Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Longtao Ma | -- | 2224 | 2022-12-08 05:27:35 | | | |
| 2 | Lindsay Dong | Meta information modification | 2224 | 2022-12-08 09:11:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hu, F.; Li, M.; Gao, G.; Fan, H.; Ma, L. The Gel-State Electrolytes in Zinc-Ion Batteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/38240 (accessed on 07 February 2026).
Hu F, Li M, Gao G, Fan H, Ma L. The Gel-State Electrolytes in Zinc-Ion Batteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/38240. Accessed February 07, 2026.
Hu, Fulong, Maoyun Li, Guowei Gao, Huiqing Fan, Longtao Ma. "The Gel-State Electrolytes in Zinc-Ion Batteries" Encyclopedia, https://encyclopedia.pub/entry/38240 (accessed February 07, 2026).
Hu, F., Li, M., Gao, G., Fan, H., & Ma, L. (2022, December 08). The Gel-State Electrolytes in Zinc-Ion Batteries. In Encyclopedia. https://encyclopedia.pub/entry/38240
Hu, Fulong, et al. "The Gel-State Electrolytes in Zinc-Ion Batteries." Encyclopedia. Web. 08 December, 2022.
Copy Citation
Zinc-ion batteries (ZIBs) are receiving increasing research attention due to their high energy density, resource abundance, low-cost, intrinsic high-safety properties, and the appropriate plating/stripping voltage. Gel-state electrolytes possess merits of having a wide electrochemical window, good flexibility, superior water retainability, and excellent compatibility with aqueous electrolytes, which makes them potential candidates for flexible batteries. However, the practical applications of ZIBs with gel-state electrolytes still have some issues of water content easily dropping, poor mechanical stability, and the interface problem.
gel-state electrolytes
zinc-ion batteries
self-healing
1. Introduction
In pursuit of rechargeable flexible/wearable battery for grid-scale electrochemical energy storage and conversion systems, metal-ion batteries are drawing much attention [1][2][3][4][5][6]. As the most extreme application of electrical energy storage devices, lithium-ion batteries (LIBs) [7][8][9][10][11][12][13] not only have the high energy density, but also show long charge/discharge life cycles [14]. Nevertheless, some inherent issues hinder its widespread application, such as the safety problems, lithium metal extrusion, dendrite growth, and the high cost, together with the limited resources of lithium metal [15]. The zinc-ion battery (ZIB) [16][17][18][19][20][21], one of the next-generation batteries, is a promising candidate to circumvent some of the above problems, due to their high theoretical capacity, low cost, high abundance, low potential, high energy density, and intrinsic safety. In addition, batteries are mainly composed of electrodes, electrolytes, and current collectors, which holistically determine battery performance. The environment for metal-ion transfers between the two electrode terminals in the battery is provided by the electrolytes, which also judges the electrochemical window, ionic conductivity, and the reversibility of the zinc plating/stripping [22].
Three main types of electrolytes are included in batteries: liquid-state, solid-state, and gel-state (Figure 1). The aqueous ZIBs with liquid-state electrolytes are usually considered as ultra-intrinsic batteries [23][24]. Nevertheless, various side reactions of dendrite growth [25], oxygen evolution reaction, hydrogen evolution reaction, [26], and metal Zn corrosion and passivation [27] impede Zn-ion batteries’ application. However, solid-state electrolytes (SSEs) are an effective strategy to free one from the above issues [28][29]. Meanwhile, the high security, excellent flexibility, good mechanical stability, and no risk of electrolyte leakage merits of SSEs show enormous potential to solve the problems of liquid-state-solution-based batteries [30]. The SSEs can be divided into solid polymer electrolytes (SPEs) [31][32] and inorganic ceramic electrolytes (ICEs) [33]. Frustratingly, the high crystalline of SSEs at room temperature will lead to ionic diffusion kinetics, low ionic conductivity, and low elastic modulus, which further increase the interfacial resistance between electrode and electrolyte, as well as high polarization voltage. On the other hand, most cathode materials of ZIBs require H+ insertion/extraction, which stems from H2O molecules of electrolytes. For ZIB systems, gel-state electrolytes containing amounts of liquid-state electrolytes have attracted extensive attention, such as hydrogel, self-healing gel, gel polymer, and thermos-reversible gel electrolytes. Compared with liquid-state electrolytes, the gel-state electrolytes can confine the activity of water, which can extend the electrochemical stability window and provide good flexibility; whereas, compared with all-solid-state electrolytes, the gel-state electrolyte can offer H+ insertion/extraction during charge/discharge process for high capacity and good cyclic stability.
