
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberta Modica | -- | 2004 | 2022-12-07 15:18:38 | | | |
| 2 | Lindsay Dong | -5 word(s) | 1999 | 2022-12-08 02:30:07 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1999 | 2022-12-09 09:43:41 | | |
Video Upload Options
Lipid metabolism is known to be involved in tumorigenesis and disease progression in many common cancer types, including colon, lung, breast and prostate, through modifications of lipid synthesis, storage and catabolism. Furthermore, lipid alterations may arise as a consequence of cancer treatment and may have a role in treatment resistance. Neuroendocrine neoplasms (NENs) are a heterogeneous group of malignancies with increasing incidence, whose mechanisms of cancer initiation and progression are far from being fully understood. Alterations of lipid metabolism may be common across various cancer types.
1. Introduction
2. Lipid Alterations as a Risk Factor in NENs
The identification of modifiable risk factors for NENs is important not only because of their rising incidence, but also because it may be a common risk factor for the onset of non-neuroendocrine malignancies in the same patient [20][21].
Saturated fat intake has been proposed as a possible explanation for the positive associations between meat intake and small intestinal cancer [22]. A prospective study included 60 patients who received a diagnosis of small intestinal adenocarcinomas and 80 with small bowel NETs, with the aim to evaluate the role of meat and fat intake in relation to cancer development. With an 8-year follow-up period, reported that the risk of small intestine NETs was greater in subjects in the highest compared with the lowest tertile of total fat intake (p trend = 0.03). Furthermore, the analyses of the continuous data confirmed a significant association between saturated fat intake and small bowel NETs. The production of bile acids from cholesterol, capable of inducing DNA damage-releasing reactive oxygen species, has been proposed as one of the potential underlying mechanisms [23].
3. Lipids Alteration in NETs
Despite the increase in knowledge of NENs’ molecular alterations, NEN onset and development are far from being understood [25][26]. Similarly, lipid alterations in NEN development are only partially explored. Neoangiogenesis may be modulated by lipid homeostasis in cancer. In particular, cholesterol metabolism and oxysterols, cholesterol-oxidized products, are known to be involved in tumor metabolism. Actually, oxysterols are capable of increasing tumor growth both directly by promoting tumor cell growth and indirectly by decreasing antitumor immune responses, inhibiting dendritic cell migration toward lymphoid organs [27].
