
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SACHIN Kumar SINGH | -- | 3424 | 2022-12-07 09:31:22 | | | |
| 2 | Vivi Li | Meta information modification | 3424 | 2022-12-08 03:07:47 | | | | |
| 3 | Vivi Li | -2 word(s) | 3422 | 2022-12-09 09:57:51 | | |
Video Upload Options
Diabetic foot ulcer (DFU) is a multifactorial disease and one of the complications of diabetes. The global burden of DFU in the health sector is increasing at a tremendous rate due to its cost management related to hospitalization, medical costs and foot amputation. Hence, to manage DFU/DWs, various attempts have been made, including treating wounds systematically/topically using synthetic drugs, herbal drugs, or tissue engineering based surgical dressings. However, less attention has been paid to the intrinsic factors that are also the leading cause of diabetes mellitus (DM) and its complications. One such factor is gut dysbiosis, which is one of the major causes of enhancing the counts of Gram-negative bacteria. These bacteria produce lipopolysaccharides, which are a major contributing factor toward insulin resistance and inflammation due to the generation of oxidative stress and immunopathy.
1. Introduction
2. Pathogenesis of Diabetic Wounds
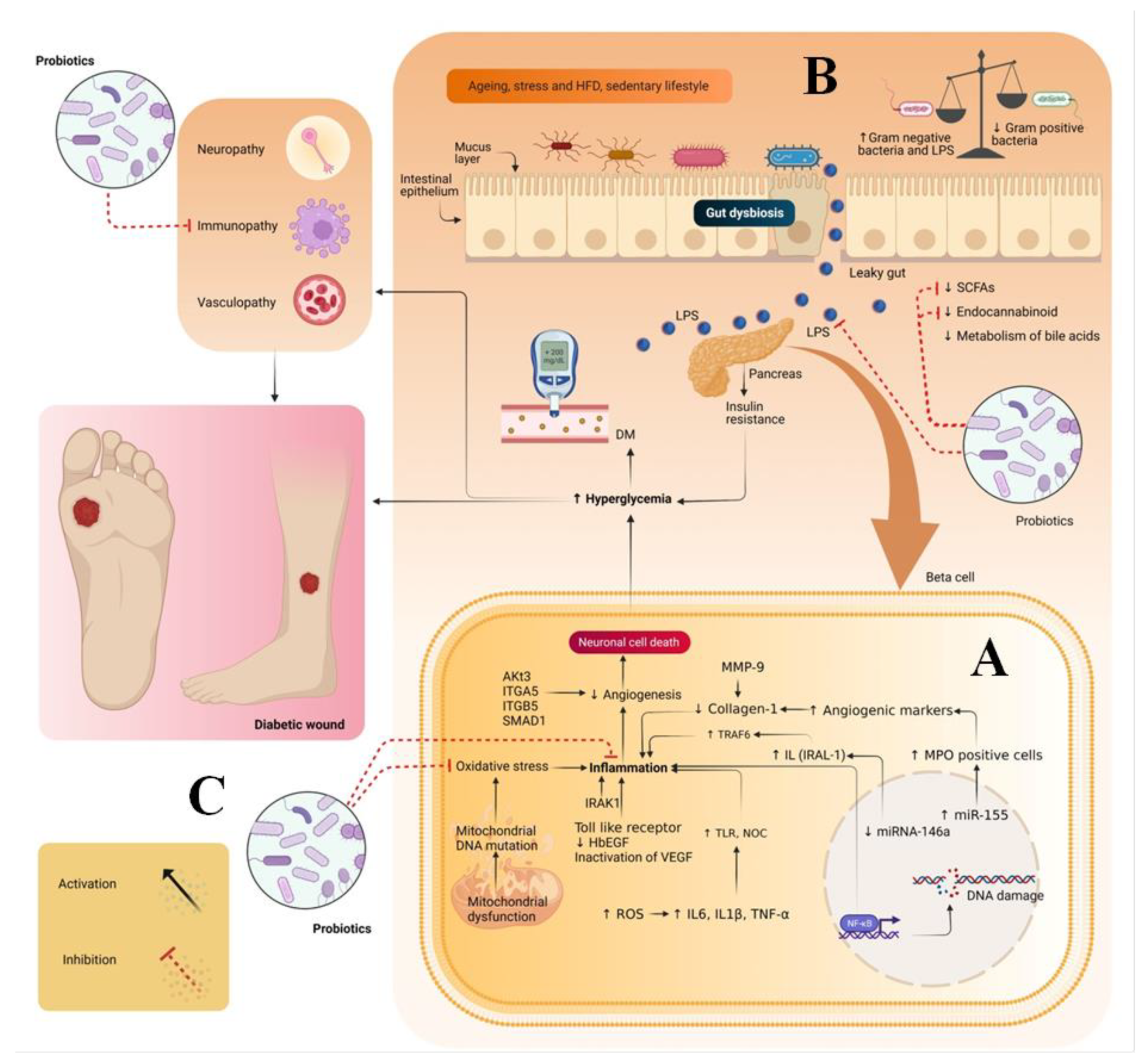
3. Therapeutic Potential of Probiotics in Treating DW
| Probiotic Strain | Assay | Results | References |
|---|---|---|---|
| Antioxidant effect | |||
| Bacillus amyloliquefaciens, Starmerella bombicola, and Lactobacillus brevis |
DPPH, ABTS |
|
[11] |
| Bifidobacterium breve, Rhamnosus GG, Probionebacterium freudenreichii and Lactobacillus retueria, | DPPH, ABTS |
|
[12] |
| BS1, BS2, BV | TAOC, MDA, SOD |
|
[13] |
| Enterococcus faecium | DPPH, Superoxide, Hydroxyl scavenging assay |
|
[14] |
| Lactobacillus acidophilus | DPPH |
|
[6] |
| Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, | DPPH |
|
[15] |
| Lactobacillus plantarum DM5 | DPPH, Superoxide anion, Hydroxyl |
|
[16] |
| Lactobacillus paracasei A-4, Lactobacillus plantarum A-7, Lactobacillus paracasei BL-12, Lactobacillus paracasei DU-8, Lactococcus lactis T-8 | DPPH |
|
[17] |
| Anti-inflammatory | |||
| Probiotic strain | Design/ participants |
