
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hasan Mehraj | -- | 1341 | 2022-12-07 01:40:20 | | | |
| 2 | Beatrix Zheng | -10 word(s) | 1331 | 2022-12-07 05:25:36 | | |
Video Upload Options
Ornamentals come in a variety of shapes, sizes, and colors to suit a wide range of climates, landscapes, and gardening needs. Compared to demand, a shortage of plant materials and diversity force the search for solutions for their constant acquisition and improvement to increase their commercial value, respectively. In vitro cultures are a suitable solution to meet expectations using callus culture, somatic embryogenesis, protoplast culture, and the organogenesis of protocorm-like bodies; many of these techniques are commercially practiced. Factors such as culture media, explants, carbohydrates, plant growth regulators, and light are associated with the success of in vitro propagation. Techniques, especially embryo rescue and somatic hybridization, are widely used to improve ornamentals. The development of synthetic seed allows season-independent seed production and preservation in the long term. Despite the advantages of propagation and the improvement of ornamentals, many barriers still need to be resolved. In contrast to propagation and crop developmental studies, there is also a high scope for molecular studies, especially epigenetic changes caused by plant tissue culture of ornamentals.
1. Callus Culture
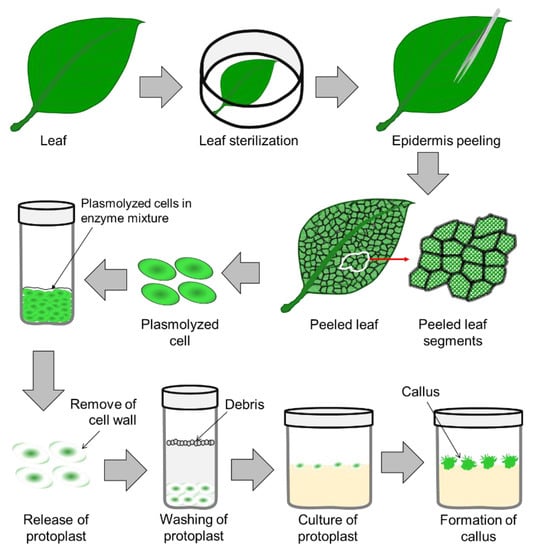
2. Protoplast Culture
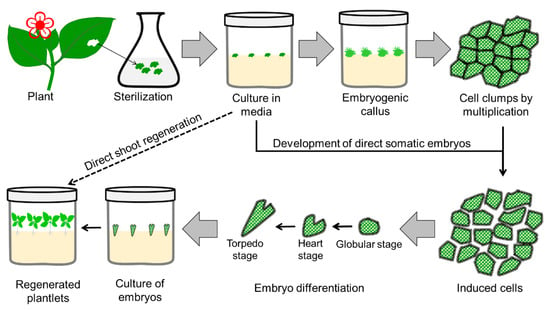
3. Somatic Embryogenesis
4. Protocorm-like Body
References
- Haberlandt, G. Culturversuehe mit isolierten Pflanzenzellen. Sitzungsber. Akad. Wiss. Wien Math. Nat. 1902, 111, 69–92.
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536.
- Bhatia, S. Plant tissue culture. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia., S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 31–107.
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59.
- Naing, A.H.; Adedeji, O.S.; Kim, C.K. Protoplast technology in ornamentals: Current progress and potential applications on genetic improvement. Sci. Hortic. 2021, 283, 110043.
- Thomas, A.; Pujari, I.; Shetty, V.; Joshi, M.B.; Rai, P.S.; Satyamoorthy, K.; Babu, V.S. Dendrobium protoplast co-culture promotes phytochemical assemblage in vitro. Protoplasma 2017, 254, 1517–1528.
- Yousuf, S.; Ashraf, F.; Kazmi, S.K.; Khan, S.; Kayani, H.A. A study on the isolation of protoplasts from the callus of Lilium longiflorum Overig. Pak. J. Bot. 2015, 47, 2391–2396.
