi-spherical | 1–8.5 | [68] | 24/37[ | 400/2 | Spherical agglomerates89 | 25–45] | |||||
| [ | 107 | ] | [ | 127 | ] | 9 | Eucalyptus spp. | Leaves | Water, 90 °C for 90 min | Zn(NO3)2·6H2O | 2/110 | 250/6 | Irregular shapes | 32.71 | ||||
| 8 | [ | 69 | ] | [ | 90 | ] | ||||||||||||
| Halomonas elongata | Centrifugation | ZnCl | 2 | Taguchi | − | Multiform shapes | 18.11 | [108][128] | 10 | Capparis zeylanica | Leaves | Water, 60 °C for 20 min | ||||||
| 9 | Lactobacillus plantarum | Cell biomass collected by centrifugation | Zn(NO3)2 | Zn(OOCCH3)2·2(H2O), | 2/80 | ·6H400/2 | Spherical | 220–40 | O,[70][91] | |||||||||
| 24/37 | − | Flower-like | 152.8–613.5 | [ | 109 | ] | [ | 129] | 11 | Ocimum tenuiflorum | Leaves | Powder | ZnSO4 | 0.5/RT | 600/0.5 | Spherical and granular | 50–63 | [71][92] |
| 10 | Streptomyces spp. | Broth dilution then centrifugation | ZnSO4 | 0.25/40 | 400/8 | Needle-like | 12–35 | [110][130] | 12 | Euphorbia sanguinea | Leaves | Water, 80 °C for 45 min | ZnCl2 | 2/90 | – | Flower-like | ||
| Fungi | 34 | [ | 72 | ] | [ | 93 | ] | |||||||||||
| 13 | Moringa oleifera | Leaves | Water, 50 °C for 105 min | Zn(NO3)2·6H2O | ||||||||||||||
| 11 | Aspergillus aeneus | Cell-free filtrate | Zn (NO3)2 | 18/RT then 100 | 500/1 | 48/28 | − | SphericalSpherical | 16–31.9 | 100–140 | [39][60[76][97] | |||||||
| ] | Gathosma betulina | Leaves | Water, 100 °C for 60 min | Zn(NO3)2·6H2O | 2/100 | 500/2 | Quasi-spherical | 15.8 | [77][98] | |||||||||
| 14 | Quince seeds | Mucilage | Water, 60 °C for 4 h | Zn(NO3)2·6H2 | ||||||||||||||
| 12 | Aspergillus fumigatus | Plating the inoculum on Martin Rose-Bengal agar medium | Zn (NO3)2 | 72/28 | − | Oblate spherical | 1.2–6.8 | [111][131] | O | 2/80 after keeping for 4 h at RT | 600/2 | Spherical | 25 | [73 | ||||
| 13 | Aspergillus fumigatus | Cell biomass | ZnSO4 | ] | [ | 72/32 | 94 | ] | ||||||||||
| − | Spherical | 60–80 | [ | 112 | ] | [ | 132 | ] | 15 | Abelmoschus esculentus | Mucilage | Soaked for 24 h at RT | Zn(OOCCH3)2·2(H2 | |||||
| 14 | O), | Aspergillus niger. | Culture filtrate | Zn(NO3) | 4/RT | 2700/2 | Spherical Rods |
29 70 |
[74][ | 48/3295] | ||||||||
| − | Near spherical | 53–69 | [ | 113 | ] | [ | 133 | ] | 16 | Panax spp. | Roots and stems | Water, 85 °C for 8 h | Zn(NO3)2 | 2.5/85 | 500/2 | Leafy flower | 480 | [75][96] |
| 15 | Agaricus bisporus | Purchased | Zn(NO3)2·6H2O | 2/90 | 400/4 | Spheroids | 32 | [114][134] | 17 | Salvadora persica | Roots | Water and methanol, 80 °C for 3 h | Zn(NO3)2·6H2OZn(CH3COO)2·2H2O | 2–3/80 | –/400400/2 | 700/2Hexagonal and rods | 24–25 | [47][68] |
| 16 | Xylaria acuta | Cultured then centrifuged | Hexagonal | 34–55 | [ | 115 | ] | [135] | ||||||||||
| 17 | Pichia kudriavzevii | Mycelia separated from the culture | Zn(CH3COO)2·2H2O | 36/35 | − | Hexagonal | 10–61 (TEM, XRD) | [116][136] | ||||||||||
| 18 | Cochliobolus geniculatus | Mycelial-free filtrate | Zn(CH3COO)2 | 72/28 | − | Quasi-spherical | 2–6 (TEM) | [117][137] | ||||||||||
| 19 | Saccharomyces cerevisiae | Cell-free filtrate | Zn(CH3COO)2·2H2O | 24/30 | − | Spherical | 20–30 | [118][138] | ||||||||||
| 20 | Cladosporium tenuissimum FCBGr | Extracellular culture | Zn(NO3)2 | 36/RT | − | Bouquet-like | 57 | [119][139] | ||||||||||
| Algae | ||||||||||||||||||
| 21 | Chlorella | Soaking method | Zn(CH3COO)2·2H2O | 1/58 | − | Hexagonal | 20 | [120][140] | ||||||||||
| 22 | Arthrospira platensis | Biomass mixed with supernatant | Zn(CH3COO)2·2H2O | 24/30 | − | Spherical | 30–55 | [121][141] | ||||||||||
| 23 | Padina gymnospora | Millipore water stirred for 2 h at 100 °C | Zn(NO3)2 AgNO3 |
2/60 | − | Spherical | 20–40 | [122][142] | ||||||||||