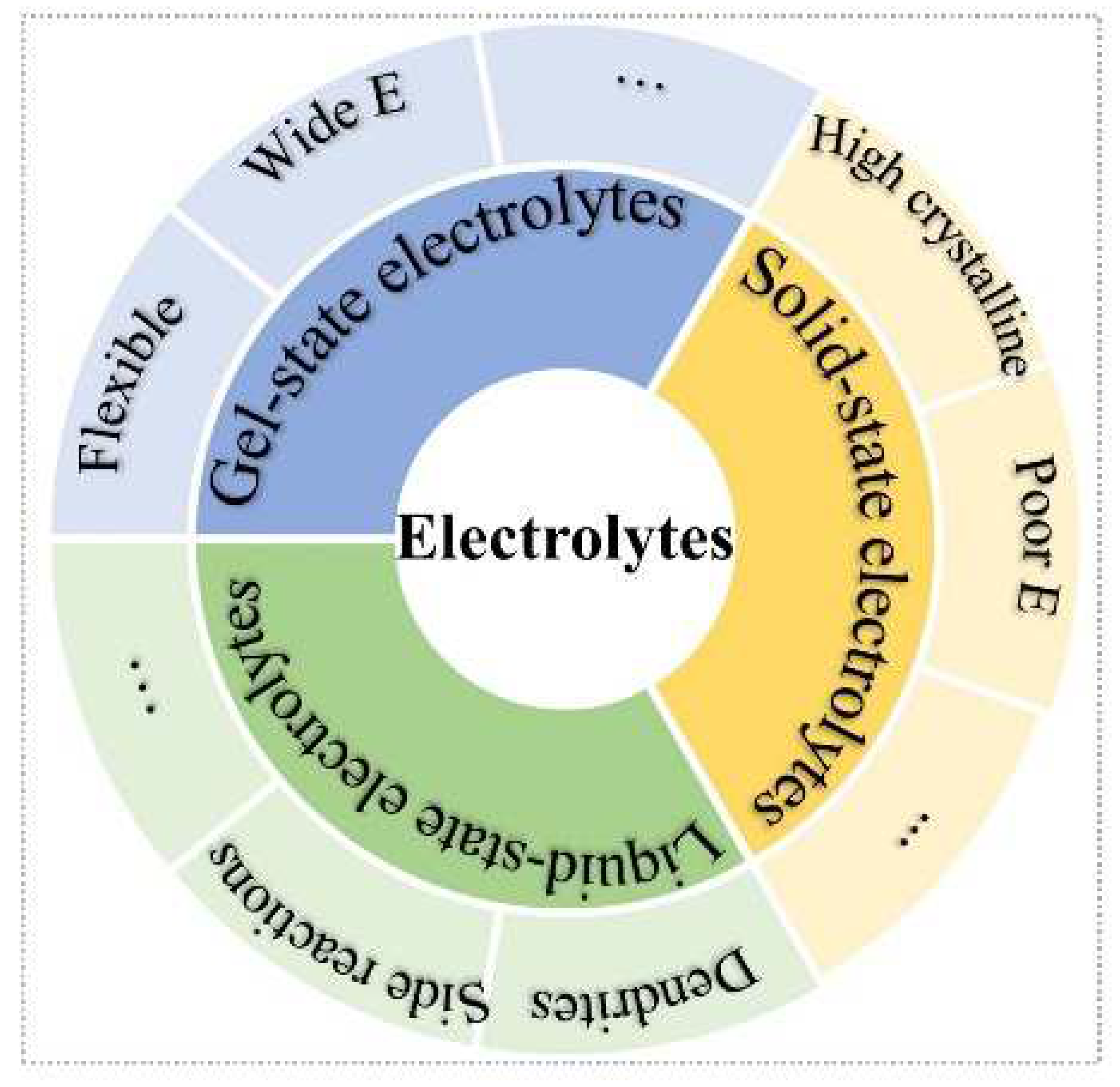
Figure 1. Schematic illustration of three types of electrolytes.