Results | References |
| Bifidobacterium animalis ssp. lactis 420 (900 billion CFU/day) |
Randomized/50 |
|
[18] |
| Lactobacillus acidophilus La-5 and Bifidobacterium BB-12 (106 CFU/g each) |
Randomized double-blind/210 |
|
[19] |
| Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus fermentum (2 × 109 CFU/g each) | Randomized double-blind/48 |
|
[20] |
| Lactobacillus acidophilus, Lactobacillus infantis, Bifidobacterium bifidum, Lactobacillus fermentum and Bifidobacterium longum (6 billion CFU each) | Randomized double-blind/ 52 |
|
[21] |
| Lactobacillus plantarum OLL2712 (5 × 109 CFU) | Randomized/ 130 |
|
[22] |
| Immunomodulatory effect | |||
| Probiotics strain | Animal model/other | Results | References |
| Bifidobacterium longum KACC 91563(100 billion CFU/g) | Male BALB/c mice |
|
[23] |
| Bifidobacterium longum CCUG 52486 (5 × 108 CFU/day) | Human |
|
[24] |
| Lactobacillus casei Shirota (1.3 × 1010 CFU/day) | Human |
|
[25] |
| Lactobacillus casei; CRL 431 (109 cells/day) | Female BALB/c mice |
|
[26] |
| Limosilactobacillus fermentum (109 CFU/mL) |
Female Balb/c mice |
|
[27] |
| Antidiabetic effect | |||
| Probiotic strain | Animal model | Results | References |
| Lactobacillus casei (4.0 × 109 CFU/rat/day) |
Rat |
|
[28] |
| Lactobacillus casei and Bifidio bifidum (1 × 107 cfu/mL) | Wistar rat |
|
[29] |
| Lactobacillus.casei (109 CFU/mL) | Mice |
|
[30] |
| Lactobacillus casei CCFM419 (109 CFU) | Mice |
|
[31] |
| Lactobacillus. Gasseri (6 × 107 cfu/g) | Rat |
|
[32] |
| Lactobacillus plantarum CCFM0236 (8 × 109 cfu/mL) | Mice |
|
[33] |
| Lactobacillus.plantarum, strain Ln4 (5 × 108 cfu/day) | Male mice |
|
[34] |
| Lactobacillus.plantarum MTCC5690 and Lactobacillus fermentum MTCC5689 (1.5 × 109 colonies/day) | C57BL/6J male mice |
|
[35] |
| Lactobacillus.rhamnoss, Lactobacillus.acidophilus, Bifidio bifidumi (6 × 108 CFU each) | Mice |
|
[36] |
References
- Awasthi, A.; Gulati, M.; Kumar, B.; Kaur, J.; Vishwas, S.; Khursheed, R.; Porwal, O.; Alam, A.; Kr, A.; Corrie, L.; et al. Review Article Recent Progress in Development of Dressings Used for Diabetic Wounds with Special Emphasis on Scaffolds. BioMed Res. Int. 2022, 2022, 1659338.