| 24 | Sargassum species | Ball-milled | Zn (NO3)2·6H2O Co(NO3)2·6H2O |
2/RT | − | Nearly spherical | 5.4–6.8 | [123][143] | ||||||||||
2.2.2. Fungal Synthesis
Fungi can release higher concentrations of secondary metabolites than bacteria. In addition, they exhibit a higher tolerance to metal concentrations, stronger binding capabilities, and better metal bioaccumulation than bacteria [124][144]. It is therefore possible that fungi have more potential than bacteria with regard to the upscaling of green-produced NPs. Moreover, fungal cells are more tolerant to intermediate products during the synthesis process; hence, they are more suitable for large-scale synthesis [125][145]. The mechanisms for generating metal and metal oxide NPs using fungal biomass or cultures are similar to the one discussed for green synthesis using bacteria. Different Aspergillus species have already been employed in the green synthesis of ZnO NPs, such as Aspergillus aeneus [39][60], Aspergillus fumigatus [111][131], Aspergillus fumigatus [112][132], and Aspergillus niger [113][133], as shown in Table 3. The ability to reduce and cap the NPs is conferred by the alcohols, phenols, and the aromatic and primary amines that are present in the fungal extracts of the various Aspergillus species [112][132]. During extracellular synthesis, fungi secrete enzymes that produce pure and monodispersed NPs. These particles are free from the cellular components that are associated with organisms [111][131]. The biomass of A. fumigatus reduced ZnSO4 and chelated the formed ZnO NPs after 72 h at 32 °C. The formed NPs were spherical with diameters ranging between 60 and 80 nm. The attained ZnO exhibited antimicrobial activities against Klebsiella pneumonia, P. aeruginosa, E. coli, S. aureus, and B. subtilis, respectively, as indicated in Figure 37. The synthesis of ZnO NPs, using the aqueous extract of Agaricus bisporus, was carried out in [114][134]. According to the FTIR results, the phenol groups contained the prominent ingredients for reducing and capping ZnO NPs. The zeta potential (−20.5 mV) validated the NPs’ stability, whereas the SEM and TEM revealed that the NPs were spheroids. ZNO NPs were produced with Zn(NO3)2·6H2O and Xylaria acuta fungal filtrate using the combustion method at 400 °C. The resultant sample was then calcined for 2 h at 700 °C to obtain hexagonal shaped NPs with diameters ranging between 34 and 55 nm [115][135].
2.2.3. Algal Synthesis
Algae are mono and multicellular aquatic photosynthesizing organisms, but unlike plants, algae are without roots and leaves [95][115]; however, similarly to plants, macroalgae have active metabolites such as alkaloids, peptides, polysaccharides, proteins, tannins, quinones, lipids, and glycerol [126][146]. These metabolites have –OH and –COOH functional groups that can chelate and stabilize ZnO NPs [126][146]; therefore, the chemical formation route of ZnO NPs during algal synthesis is similar to that of plants [36][57]. Khalafi et al. [120][140] propose a mechanism of ZnO NP formation using a Chlorella extract (Figure 48). Based on the assumption that chlorella contains a significant amount of carbohydrates (20%), it was presumed that carbohydrates predominantly served as Zn2+ reducing agents and as stabilizers of the prepared ZnO NPs. Moreover, the biosynthesis of green ZnO NPs was assumed to occur through a donor–acceptor mechanism that occurred between the oxygen atoms of functional moieties in chlorella and Zn2+ wherein the −OH groups on the carbohydrates transfers an electron to the electrotrophilic Zn species, thus resulting in the oxidation of the −OH group and Zn2+ reduction to Zn atoms.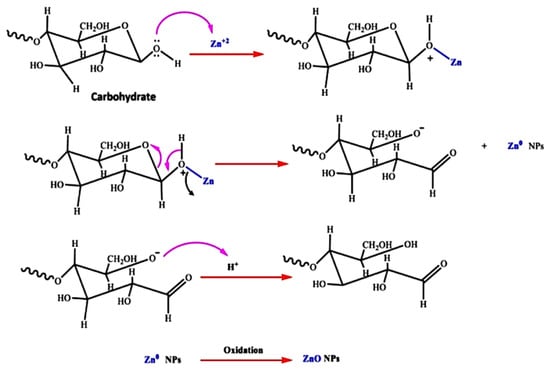
Figure 48.