2. Working Principle and Mechanism of Gel-Electrolyte-Based ZIBs
The structure and working principle of ZIBs are similar to those of LIBs, which include three main parts: cathode, anode, and electrolyte. The cathode materials include manganese-based materials, Prussian blue-based materials, vanadium-based materials, organic-based materials, etc., while the anode is metal Zn. After the ZIBs are assembled, there will be a certain potential difference between the two poles of the battery, and a redox reaction will occur between the positive and negative electrodes during the charging and discharging process of the battery. The charge shuttles through the movement of Zn ions between the two electrodes. Upon charging the battery, the Zn2+ ions on the anode side obtains electrons and are reduced to Zn metal, and the inserted Zn2+ ions are extracted from cathode materials. While in the discharging process, the process is completely reversed.
On the other hand, Zn metal easily reacts with the electrolyte to form a solid electrolyte interface (SEI) film on the metal surface, which conducts electricity at the ionic level and insulates at the electronic level. Moreover, the usually formed SEI film is uneven, so that the surface of Zn metal cannot be fully passivated, which eventually leads to continuous side reactions between the Zn metal and the electrolyte. Therefore, it is very important to study the electrolyte of ZIB battery and solve the above problems by ion doping.
3. Gel-State Electrolytes
The gel-state electrolytes with liquid solution contained and good flexibility can play the dual role of electrolyte and separator. They are usually formed by the coagulation of colloidal particles or polymers in a sol or solution under certain conditions. The formation of a spatial network structure enables them to maintain the original structure even with liquid solutions absorbed. Gel-state electrolytes tend to exhibit semi-solid electrolyte characteristics between liquid and solid, which usually have high ionic mobility, mechanical flexibility, light weight, and good adhesion. The superior flexibility and good adhesion properties promote gel-state electrolytes to be used in flexible wearable electronics. However, the commercial application of gel-state-electrolytes-based ZIBs is still in its infancy, so the gel-state electrolytes for ZIBs still need to be continually developed and optimized. High-performance gel-state electrolytes should possess high ionic conductivity, flexibility, mechanical stability, certain electrochemical stability, environmental friendliness, and multifunctional integration. Therefore, the universal gel-state electrolytes —such as polyacrylamide (PAM) [34], poly(ethylene oxide) (PEO) [35], polyvinyl alcohol (PVA) [36], poly(acrylic acid) (PAA) [37], poly(vinyl chloride) (PVC) [38], PCDF, poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) [39], poly(ethylene glycol) diglycidylether (PEGDGE) [40], and tetraethylene glycol diacrylate (TEGDA) [41]—have become a research hotspot for the majority of scientific researchers.
3.1. Hydrogel Electrolytes
The hydrogels are one of the most typical gel-state electrolytes, which could synthesize by crosslinking, dual-network crosslinking, and self-assembly. According to different sources, hydrogels can be divided into synthetic polymer hydrogels and natural polymer hydrogels. For natural polymer hydrogels, they can be directly obtained from nature, which show good biocompatibility and abundant reserves. Discouragingly, they always have poor mechanical properties and instability. In sharp comparison, the synthetic polymer hydrogels have good stability and designability due to controllable physical or chemical procedure utilized. The current synthetic methods for synthesizing hydrogels include physical and chemical monomer crosslinking. When the hydrogel is synthesized through increasing the degree of crosslinking, the modulus and the stretch ability will be increased and decreased, respectively. Additionally, the hydrogel with good solubility to salts and the liquid-like flow ability stem from a superior water absorbency. Nevertheless, physical crosslinking is formed mainly through intermolecular interactions, which will form a cluster structure where ineffective and uniform crosslinking defects exist. Chemical crosslinking, on the other hand, will form certain covalent bonds and possess certain thermal stability.
3.2. Self-Healing Gel Electrolytes
Self-healing, as the magical additional function of the hydrogel, could be realized by introducing reversible weak interactions into electrolytes, such as reversible ionic bonding, covalent bonding, H-bonding, etc. The self-healing functional could not only achieve the ZIBs automatic repair to improve the stability and lifespan but also increase the social benefits and reduce waste of resources. Therefore, developing ZIBs with self-healing and additional functions, such as rechargeability, high-energy, flexibility for wearable applications, and others, may potentially boost the application to electronic devices.
