Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Longtao Ma and Version 2 by Lindsay Dong.
Zinc-ion batteries (ZIBs) are receiving increasing research attention due to their high energy density, resource abundance, low-cost, intrinsic high-safety properties, and the appropriate plating/stripping voltage. Gel-state electrolytes possess merits of having a wide electrochemical window, good flexibility, superior water retainability, and excellent compatibility with aqueous electrolytes, which makes them potential candidates for flexible batteries. However, the practical applications of ZIBs with gel-state electrolytes still have some issues of water content easily dropping, poor mechanical stability, and the interface problem.
- gel-state electrolytes
- zinc-ion batteries
- self-healing
1. Introduction
In pursuit of rechargeable flexible/wearable battery for grid-scale electrochemical energy storage and conversion systems, metal-ion batteries are drawing much attention [1][2][3][4][5][6][1,2,3,4,5,6]. As the most extreme application of electrical energy storage devices, lithium-ion batteries (LIBs) [7][8][9][10][11][12][13][7,8,9,10,11,12,13] not only have the high energy density, but also show long charge/discharge life cycles [14]. Nevertheless, some inherent issues hinder its widespread application, such as the safety problems, lithium metal extrusion, dendrite growth, and the high cost, together with the limited resources of lithium metal [15]. The zinc-ion battery (ZIB) [16][17][18][19][20][21][16,17,18,19,20,21], one of the next-generation batteries, is a promising candidate to circumvent some of the above problems, due to their high theoretical capacity, low cost, high abundance, low potential, high energy density, and intrinsic safety. In addition, batteries are mainly composed of electrodes, electrolytes, and current collectors, which holistically determine battery performance. The environment for metal-ion transfers between the two electrode terminals in the battery is provided by the electrolytes, which also judges the electrochemical window, ionic conductivity, and the reversibility of the zinc plating/stripping [22].
Three main types of electrolytes are included in batteries: liquid-state, solid-state, and gel-state (Figure 1). The aqueous ZIBs with liquid-state electrolytes are usually considered as ultra-intrinsic batteries [23][24][23,24]. Nevertheless, various side reactions of dendrite growth [25], oxygen evolution reaction, hydrogen evolution reaction, [26], and metal Zn corrosion and passivation [27] impede Zn-ion batteries’ application. However, solid-state electrolytes (SSEs) are an effective strategy to free one from the above issues [28][29][28,29]. Meanwhile, the high security, excellent flexibility, good mechanical stability, and no risk of electrolyte leakage merits of SSEs show enormous potential to solve the problems of liquid-state-solution-based batteries [30]. The SSEs can be divided into solid polymer electrolytes (SPEs) [31][32][31,32] and inorganic ceramic electrolytes (ICEs) [33]. Frustratingly, the high crystalline of SSEs at room temperature will lead to ionic diffusion kinetics, low ionic conductivity, and low elastic modulus, which further increase the interfacial resistance between electrode and electrolyte, as well as high polarization voltage. On the other hand, most cathode materials of ZIBs require H+ insertion/extraction, which stems from H2O molecules of electrolytes. For ZIB systems, gel-state electrolytes containing amounts of liquid-state electrolytes have attracted extensive attention, such as hydrogel, self-healing gel, gel polymer, and thermos-reversible gel electrolytes. Compared with liquid-state electrolytes, the gel-state electrolytes can confine the activity of water, which can extend the electrochemical stability window and provide good flexibility; whereas, compared with all-solid-state electrolytes, the gel-state electrolyte can offer H+ insertion/extraction during charge/discharge process for high capacity and good cyclic stability.
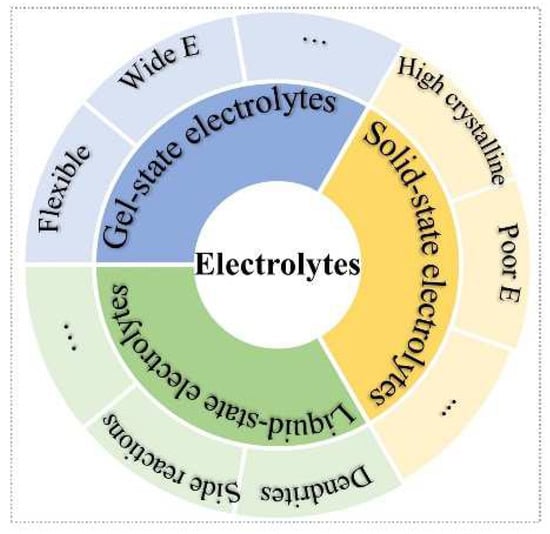

Figure 1.
Schematic illustration of three types of electrolytes.
2. Working Principle and Mechanism of Gel-Electrolyte-Based ZIBs
The structure and working principle of ZIBs are similar to those of LIBs, which include three main parts: cathode, anode, and electrolyte. The cathode materials include manganese-based materials, Prussian blue-based materials, vanadium-based materials, organic-based materials, etc., while the anode is metal Zn. After the ZIBs are assembled, there will be a certain potential difference between the two poles of the battery, and a redox reaction will occur between the positive and negative electrodes during the charging and discharging process of the battery. The charge shuttles through the movement of Zn ions between the two electrodes. Upon charging the battery, the Zn2+ ions on the anode side obtains electrons and are reduced to Zn metal, and the inserted Zn2+ ions are extracted from cathode materials. While in the discharging process, the process is completely reversed.
On the other hand, Zn metal easily reacts with the electrolyte to form a solid electrolyte interface (SEI) film on the metal surface, which conducts electricity at the ionic level and insulates at the electronic level. Moreover, the usually formed SEI film is uneven, so that the surface of Zn metal cannot be fully passivated, which eventually leads to continuous side reactions between the Zn metal and the electrolyte. Therefore, it is very important to study the electrolyte of ZIB battery and solve the above problems by ion doping.