- Pati, P.K.; Sharma, M.; Ahuja, P.S. Rose protoplast isolation and culture and heterokaryonselection by immobilization in extra thin alginate film. Protoplasma 2008, 233, 165–171.
- Adedeji, O.S.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived calli of Chrysanthemum cv. White ND. Plant Cell Tissue Organ Cult. 2020, 141, 571–581.
- Kang, H.H.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived callus of Petunia hybrida Cv. Mirage Rose. Biology 2020, 9, 228.
- Shiba, T.; Mii, M. Plant regeneration from mesophyll-and cell suspension-derived protoplasts of Dianthus acicularis and characterization of regenerated plants. Vitr. Cell Dev. Biol. Plant 2005, 41, 794.
- Liqing, Z.; Bochu, W.; Jing, Z.; Lingxi, C.; Chuanyun, D.; Chuanren, D. Protoplast isolation of callus in Echinacea augustifolia. Colloids Surf. B Biointerfaces 2005, 44, 1–5.
- Nassour, M.; Dorion, N. Plant regeneration from protoplasts of micropropagated Pelargonium x hortorum ‘Alain’: Effect of some environmental and medium factors on protoplast system efficiency. Plant Sci. 2002, 163, 169–176.
- Nassour, M.; Chasseriaux, G.; Dorion, N. Optimization of protoplast-to-plant system for Pelargonium× hortorum ‘Alain’ and genetic stability of the regenerated plants. Plant Sci. 2003, 165, 121–128.
- Rahmani, M.S.; Pijut, P.M.; Shabanian, N. Protoplast isolation and genetically true-to-type plant regeneration from leaf-and callus-derived protoplasts of Albizia julibrissin. Plant Cell Tissue Organ Cult. 2016, 127, 475–488.
- Lang, I.; Sassmann, S.; Schmidt, B.; Komis, G. Plasmolysis: Loss of turgor and beyond. Plants 2014, 3, 583–593.
- Pan, Z.G.; Liu, C.Z.; Zobayed, S.M.A.; Saxena, P.K. Plant regeneration from mesophyll protoplasts of Echinacea purpurea. Plant Cell Tissue Organ Cult. 2004, 77, 251–255.
- Zhou, J.; Wang, B.; Zhu, L. Conditioned culture for protoplasts isolated from Chrysanthemum: An efficient approach. Colloids Surf. B Biointerfaces 2005, 45, 113–119.
- Duquenne, B.; Eeckhaut, T.; Werbrouck, S. Effect of enzyme concentrations on protoplast isolation and protoplast culture of Spathiphyllum and Anthurium. Plant Cell Tissue Organ Cult. 2007, 91, 165–173.
- Pongchawee, K.; Na-Nakorn, U.; Lamseejan, S.; Poompuang, S.; Phansiri, S. Factors affecting the protoplast isolation and culture of Anubias nana Engler. Int. J. Bot. 2006, 2, 193–200.
- Meyer, L.; Serek, M.; Winkelmann, T. Protoplast isolation and plant regeneration of different genotypes of Petunia and Calibrachoa. Plant Cell Tissue Organ Cult. 2009, 99, 27–34.
- Li, J.; Liao, X.; Zhou, S.; Liu, S.; Jiang, L.; Wang, G. Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty’. Vitr. Cell Dev. Biol. Plant 2018, 54, 87–93.
- Teo, C.K.H.; Neumann, K.H. The culture of protoplasts isolated from Renantanda Rosalind Cheok. Orchid Rev. 1978, 86, 156–158.
- Teo, C.K.H.; Neumann, K.H. The isolation and hybridization of protoplasts from orchids. Orchid Rev. 1978, 86, 186–189.
- Kobayashi, S.; Kameya, T.; Ichihashi, S. Plant regeneration from protoplasts derived from callus of Phalaenopsis. Plant Tiss. Cult. Lett. 1993, 10, 267–270.