The healable gel electrolyte can be divided into two categories. One is the extrinsic healing, and the other is the intrinsic healing. Regarding the first category, this relies on the preliminarily embedded microcapsules or vascular networks, while its wide application is limited by self-healing times. Interestingly, intrinsic healing occurs by some reversible interactions, such as ionic interactions, hydrogen bonding, and so on. In this way, it can not only realize self-healing function but also enhanced mechanical properties.
3.3. Gel Polymer Electrolytes
Gel polymer electrolyte (GPE) is an electrolyte material that forms a stable gel by a metal salt medium, a solvent, a liquid plasticizer, and a polymer host matrix. The polymer and plasticizer are both continuous phases. The GPE reduces the production safety problems such as electrode corrosion and oxidative combustion caused by the leakage of liquid electrolytes. Up to now, GPEs have become one of the development trends in the commercial application of metal-ion batteries. The phase existence state of the GPE is complex and consists of three phases: crystalline phase, amorphous phase, and liquid phase. The crystalline phase consists of the crystalline part of the polymer, the amorphous phase consists of the amorphous part of the polymer swollen by the plasticizer, and the liquid phase consists of the plasticizer and metal salts in the polymer pores. GPE polymers are in a crosslinked state, similar to hydrogel electrolytes with two ways of physical and chemical crosslinking. The commonly used gel polymers in ZIB batteries include polyvinylidene fluoride (PVDF), vinylidene fluoride hexafluoropropylene copolymer [P(VDFHFP)], PEO, polyacrylonitrile (PAN), polymethacrylic acid Methyl ester (PMMA), and so on. A carboxymethyl cellulose/Zn salts (CMC/ZnSs) complex-based GPE is fabricated by dissolving the Zn salts into CMC with a mass ratio ranging within 0–30 wt% in 1 wt%, in which the Zn salts include zinc acetate (ZnA), zinc sulphate (ZnS), and zinc triflate (ZnT) [42].
3.4. Thermoreversible Gel Electrolytes
Recently, high energy and high-power densities for the batteries have been obstructed by the problem of thermal runaway. Meanwhile, the batteries may cause a series of safety problems in the process of rapid charging and discharging, such as catching fire or exploding. While the application of fusible switches, extinguishing agents, and shut-off current collectors can combat the above issues, these measures do not allow the battery to return to its original state after cooling. Fortunately, this defect can be well-resolved by the application of thermos-reversible gel electrolytes.
Thermoreversible gel electrolytes can be prepared from various polymers and high-temperature solvents by gel casting [43], such as PVDF/dimerhylformamide (DFM), poly(ethylene terephthalate) (PET)/N-methy1-2-pyrrolidinone (NMP), poly(hydroxybutyric acid) (PHBA)/N,N-dimethylacetamide (DMA), and so on. Each of above exhibits excellent ionic conductivity, even at −20 °C (up to 10−3 Scm−1) and storage modulus (approximately 105 Pa) [44]. These electrolytes are usually liquid below room temperature and can quickly transform into solid gels once heated above a critical temperature. Encouragingly, this phase transition is reversible and shows the excellent temperature sensitivity due to their physical entanglement and non-covalent interactions of laterally associating polymer helices in extended junction regions. Therefore, thermos-reversible gel electrolytes may be good candidate material for designing advanced batteries with intelligent thermal loading. The ploy(ethyleneoxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) and poly(N-isopropylacrylamide-acrylamide) (PNIPAM/AM) have the properties of thermos-reversible properties, which were treated as smart materials.