4. Treatment-Related Lipid Alterations in NENs
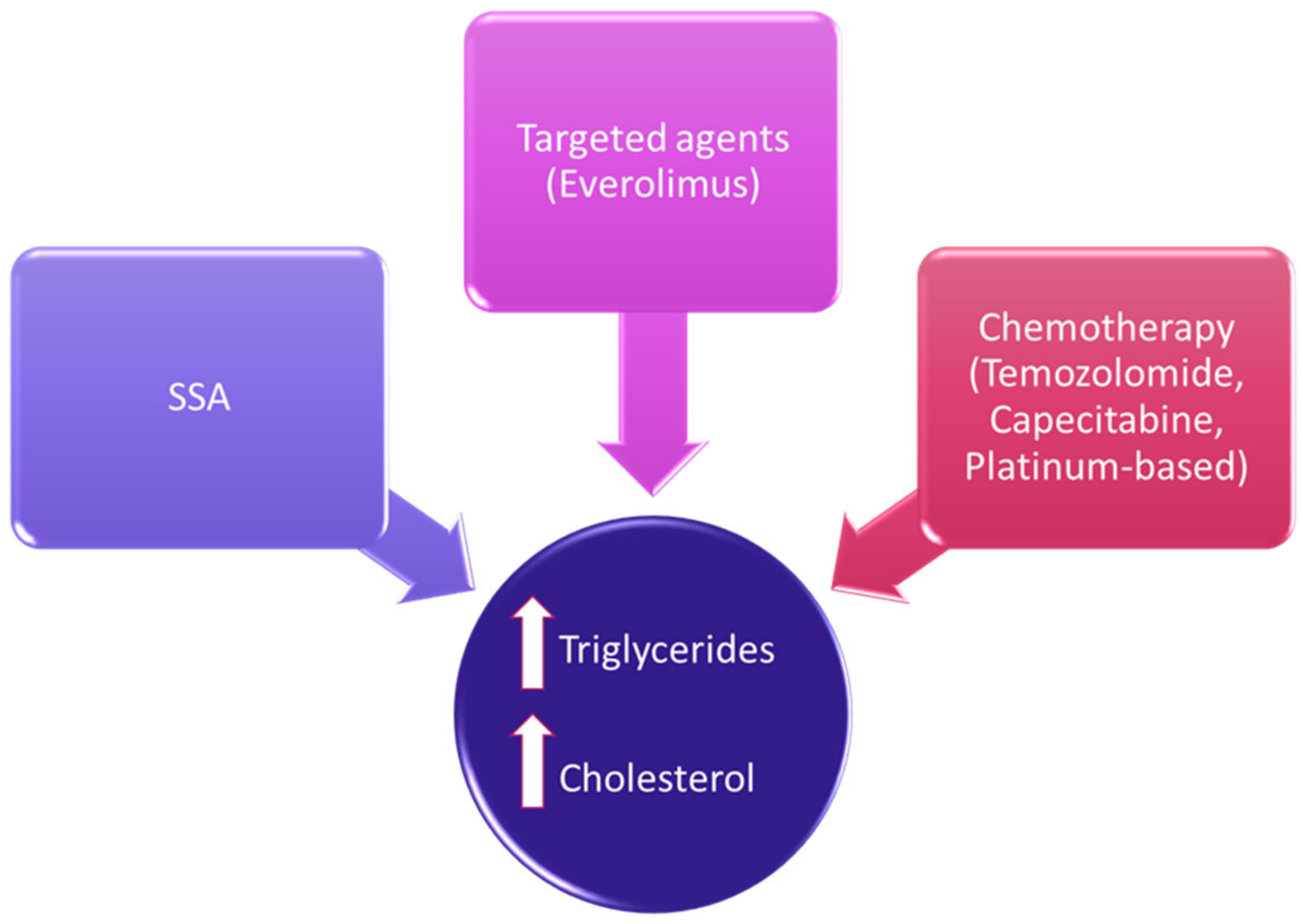
5. Lipids as Target for Anticancer Therapies
The ability of cancer cells to expand their lipidic membrane is crucial for their survival. For this reason, some studies have focused the attention on possible molecular-targeting drugs that could interfere with lipidic membrane stability. An interesting study proposed squalene epoxidase (SQLE), a key rate-limiting enzyme involved in the cholesterol biosynthetic pathway, as a potential therapeutic target in a specific subset of aggressive and difficult to treat NENs, small-cell lung cancer (SCLC).
References
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342.
- Muscogiuri, G.; Altieri, B.; Albertelli, M.; Dotto, A.; Modica, R.; Barrea, L.; Fanciulli, G.; Feola, T.; Baldelli, R.; Ruggeri, R.M.; et al. Epidemiology of pancreatic neuroendocrine neoplasms: A gender perspective. Endocrine 2020, 69, 441–450.
- Ruggeri, R.M.; Benevento, E.; De Cicco, F.; Fazzalari, B.; Guadagno, E.; Hasballa, I.; Tarsitano, M.G.; Isidori, A.M.; Colao, A.; Faggiano, A.; et al. Neuroendocrine neoplasms in the context of inherited tumor syndromes: A reappraisal focused on targeted therapies. J. Endocrinol. Investig. 2022, 1–22.
- Zatelli, M.C.; Guadagno, E.; Messina, E.; lo Calzo, F.; Faggiano, A.; Colao, A.; Albertelli, M.; Bianchi, A.; Circelli, L.; de Cicco, F.; et al. Open issues on G3 neuroendocrine neoplasms: Back to the future. Endocr. Relat. Cancer 2018, 25, R375–R384.
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154.
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686.
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072.
- De Carvalho, C.C.C.R.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583.
- Lydic, T.A.; Goo, Y.-H. Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clin. Transl. Med. 2018, 7, 4.
- Ferro, M.; Terracciano, D.; Buonerba, C.; Lucarelli, G.; Bottero, D.; Perdonà, S.; Autorino, R.; Serino, A.; Cantiello, F.; Damiano, R.; et al. The emerging role of obesity, diet and lipid metabolism in prostate cancer. Future Oncol. 2017, 13, 285–293.
- Tworoger, S.S.; Huang, T. Obesity and Ovarian Cancer; Springer International Publishing: Cham, Switzerland, 2016.
- Hoy, A.J.; Balaban, S.; Saunders, D. Adipocyte–Tumor Cell Metabolic Crosstalk in Breast Cancer. Trends Mol. Med. 2017, 23, 381–392.
- Rysman, E.; Brusselmans, K.; Scheys, K.; Timmermans, L.; Derua, R.; Munck, S.; Van Veldhoven, P.P.; Waltregny, D.; Daniëls, V.W.; Machiels, J.; et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010, 70, 8117–8126.
- De Schrijver, E.; Brusselmans, K.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003, 63, 3799–3804.
