Lipid metabolism is known to be involved in tumorigenesis and disease progression in many common cancer types, including colon, lung, breast and prostate, through modifications of lipid synthesis, storage and catabolism. Furthermore, lipid alterations may arise as a consequence of cancer treatment and may have a role in treatment resistance. Neuroendocrine neoplasms (NENs) are a heterogeneous group of malignancies with increasing incidence, whose mechanisms of cancer initiation and progression are far from being fully understood. Alterations of lipid metabolism may be common across various cancer types.
- lipid metabolism
- cholesterol
- metabolic syndrome
- neuroendocrine neoplasm
1. Introduction
2. Lipid Alterations as a Risk Factor in NENs
The identification of modifiable risk factors for NENs is important not only because of their rising incidence, but also because it may be a common risk factor for the onset of non-neuroendocrine malignancies in the same patient [20][21][42,43].
Saturated fat intake has been proposed as a possible explanation for the positive associations between meat intake and small intestinal cancer [22][44]. A prospective study included 60 patients who received a diagnosis of small intestinal adenocarcinomas and 80 with small bowel NETs, with the aim to evaluate the role of meat and fat intake in relation to cancer development. WThis study, with an 8-year follow-up period, reported that the risk of small intestine NETs was greater in subjects in the highest compared with the lowest tertile of total fat intake (p trend = 0.03). Furthermore, the analyses of the continuous data confirmed a significant association between saturated fat intake and small bowel NETs. The production of bile acids from cholesterol, capable of inducing DNA damage-releasing reactive oxygen species, has been proposed as one of the potential underlying mechanisms [23][45].
Evaluation of lipid profiles was also investigated in patients with pheochromocytomas or paragangliomas (PPGLs). Indeed, hyperlipidemia in PPGLs patients may be partially related to an excess of catecholamines, and the occurrence of other malignancies has been reported [21][43]. The correlation between catecholamines and lipid levels was evaluated in a retrospective study enrolling 54 patients before and after surgery for PPGLs [24][49]. The prevalence of hyperlipidemia in these patients was 46%. On multivariate analysis, preoperative lipid levels were not significantly associated with any variable, including preoperative symptoms, body mass index, tumor location, tumor size, germline mutation or lipid-lowering medication. Preoperative serum and urinary metanephrine levels, elevated in 94.4% of patients, were associated with elevated total cholesterol and LDL levels. Interestingly, surgical resection of PPGLs was associated with an improvement in both total cholesterol, HDL and LDL levels. These findings may support a relationship between catecholamine excess and hyperlipidemia, although lipid alterations do not seem to represent a risk factor. Nevertheless, screening and monitoring of the lipid profile in PPGL patients seems appropriate [24][49]. The evaluation of lipid alterations as risk factors in NENs is mainly retrospective and focused on GEP-NENs in the context of metabolic syndrome. Nevertheless, the relevance of the lipid profile in NEN patients highlights the importance of evaluating the lipid profile, even when diagnosis is suspected.3. Lipids Alteration in NETs
Despite the increase in knowledge of NENs’ molecular alterations, NEN onset and development are far from being understood [25][26][50,51]. Similarly, lipid alterations in NEN development are only partially explored. Neoangiogenesis may be modulated by lipid homeostasis in cancer. In particular, cholesterol metabolism and oxysterols, cholesterol-oxidized products, are known to be involved in tumor metabolism. Actually, oxysterols are capable of increasing tumor growth both directly by promoting tumor cell growth and indirectly by decreasing antitumor immune responses, inhibiting dendritic cell migration toward lymphoid organs [27][52].