3.5. Additional Functions of Gel-State Electrolytes
3.5.1. Antifreeze Gel Electrolytes
Conventional GPEs inevitably freeze at low temperatures and lose their elasticity, which seriously affects the electrochemical performance of ZIBs and their practical applications in extremely cold environments. Therefore, it is necessary to develop antifreeze GPEs to adapt to low-temperature scenarios [45]. The ideal antifreeze GPE needs to meet the requirements of having a low freezing point, being non-toxic and harmless, and possessing excellent ionic conductivity and mechanical properties. For example, hydrogel electrolytes, as typical gel electrolytes, can be divided into the following three types according to their bound water content: (1) strongly bound water (SBW), (2) weakly bound water, and (3) non-bound water (NBW). As the water will be frozen at 0 °C, the freezing temperature of NBW is 0 °C. However, the SBW have a lower freezing temperature (approximately −100 °C) compared with NBW, due to little free water content. Three strategies are proposed to address the problem of the freezing temperature of gel electrolytes. One of most effective methods to lower the freezing point is introducing salt solutions into electrolytes. This is similar to using sodium chloride (NaCl) to solve the problem of road icing. For example, high-concentration zinc salts (ZnCl2) are mixed with xanthan gum to prepare new quasi-solid-state electrolytes for ZIBs, which could depress its freezing point to −20 °C [46]. On the basis of this electrolyte, the ZIBs exhibits a discharge capacity of 119 mAh g−1 at −20 °C, and maintains 83% of the initial capacity after 450 cycles at 0.5 A g−1 at the same temperature of −20 °C.
3.5.2. Self-Protected Gel Electrolytes
Most batteries could explode under hazardous conditions, such as overpressure and overheating. To dissipate the heat accumulated during battery operation, many studies have adopted physical safety designs, such as fuse circuit breakers and fire-extinguishing agents to prevent safety issues. Unfortunately, these methods only provide one-time protection and cannot achieve reversible recovery. Advanced methods for developing dehydration-resistant gel-state electrolyte and thermally stable energy storage devices have a view to providing new ideas for developing ZIBs at extreme high temperatures. Similar to the method of antifreeze GPEs, this can also be achieved by adding salt and polyol and modifying the network of gel. This method can effectively reduce the presence of free water, which, in turn, reduces the evaporation of free water inside the battery. Introducing inorganic particles into electrolyte is also regarded as an effective approach for reducing dehydration. For example, adding silica (SiO2) into PVA can effectively alleviate free water from dehydration [47]. In addition, a temperature-responsive poly(N-isopropylacrylamide-co-N-methacrylic acid propylene amide) (PNIPAM/NMAM) GPE that is solid at room temperature is designed to avoid liquid leakage [48]. As the temperature increases, hydrogen bonds are gradually formed between the PNIPAM/NMAM molecular chains, which cut off the ion transport channels inside the electrolyte and spread on the surface of the electrolyte. The upper surface changes from hydrophilic to hydrophobic, which limits the movement of free ions. This temperature-responsive GPE can resist thermal runaway and achieve reversible protection.
References
- Wang, S.; Ma, J.; Shi, X.; Zhu, Y.; Wu, Z.S. Recent Status and Future Perspectives of Ultracompact and Customizable Micro-Supercapacitors. Nano Res. Energy 2022, 1, e9120018.
- Li, L.; Chen, H.; He, E.; Wang, L.; Ye, T.; Lu, J.; Jiao, Y.; Wang, J.; Gao, R.; Peng, H.; et al. High-Energy-Density Magnesium-Air Battery Based on Dual-Layer Gel Electrolyte. Angew. Chem. Int. Ed. 2021, 60, 15317.
- Bai, S.; Kim, B.; Kim, C.; Tamwattana, O.; Park, H.; Kim, J.; Lee, D.; Kang, K. Permselective Metal-Organic Framework Gel Membrane Enables Long-Life Cycling of Rechargeable Organic Batteries. Nat. Nanotechnol. 2021, 16, 77.
- Li, Z.; Gadipelli, S.; Li, H.; Howard, C.A.; Brett, D.J.L.; Shearing, P.R.; Guo, Z.; Parkin, I.P.; Li, F. Tuning the Interlayer Spacing of Graphene Laminate Films for Efficient Pore Utilization Towards Compact Capacitive Energy Storage. Nat. Energy 2020, 5, 160.
- Chen, Z.; Zhao, Y.; Mo, F.; Huang, Z.; Li, X.; Wang, D.; Liang, G.; Yang, Q.; Chen, A.; Li, Q.; et al. Metal-Tellurium Batteries: A Rising Energy Storage System. Small Struct. 2020, 1, 2000005.