- Swinnen, J.V.; Roskams, T.; Joniau, S.; Van Poppel, H.; Oyen, R.; Baert, L.; Heyns, W.; Verhoeven, G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer 2002, 98, 19–22.
- Zhang, J.; Li, Q.; Wu, Y.; Wang, D.; Xu, L.; Zhang, Y.; Wang, S.; Wang, T.; Liu, F.; Zaky, M.Y.; et al. Cholesterol content in cell membrane maintains surface levels of ErbB2 and confers a therapeutic vulnerability in ErbB2-positive breast cancer. Cell Commun. Signal. 2019, 17, 15.
- Li, Y.C.; Park, M.J.; Ye, S.-K.; Kim, C.-W.; Kim, Y.-N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006, 168, 1107–1118.
- Pike, L.J. Growth factor receptors, lipid rafts and caveolae: An evolving story. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2005, 1746, 260–273.
- Casaletto, J.B.; McClatchey, A.I. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 2012, 12, 387–400.
- Massironi, S.; Campana, D.; Pusceddu, S.; Albertelli, M.; Faggiano, A.; Panzuto, F.; Smiroldo, V.; Andreasi, V.; Rossi, R.; Maggio, I.; et al. Second primary neoplasms in patients with lung and gastroenteropancreatic neuroendocrine neoplasms: Data from a retrospective multi-centric study. Dig. Liver Dis. 2021, 53, 367–374.
- Canu, L.; Puglisi, S.; Berchialla, P.; De Filpo, G.; Brignardello, F.; Schiavi, F.; Ferrara, A.M.; Zovato, S.; Luconi, M.; Pia, A.; et al. A multicenter epidemiological study on second malignancy in non-syndromic pheochromocytoma/paraganglioma patients in Italy. Cancers 2021, 13, 5831.
- Wu, A.H.; Yu, M.C.; Mack, T.M. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int. J. Cancer 1997, 70, 512–517.
- Cross, A.J.; Leitzmann, M.F.; Subar, A.F.; Thompson, F.E.; Hollenbeck, A.R.; Schatzkin, A. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res. 2008, 68, 9274–9279.
- Good, M.L.; Malekzadeh, P.; Ruff, S.M.; Gupta, S.; Copeland, A.; Pacak, K.; Nilubol, N.; Kebebew, E.; Patel, D. Surgical Resection of Pheochromocytomas and Paragangliomas is Associated with Lower Cholesterol Levels. World J. Surg. 2020, 44, 552–560.
- Colao, A.; De Nigris, F.; Modica, R.; Napoli, C. Clinical Epigenetics of Neuroendocrine Tumors: The Road Ahead. Front. Endocrinol. 2020, 11, 604341, PMCID:PMC7770585.
- Melone, V.; Salvati, A.; Palumbo, D.; Giurato, G.; Nassa, G.; Rizzo, F.; Palo, L.; Giordano, A.; Incoronato, M.; Vitale, M.; et al. Identification of functional pathways and molecular signatures in neuroendocrine neoplasms by multi-omics analysis. J. Transl. Med. 2022, 20, 306.
- Raccosta, L.; Fontana, R.; Maggioni, D.; Lanterna, C.; Villablanca, E.; Paniccia, A.; Musumeci, A.; Chiricozzi, E.; Trincavelli, M.L.; Daniele, S.; et al. The oxysterol-cxcr2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med. 2013, 210, 1711–1728.
- Soncini, M.; Corna, G.; Moresco, M.; Coltella, N.; Restuccia, U.; Maggioni, D.; Raccosta, L.; Lin, C.-Y.; Invernizzi, F.; Crocchiolo, R.; et al. 24-hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc. Natl. Acad. Sci. USA 2016, 113, E6219–E6227.
- Sadanandam, A.; Wullschleger, S.; Lyssiotis, C.A.; Grötzinger, C.; Barbi, S.; Bersani, S.; Körner, J.; Wafy, I.; Mafficini, A.; Lawlor, R.T.; et al. A cross-species analysis in pancreatic neuroendocrine tumors reveals molecular subtypes with distinctive clinical, metastatic, developmental, and metabolic characteristics. Cancer Discov. 2015, 5, 1296–1313.
- Pereira, S.S.; Pereira, R.; Santos, A.P.; Costa, M.M.; Morais, T.; Sampaio, P.; Machado, B.; Afonso, L.P.; Henrique, R.; Monteiro, M.P. Higher IL-6 peri-tumoural expression is associated with gastro-intestinal neuroendocrine tumour progression. Pathology 2019, 51, 593–599.