Soncini et al. investigated whether the neutrophil-dependent angiogenic switch occurring during pNET formation was dependent on oxysterols. TIn this study, the scholaauthors analyzed the role of oxysterols in the spontaneous development of pNETs in RIP1-Tag2 mice, demonstrating an upregulation of the Cyp46a1 enzyme. This leads to an increased synthesis of the oxysterol 24S-HC in pancreatic islets. In addition, the oxysterol 24S-HC accumulation leads to the positioning of neutrophils close to hypoxic regions that require formation of neo-vessels, with the direct induction of neoangiogenesis. The oxysterol accumulation has possible therapeutic consequences: in fact, in this experiment, the RIP1-Tag2 mice treated with the squalene synthase inhibitor showed a significant reduction in proangiogenic neutrophils and angiogenic islets, confirming the previously described link between cholesterol metabolism and neutrophil-mediated angiogenesis [28][53]. Moreover, comparing the RIP1-Tag2-engineered mouse model of pNETs and their cognate human cancer, a similar transcriptomic profile has been described [29][54]. Cyp46a1 is overexpressed in some human pNET samples, and there is a linear correlation among Cyp46a1, VEGF and tumor diameter in G1 pNET patients. This model could represent a useful tool to test combination therapies of drugs currently used in pNET patients and cholesterol-lowering compounds endowed with a well-established pharmacologic profile (e.g., statins). On the other side, HDL cholesterol could have an indirect protective role on tumor progression, as supposed by Pereira et al. They evaluated 39 patients with well-differentiated GEP-NETs, finding that elevated IL-6 expression was associated with disease progression, and IL-6 peritumoral expression was higher in patients with low HDL cholesterol (p = 0.02). Even if a definite correlation between elevated IL-6 expression and low HDL cholesterol could not be demonstrated because of the presence of a cancer-related proinflammatory microenvironment, these data suggest that reducing inflammation could potentially increase HDL cholesterol levels and consequently could improve survival [30][56]. Importantly, low HDL levels