- Jiang, L.; Lu, Y.; Zhao, C.; Liu, L.; Zhang, J.; Zhang, Q.; Shen, X.; Zhao, J.; Yu, X.; Li, H.; et al. Building Aqueous K-Ion Batteries for Energy Storage. Nat. Energy 2019, 4, 495.
- Zhang, C.; Fei, B.; Yang, D.; Zhan, H.; Wang, J.; Diao, J.; Li, J.; Henkelman, G.; Cai, D.; Biendicho, J.J.; et al. Robust Lithium–Sulfur Batteries Enabled by Highly Conductive Wse2-Based Superlattices with Tunable Interlayer Space. Adv. Funct. Mater. 2022, 32, 2201322.
- Huo, H.; Gao, J.; Zhao, N.; Zhang, D.; Holmes, N.G.; Li, X.; Sun, Y.; Fu, J.; Li, R.; Guo, X.; et al. A Flexible Electron-Blocking Interfacial Shield for Dendrite-Free Solid Lithium Metal Batteries. Nat. Commun. 2021, 12, 176.
- Chang, Z.; Qiao, Y.; Yang, H.; Cao, X.; Zhu, X.; He, P.; Zhou, H. Sustainable Lithium-Metal Battery Achieved by a Safe Electrolyte Based on Recyclable and Low-Cost Molecular Sieve. Angew. Chem. Int. Ed. 2021, 60, 15572.
- Yu, X.; Wang, L.; Ma, J.; Sun, X.; Zhou, X.; Cui, G. Selectively Wetted Rigid–Flexible Coupling Polymer Electrolyte Enabling Superior Stability and Compatibility of High-Voltage Lithium Metal Batteries. Adv. Energ. Mater. 2020, 10, 1903939.
- Liu, Q.; Cai, B.; Li, S.; Yu, Q.; Lv, F.; Kang, F.; Wang, Q.; Li, B. Long-Cycling and Safe Lithium Metal Batteries Enabled by the Synergetic Strategy of Ex Situ Anodic Pretreatment and an in-Built Gel Polymer Electrolyte. J. Mater. Chem. A 2020, 8, 7197.
- Li, L.; Wang, M.; Wang, J.; Ye, F.; Wang, S.; Xu, Y.; Liu, J.; Xu, G.; Zhang, Y.; Zhang, Y.; et al. Asymmetric Gel Polymer Electrolyte with High Lithium Ion Conductivity for Dendrite-Free Lithium Metal Batteries. J. Mater. Chem. A 2020, 8, 8033.
- Chen, Y.; Wang, Z.; Li, X.; Yao, X.; Wang, C.; Li, Y.; Xue, W.; Yu, D.; Kim, S.Y.; Yang, F.; et al. Li Metal Deposition and Stripping in a Solid-State Battery Via Coble Creep. Nature 2020, 578, 251.
- Park, J.H.; Kwak, M.J.; Hwang, C.; Kang, K.N.; Liu, N.; Jang, J.H.; Grzybowski, B.A. Self-Assembling Films of Covalent Organic Frameworks Enable Long-Term, Efficient Cycling of Zinc-Ion Batteries. Adv. Mater. 2021, 33, e2101726.
- Wang, G.; He, P.; Fan, L.Z. Asymmetric Polymer Electrolyte Constructed by Metal–Organic Framework for Solid-State, Dendrite-Free Lithium Metal Battery. Adv. Funct. Mater. 2020, 31, 2007198.
- Liu, C.; Tian, Y.; An, Y.; Yang, Q.; Xiong, S.; Feng, J.; Qian, Y. Robust and Flexible Polymer/Mxene-Derived Two Dimensional Tio2 Hybrid Gel Electrolyte for Dendrite-Free Solid-State Zinc-Ion Batteries. Chem. Eng. J. 2022, 430, 132748.
- Chen, Q.; Zhao, J.; Chen, Z.; Jin, Y.; Chen, J. High Voltage and Self-Healing Zwitterionic Double-Network Hydrogels as Electrolyte for Zinc-Ion Hybrid Supercapacitor/Battery. Int. J. Hydrogen Energ. 2022, 47, 23909.