- Awasthi, A.; Singh, S.K.; Kumar, B.; Gulati, M.; Kumar, R.; Wadhwa, S.; Khursheed, R.; Corrie, L.; KR, A.; Kumar, R.; et al. Treatment Strategies Against Diabetic Foot Ulcer: Success so Far and the Road Ahead. Curr. Diabetes Rev. 2020, 17, 421–436.
- Awasthi, A.; Vishwas, S.; Gulati, M.; Corrie, L.; Kaur, J.; Khursheed, R.; Alam, A.; Alkhayl, F.F.A.; Khan, F.R.; Nagarethinam, S.; et al. Expanding Arsenal against Diabetic Wounds Using Nanomedicines and Nanomaterials: Success so Far and Bottlenecks. J. Drug Deliv. Sci. Technol. 2022, 74, 103534.
- Yahfoufi, N.; Mallet, J.F.; Graham, E.; Matar, C. Role of Probiotics and Prebiotics in Immunomodulation. Curr. Opin. Food Sci. 2018, 20, 82–91.
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66.
- Tavakoli, M.; Habibi Najafi, M.B.; Mohebbi, M. Effect of the Milk Fat Content and Starter Culture Selection on Proteolysis and Antioxidant Activity of Probiotic Yogurt. Heliyon 2019, 5, e01204.
- Sharma, S.; Singh, A.; Sharma, S.; Kant, A.; Sevda, S.; Taherzadeh, M.J.; Garlapati, V.K. Functional Foods as a Formulation Ingredients in Beverages: Technological Advancements and Constraints. Bioengineered 2021, 12, 11055–11075.
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res. Int. 2018, 2018, 8063647.
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinia, H. Probiotic and Resveratrol Normalize GLP-1 Levels and Oxidative Stress in the Intestine of Diabetic Rats. Metab. Open 2021, 10, 100093.
- Denkova, R.; Goranov, B.; Teneva, D.; Kostov, G. Antimicrobial Activity of Probiotic Microorganisms: Mechanisms of Interaction and Methods of Examination, 1st ed.; Z.-Mendez Villas: Polvdiv, Bulgaria, 2017; pp. 201–212.
- Oh, B.T.; Jeong, S.Y.; Velmurugan, P.; Park, J.H.; Jeong, D.Y. Probiotic-Mediated Blueberry (Vaccinium Corymbosum L.) Fruit Fermentation to Yield Functionalized Products for Augmented Antibacterial and Antioxidant Activity. J. Biosci. Bioeng. 2017, 124, 542–550.
- Helmy, S.A. Chemical Composition and Antimicrobial Activity of Some Essential Oils and Their Major Constituents. Int. J. Acad. Res. 2012, 4, 124–137.
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics Isolated from Yaks Improves the Growth Performance, Antioxidant Activity, and Cytokines Related to Immunity and Inflammation in Mice. Microb. Cell Fact. 2019, 18, 112.
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int. J. Biol. Macromol. 2014, 70, 450–454.
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of Probiotics and Antioxidant Activity of Cashew Milk-Based Yogurt Fermented with Selected Strains of Probiotic lactobacillus Spp. LWT 2022, 153, 112482.
- Das, D.; Goyal, A. Antioxidant Activity and γ-Aminobutyric Acid (GABA) Producing Ability of Probiotic Lactobacillus plantarum DM5 Isolated from Marcha of Sikkim. LWT—Food Sci. Technol. 2015, 61, 263–268.
- Uugantsetseg, E.; Batjargal, B. Antioxidant Activity of Probiotic Lactic Acid Bacteria Isolated from Mongolian Airag. Mong. J. Chem. 2014, 15, 73–78.
- Mäkelä, S.M.; Forssten, S.D.; Kailajärvi, M.; Langén, V.L.; Scheinin, M.; Tiihonen, K.; Ouwehand, A.C. Effects of Bifidobacterium animalis Ssp. Lactis 420 on Gastrointestinal Inflammation Induced by a Nonsteroidal Anti-Inflammatory Drug: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Br. J. Clin. Pharmacol. 2021, 87, 4625–4635.
- Shadnoush, M.; Hosseini, R.S.; Khalilnezhad, A.; Navai, L.; Goudarzi, H.; Vaezjalali, M. Effects of Probiotics on Gut Microbiota in Patients with Inflammatory Bowel Disease: A Double-Blind, Placebo-Controlled Clinical Trial. Korean J. Gastroenterol. 2015, 65, 215–221.
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235.
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A Randomized Double-Blind Placebo-Controlled Trial of Probiotics in Post-Surgical Colorectal Cancer. BMC Gastroenterol. 2019, 19, 131.