The probable method for the algal production of ZnO NPs, using aqueous
2.2.4. Preparation of ZnO NPs Using Biological Derivatives Synthesis
Apart from macro- and microorganisms, several biological derivatives have been used as precursors to synthesize NPs; however, only a handful reports discuss the use of biological derivatives for the green fabrication of ZnO, as shown in Table 4. Moreover, the polysaccharide pullulan was stirred together with Zn(NO3)2·6H2O for 5 h at RT. This was followed by a 1 h heat treatment at 400 °C to yield spherical and hexagonal ZnO NPs with an average diameter of 58.13 nm [127][147]. El-Saied and Ibrahim, 2020, used chitosan to synthesize ZnO NPs, with average size of 55 to 70 nm [128][148]. Vijayakumar et al. [129][149] prepared egg albumen wrapped ZnO NPs, with diameters ranging between 20 and 60 nm, and with spherical and diagonal platelets. Additionally, artemia eggshells were used to hydrothermally produce ellipsoidal ZnO NPs. The prepared NPs had a 50 nm diameter on average, according to TEM [130][150]. Highly crystalline crustin-capped ZnO NPs were produced after the coprecipitation of the salt with an extract of crustin at RT for 2 h. The average size of the resultant NPs were 50 nm [131][151]. Amino acid-capped NPs were produced via a wet chemical synthesis, prior to calcining at 550 °C for 3.5 h. The produced sheet-like NPs had diameters ranging between 22.46 and 40.29 nm [132][152]. Smaller ZnO NPs were produced with the aid of tannic acid, as both the size influencer and capping agent were treated at 70 °C for 1.5 h without further heat treatment. The particles had a spherical morphology, with diameters ranging between 26 and 34 nm [133][153]. A separate study reported that honey and cow urine, respectively, yielded spherical and hexagonal leaf-like ZnO NPs [134][154].Table 4.
ZnO nanoparticles prepared from biological derivatives.
| Serial No. | Bio-Product | Precursors | Biosynthesis Temp (°C)/Duration |
Thermal Treatment Temp (°C)/Time (h) |
Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Alginate | Purchased ZnO | − | Irregular spheres | 20–90 | [135][155] | |
| 2 | Starch | Zn(NO3)2·6H2O HAuCl4·3H2O |
Stirred at 90–100 °C | Oven dried at 80 °C Calcined at 600 °C for 4 h |
Spherical | 18.9 | [136][156] |
| 3 | Lignin | Zn(NO3)2·6H2O | Oven at 120 °C for 4 h | Oven dried at 120 °C for 4 h | Rods | 5–10 | [137][157] |
| 4 | Pullulan | Zn(NO3)2·6H2O, | Stirred at RT for 5 h | Oven dried at 60 °C for 24 h Calcined at 400 °C for 1 h |
Spherical and hexagonal | 58.13 | [110][130] |
| 5 | Chitosan | Zn(NO3)2·6H2O | Stirred at 80 °C for 2 h | Oven dried at 80 °C for 24 h | Rods | 50–70 | [128][148] |
| 6 | Egg albumen | Zn(CH3COO)2 | Hydrothermal, at 85 °C for 3 h | − | Spherical and diagonal platelet | 20–60 | [129][149] |
| 7 | Artemia eggshell | Zn(CH3COO)2 | Hydrothermal, 180 °C for 20 h | − | Ellipsoidal | 50 | [130][150] |
| 8 | Crustin | Zn(CH3COO)2 | Co-precipitation, magnetically stirred for 2 h | Vacuum dried at 30 °C | 50 | [131][151] | |
| 9 | Amino acids | Zn(NO3)2·6H2O | Stirred at 22 °C for 30 min prior to increasing the temperature to 150 °C | Calcined at 550 °C for 3 h 20 min |
Sheet-like particles | 22.46–40.29 | [132][152] |
| 10 | Tannic acid | Zn powder | Stirred at 70 °C for 1 h and 30 min | − | Spherical | 26–34 | [133][153] |
| 11 | Honey and cow urine | Zn(NO3)2·6H2O | Combustion method, further stirred at 100 °C | − | Hexagonal, leaf-like structure (cow urine) Spherical (honey) |
27 44 |
[134][154] |