- Zhang, X.; Li, J.; Liu, D.; Liu, M.; Zhou, T.; Qi, K.; Shi, L.; Zhu, Y.; Qian, Y. Ultra-Long-Life and Highly Reversible Zn Metal Anodes Enabled by a Desolvation and Deanionization Interface Layer. Energ. Environ. Sci. 2021, 14, 3120.
- Yang, H.; Qiao, Y.; Chang, Z.; Deng, H.; Zhu, X.; Zhu, R.; Xiong, Z.; He, P.; Zhou, H. Reducing Water Activity by Zeolite Molecular Sieve Membrane for Long-Life Rechargeable Zinc Battery. Adv. Mater. 2021, 33, e2102415.
- Cao, L.; Li, D.; Deng, T.; Li, Q.; Wang, C. Hydrophobic Organic-Electrolyte-Protected Zinc Anodes for Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2020, 59, 19292.
- Jia, H.; Wang, Z.; Dirican, M.; Qiu, S.; Chan, C.Y.; Fu, S.; Fei, B.; Zhang, X. A Liquid Metal Assisted Dendrite-Free Anode for High-Performance Zn-Ion Batteries. J. Mater. Chem. A 2021, 9, 5597.
- Liu, C.; Lu, Q.; Omar, A.; Mikhailova, D. A Facile Chemical Method Enabling Uniform Zn Deposition for Improved Aqueous Zn-Ion Batteries. Nanomaterials 2021, 11, 764.
- Yang, H.; Qiao, Y.; Chang, Z.; Deng, H.; He, P.; Zhou, H. A Metal-Organic Framework as a Multifunctional Ionic Sieve Membrane for Long-Life Aqueous Zinc-Iodide Batteries. Adv. Mater. 2020, 32, e2004240.
- Mo, F.; Chen, Z.; Liang, G.; Wang, D.; Zhao, Y.; Li, H.; Dong, B.; Zhi, C. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-Mno2 Batteries with Superior Rate Capabilities. Adv. Energ. Mater. 2020, 10, 2000035.
- Pei, Z. Symmetric Is Nonidentical: Operation History Matters for Zn Metal Anode. Nano Res. Energy 2022, 1, e9120023.
- Guo, S.; Qin, L.; Zhang, T.; Zhou, M.; Zhou, J.; Fang, G.; Liang, S. Fundamentals and Perspectives of Electrolyte Additives for Aqueous Zinc-Ion Batteries. Energy Storage Mater. 2021, 34, 545.
- Yan, H.; Li, S.; Nan, Y.; Yang, S.; Li, B. Ultrafast Zinc–Ion–Conductor Interface toward High-Rate and Stable Zinc Metal Batteries. Adv. Energy Mate. 2021, 11, 2100186.
- Guo, J.; Zheng, J.; Zhang, W.; Lu, Y. Recent Advances of Composite Solid-State Electrolytes for Lithium-Based Batteries. Energ. Fuel. 2021, 35, 11118.
- Ma, L.; Chen, S.; Li, X.; Chen, A.; Dong, B.; Zhi, C. Liquid-Free All-Solid-State Zinc Batteries and Encapsulation-Free Flexible Batteries Enabled by in Situ Constructed Polymer Electrolyte. Angew. Chem. Int. Ed. 2020, 59, 23836.
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795.
- Liu, D.; Tang, Z.; Luo, L.; Yang, W.; Liu, Y.; Shen, Z.; Fan, X.H. Self-Healing Solid Polymer Electrolyte with High Ion Conductivity and Super Stretchability for All-Solid Zinc-Ion Batteries. ACS Appl. Mater. Inter. 2021, 13, 36320.
- Liu, Q.; Liu, R.; He, C.; Xia, C.; Guo, W.; Xu, Z.L.; Xia, B.Y. Advanced Polymer-Based Electrolytes in Zinc–Air Batteries. eScience 2022, 2, 453.
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088.
- Wang, D.; Li, H.; Liu, Z.; Tang, Z.; Liang, G.; Mo, F.; Yang, Q.; Ma, L.; Zhi, C. A Nanofibrillated Cellulose/Polyacrylamide Electrolyte-Based Flexible and Sewable High-Performance Zn-MnO2 Battery with Superior Shear Resistance. Small 2018, 14, e1803978.