- Trial, A.A.R.P. Nutrients Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus Plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients. 2020, 12, 374.
- Choi, M.; Lee, Y.; Lee, N.K.; Bae, C.H.; Park, D.C.; Paik, H.D.; Park, E. Immunomodulatory Effects by Bifidobacterium longum KACC 91563 in Mouse Splenocytes and Macrophages. J. Microbiol. Biotechnol. 2019, 29, 1739–1744.
- Enani, S.M.; Childs, C.E.; Przemska, A.; Maidens, C.; Dong, H.; Rowland, I.; Tuohy, K.; Todd, S.; Gosney, M.; Yaqoob, P. Effects of a Novel Probiotic, Bifidobacterium longum Bv. Infantis CCUG 52486 with Prebiotic on the B-Cell Response to Influenza Vaccination. Proc. Nutr. Soc. 2014, 73, 52486.
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory Effects of a Probiotic Drink Containing Lactobacillus casei Shirota in Healthy Older Volunteers. Eur. J. Nutr. 2013, 52, 1853–1863.
- Villena, J.; Salva, S.; Agüero, G.; Alvarez, S. Immunomodulatory and Protective Effect of Probiotic Lactobacillus casei against Candida Albicans Infection in Malnourished Mice. Microbiol. Immunol. 2011, 55, 434–445.
- D’ambrosio, S.; Ventrone, M.; Fusco, A.; Casillo, A.; Dabous, A.; Cammarota, M.; Corsaro, M.M.; Donnarumma, G.; Schiraldi, C.; Cimini, D. Limosilactobacillus fermentum from Buffalo Milk Is Suitable for Potential Biotechnological Process Development and Inhibits Helicobacter Pylori in a Gastric Epithelial Cell Model. Biotechnol. Rep. 2022, 34, e00732.
- Zhang, Y.; Guo, X.; Guo, J.; He, Q.; Li, H.; Song, Y.; Zhang, H. Lactobacillus casei Reduces Susceptibility to Type 2 Diabetes via Microbiota-Mediated Body Chloride Ion Influx. Sci. Rep. 2014, 4, 5654.
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus casei and Bifidobacterium Bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102.
- Asgharzadeh, F.; Tanomand, A.; Ashoori, M.R.; Asgharzadeh, A.; Zarghami, N. Investigating the Effects of Lactobacillus casei on Some Biochemical Parameters in Diabetic Mice. J. Endocrinol. Metab. Diabetes S. Afr. 2017, 22, 47–50.
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 Attenuates Type 2 Diabetes via a Gut Microbiota Dependent Mechanism. Food Funct. 2017, 8, 3155–3164.
- Niibo, M.; Shirouchi, B.; Umegatani, M.; Morita, Y.; Ogawa, A.; Sakai, F.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 Improves Insulin Secretion in a Diabetic Rat Model. J. Dairy Sci. 2019, 102, 997–1006.
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Jiang, T.; Fang, S.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Effects of Lactobacillus plantarum CCFM0236 on Hyperglycaemia and Insulin Resistance in High-Fat and Streptozotocin-Induced Type 2 Diabetic Mice. J. Appl. Microbiol. 2016, 121, 1727–1736.
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S. Il Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic MRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643.
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in Glucose Tolerance and Insulin Sensitivity by Probiotic Strains of Indian Gut Origin in High-Fat Diet-Fed C57BL/6J Mice. Eur. J. Nutr. 2018, 57, 279–295.
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics Modulate Gut Microbiota and Improve Insulin Sensitivity in DIO Mice. J. Nutr. Biochem. 2017, 50, 16–25.
- Peral, M.C.; Rachid, M.M.; Gobbato, N.M.; Huaman Martinez, M.A.; Valdez, J.C. Interleukin-8 Production by Polymorphonuclear Leukocytes from Patients with Chronic Infected Leg Ulcers Treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 2010, 16, 281–286.
- Ahmadi Majd, S.; Khorasgani, M.R.; Talebi, A. Study of Diabetic Cutaneous Wound Healing in Rats Treated with Lactobacillus casei and Its Exopolysaccharide. Int. J. Adv. Biotechnol. Res. 2016, 7, 2083–2092.
- Mohseni, S.; Bayani, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Bayani, M.A.; Jafari, P.; Asemi, Z. The Beneficial Effects of Probiotic Administration on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes. Metab. Res. Rev. 2018, 34, e2970.
- González, M.P.B.; Quiñones-Gutiérrez, Y. Antibiosis of Cefotaxime/Clindamycin and Lactobacillus acidophilus on Related Bacteria to Diabetic Foot Ulcer. Food Nutr. Sci. 2018, 9, 277–289.