- López-López, Á.; Godzien, J.; Soldevilla, B.; Gradillas, A.; López-Gonzálvez, Á.; Lens-Pardo, A.; La Salvia, A.; Riesco-Martínez, M.D.C.; García-Carbonero, R.; Barbas, C. Oxidized lipids in the metabolic profiling of neuroendocrine tumors—Analytical challenges and biological implications. J. Chromatogr. A 2020, 1625, 461233.
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Investig. 2010, 120, 142–156.
- Santos, C.R.; Schulze, A.; Claudio Santos, C.R. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623.
- Martín-Sierra, C.; Laranjeira, P.; Domingues, M.; Paiva, A. Lipoxidation and cancer immunity. Redox Biol. 2019, 23, 101103.
- Faggiano, A.; Calzo, F.L.; Pizza, G.; Modica, R.; Colao, A. The safety of available treatments options for neuroendocrine tumors. Expert Opin. Drug Saf. 2017, 16, 1149–1161.
- Lamberti, G.; Faggiano, A.; Brighi, N.; Tafuto, S.; Ibrahim, T.; Brizzi, M.P.; Pusceddu, S.; Albertelli, M.; Massironi, S.; Panzuto, F.; et al. Nonconventional Doses of Somatostatin Analogs in Patients with Progressing Well-Differentiated Neuroendocrine Tumor. J. Clin. Endocrinol. Metab. 2020, 105, 194–200.
- Filippatos, T.; Liberopoulos, E.; Pavlidis, N.; Elisaf, M.; Mikhailidis, D. Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat. Rev. 2009, 35, 175–184.
- Caron, P.J.; Petersenn, S.; Houchard, A.; Sert, C.; Bevan, J.S. Glucose and lipid levels with lanreotide autogel 120 mg in treatment-naïve patients with acromegaly: Data from the PRIMARYS study. Clin. Endocrinol. 2017, 86, 541–551.
- Niederle, B.; Pape, U.-F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology 2016, 103, 125–138.
- Oberg, K.; Norheim, I.; Lind, E.; Alm, G.; Lundqvist, G.; Wide, L.; Jonsdottir, B.; Magnusson, A.; Wilander, E. Treatment of malignant carcinoid tumors with human leukocyte interferon: Long-term results. Cancer Treat. Rep. 1986, 70, 1297–1304.
- Ruiz-Moreno, M.; Carreño, V.; Rúa, M.; Cotonat, T.; Serrano, B.; Santos, M.; Marriott, E. Increase in triglycerides during alpha-interferon treatment of chronic viral hepatitis. J. Hepatol. 1992, 16, 384.
- Laplante, M.; Sabatini, D.M. An Emerging Role of mTOR in Lipid Biosynthesis. Curr. Biol. 2009, 19, R1046–R1052.
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513.
- Sharma, M.; Tuaine, J.; McLaren, B.; Waters, D.L.; Black, K.; Jones, L.M.; McCormick, S.P.A. Chemotherapy agents alter plasma lipids in breast cancer patients and show differential effects on lipid metabolism genes in liver cells. PLoS ONE 2016, 11, e0148049.
- He, T.; Wang, C.; Tan, Q.; Wang, Z.; Li, J.; Chen, T.; Cui, K.; Wu, Y.; Sun, J.; Zheng, D.; et al. Adjuvant chemotherapy-associated lipid changes in breast cancer patients: A real-word retrospective analysis. Medicine 2020, 99, e21498.
- Caragher, S.; Miska, J.; Shireman, J.; Park, C.H.; Muroski, M.; Lesniak, M.S.; Ahmed, A.U. Temozolomide treatment increases fatty acid uptake in glioblastoma stem cells. Cancers 2020, 12, 3126.
- Uche, A.; Vankina, R.; Gong, J.; Cho, M.; Yeh, J.J.; Kim, P.; Pan, K. Capecitabine-induced hypertriglyceridemia: A rare but clinically relevant treatment-related adverse event. J. Gastrointest. Oncol. 2018, 9, 1213–1219.
- Koufaki, M.; Polychroniou, V.; Calogeropoulou, T.; Tsotinis, A.; Drees, M.; Fiebig, H.H.; LeClerc, S.; Hendriks, A.H.R.; Makriyannis, A. Alkyl and alkoxyethyl antineoplastic phospholipids. J. Med. Chem. 1996, 39, 2609–2614.
- Gallo, M.; NIKE Group; Guarnotta, V.; De Cicco, F.; Rubino, M.; Faggiano, A.; Colao, A. Immune checkpoint blockade for Merkel cell carcinoma: Actual findings and unanswered questions. J. Cancer Res. Clin. Oncol. 2019, 145, 429–443.
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655.