could be a marker of inflammation. Furthermore, NETs had higher abundances, compared to a non-cancer control group, of oxidized lysoglycerophospholipids, indicating that these tumors were under significant oxidative stress [31][57]. Oxidized lipids in cancer metabolism and their possible relationship with the immune response open the door to innovative therapeutic possibilities and a deeper understanding of the metabolic wiring of cancer cells [32][33][34][58,59,60].4. Treatment-Related Lipid Alterations in NENs
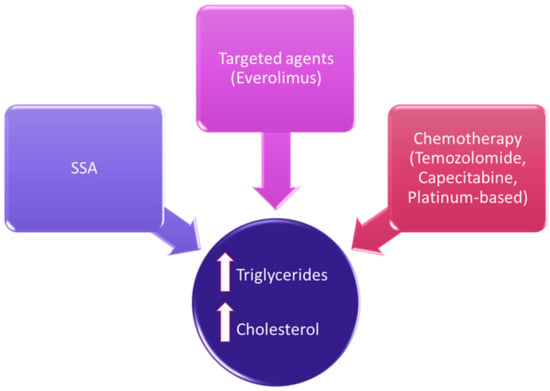
Therapeutic options in NENs have considerably expanded in the last decades, and the evaluation of their safety profile should include lipid homeostasis [35][12]. Besides a direct effect on lipid metabolism, there could be other indirect effects due to metabolic alterations (Figure 1).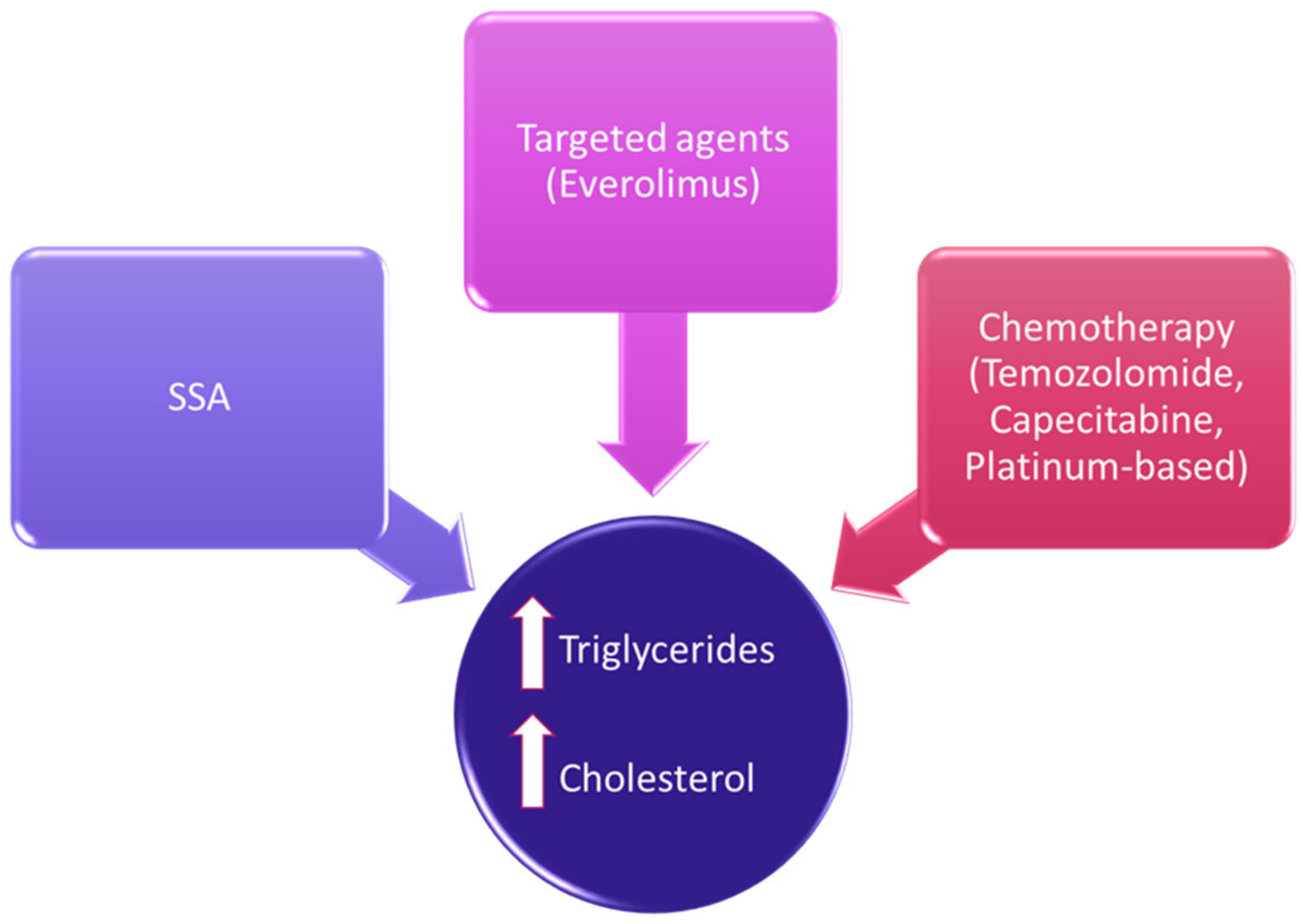
5. Lipids as Target for Anticancer Therapies
The ability of cancer cells to expand their lipidic membrane is crucial for their survival. For this reason, some studies have focused the attention on possible molecular-targeting drugs that could interfere with lipidic membrane stability. An interesting study proposed squalene epoxidase (SQLE), a key rate-limiting enzyme involved in the cholesterol biosynthetic pathway, as a potential therapeutic target in a specific subset of aggressive and difficult to treat NENs, small-cell lung cancer (SCLC).
Moreover, the potential anticancer role of other compounds named alkyl phospholipids, has been spurred forward by recent data [48][79]. Alkyl phospholipids are synthetic analogues of naturally occurring membrane phospholipid esters. These agents have demonstrated significant effectiveness against skin malignancies and several types of hematological malignancies. Lipid metabolism may represent a target, even as a supportive anticancer therapy, as proposed in combination with immune checkpoint inhibitors (ICIs), currently approved drugs even for the treatment of Merkel cell carcinoma, a rare and aggressive cutaneous NEN [49][82]. It has been reported that the anticancer response of CD8+ T cells could be potentiated by modulating cholesterol metabolism [50][83].