- Ma, L.; Ying, Y.; Chen, S.; Huang, Z.; Li, X.; Huang, H.; Zhi, C. Electrocatalytic Iodine Reduction Reaction Enabled by Aqueous Zinc-Iodine Battery with Improved Power and Energy Densities. Angew. Chem. Int. Ed. 2021, 60, 3791.
- Velez, A.A.I.; Reyes, E.; Diaz Barrios, A.; Santos, F.; Fernandez Romero, A.J.; Tafur, J.P. Properties of the Pva-Vavtd Koh Blend as a Gel Polymer Electrolyte for Zinc Batteries. Gels 2021, 7, 256.
- Zhang, J.; Huang, Y.; Li, Z.; Gao, C.; Jin, S.; Zhang, S.; Wang, X.; Zhou, H. Polyacrylic Acid Assisted Synthesis of Free-Standing Mno2/CNTs Cathode for Zinc-Ion Batteries. Nanotechnology 2020, 31, 375401.
- Prasanna, C.M.S.; Suthanthiraraj, S.A. Effective Influences of 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl) Imide (Emimtfsi) Ionic Liquid on the Ion Transport Properties of Micro-Porous Zinc-Ion Conducting Poly (Vinyl Chloride) /Poly (Ethyl Methacrylate) Blend-Based Polymer Electrolytes. J. Poly. Res. 2016, 23, 140.
- Chinya, I.; Pal, A.; Sen, S. Flexible, Hybrid Nanogenerator Based on Zinc Ferrite Nanorods Incorporated Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) Nanocomposite for Versatile Mechanical Energy Harvesting. Mater. Res. Bull. 2019, 118, 110515.
- Cunha, R.H.; Nele, M.; Dias, M.L. Reaction and Thermal Behavior of Vitrimer-Like Polyhydroxy Esters Based on Polyethylene Glycol Diglycidyl Ether. J. Appl. Polym. Sci. 2020, 137, 49329.
- Obata, M.; Tanaka, S.; Mizukoshi, H.; Ishihara, E.; Takahashi, M.; Hirohara, S. Raft Synthesis of Polystyrene-Block-Poly(Polyethylene Glycol Monomethyl Ether Acrylate) for Zinc Phthalocyanine-Loaded Polymeric Micelles as Photodynamic Therapy Photosensitizers. J. Polym. Sci. Pol. Chem. 2018, 56, 560.
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H. High Electrochemical and Mechanical Performance of Zinc Conducting-Based Gel Polymer Electrolytes. Sci. Rep. 2021, 11, 13268.
- Li, X.; Wang, H.; Sun, X.; Li, J.; Liu, Y.N. Flexible Wide-Temperature Zinc-Ion Battery Enabled by an Ethylene Glycol-Based Organohydrogel Electrolyte. ACS Appl. Energ. Mater. 2021, 4, 12718.
- Voice, A.M.; Southall, J.P.; Rogers, V.; Matthews, K.H.; Davies, G.R.; Mclntyre, J.E.; Ward, I.M. Thermoreversible Polymer Gel Electrolytes. Polymer Vol. 1994, 35, 3363.
- Quan, Y.; Chen, M.; Zhou, W.; Tian, Q.; Chen, J. High-Performance Anti-Freezing Flexible Zn-Mno2 Battery Based on Polyacrylamide/Graphene Oxide/Ethylene Glycol Gel Electrolyte. Front Chem. 2020, 8, 603.
- Chen, Y.; Zhao, J.; Wang, Y. Quasi-Solid-State Zinc Ion Rechargeable Batteries for Subzero Temperature Applications. ACS Appl. Energ. Mater. 2020, 3, 9058.
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous Nanocomposite Gel Polymer Electrolyte with High Ionic Conductivity and Superior Electrolyte Retention Capability for Long-Cycle-Life Flexible Zinc–Air Batteries. Nano Energy 2019, 56, 454.
- Zhang, H.; Xue, P.; Liu, J.; Xu, X. Thermal-Switching and Repeatable Self-Protective Hydrogel Polyelectrolytes for Energy Storage Applications of Flexible Electronics. ACS Appl. Energ. Mater. 2021, 4, 6116.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
08 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