- Kunasakdakul, K.; Smitamana, P. Dendrobium Pratum Red protoplast. Thai J. Agric. Sci. 2003, 36, 1–8.
- Khentry, Y.; Paradornuvat, A.; Tantiwiwat, S.; Phansiri, S.; Thaveechai, N. Protoplast isolation and culture of Dendrobium Sonia “Bom 17”. Kasetsart J. (Nat. Sci.) 2006, 40, 361–369.
- Shrestha, B.R.; Tokuhara, K.; Mii, M. Plant regeneration from cell suspension-derived protoplasts of Phalaenopsis. Plant Cell Rep. 2007, 26, 719–725.
- Tee, C.S.; Lee, P.S.; Kiong, A.L.P.; Mahmood, M. Optimisation of protoplast isolation protocols using in vitro leaves of Dendrobium crumenatum (pigeon orchid). Afr. J. Agric. Res. 2011, 5, 2685–2693.
- Cui, J.; Mackenzie, K.K.; Eeckhaut, T.; Müller, R.; Lütken, H. Protoplast isolation and culture from Kalanchoë species: Optimization of plant growth regulator concentration for efficient callus production. Plant Cell Tissue Organ Cult. 2019, 138, 287–297.
- Furuta, H.; Shinoyama, H.; Nomura, Y.; Maeda, M.; Makara, K. Production of intergeneric somatic hybrids of chrysanthemum and wormwood (Artemisia sieversiana JF Ehrh. ex. Willd) with rust (Puccinia horiana Henning) resistance by electrofusion of protoplasts. Plant Sci. 2004, 166, 695–702.
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708.
- Reinert, J. Über die kontrolle der morphogenese und die induktion von adventivembryonen an gewebekulturen aus karotten. Planta 1959, 53, 318–333.
- Backs-Hüsemann, D.; Reinert, J. Embryobildung durch isolierte Einzelzellen aus Gewebekulturen vonDaucus carota. Protoplasma 1970, 70, 49–60.
- Hossain, M.M.; Kant, R.; Van, P.T.; Winarto, B.; Zeng, S.; Teixeira da Silva, J.A. The application of biotechnology to orchids. Crit. Rev. Plant Sci. 2013, 32, 69–139.
- Mujib, A. Somatic Embryogenesis in Ornamentals and Its Applications; Springer: New Delhi, India, 2016; Volume 267, pp. 1–267.
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; AlcazarMagaña, A.; Wrobel, K.; Loyola-Vargas, V.M. Somatic embryogenesis: Identified factors that lead to embryogenic repression. a case of species of the same genus. PLoS ONE 2015, 10, e0126414.
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Cham, Switzerland, 2018; pp. 1–296.
- Mahendran, G.; Bai, V.N. Direct somatic embryogenesis and plant regeneration from seed derived protocorms of Cymbidium bicolor Lindl. Sci. Hortic. 2012, 135, 40–44.
- Deb, C.R.; Pongener, A. Studies on the in vitro regenerative competence of aerial roots of two horticultural important Cymbidium species. J. Plant Biochem. Biotechnol. 2012, 21, 235–241.
- Chang, C.; Chang, W.C. Plant regeneration from callus of Cymbidium ensifolium var ‘Misericors’. Plant Cell Rep. 1998, 17, 251–255.
- Teixeira da Silva, J.A.; Chan, M.-T.; Sanjaya; Chai, M.-L.; Tanaka, M. Priming abiotic factors for optimal hybrid Cymbidium (Orchidaceae) PLB and callus induction, plantlet formation, and their subsequent cytogenetic stability analysis. Sci. Hortic. 2006, 109, 368–378.
- Teixeira da Silva, J.A.; Singh, N.; Tanaka, M. Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tissue Organ Cult. 2006, 84, 135–144.
- Teixeira da Silva, J.A.; Winarto, B. Somatic embryogenesis in two orchid genera (Cymbidium, Dendrobium). In In Vitro Embryogenesis in Higher Plants. Methods in Molecular Biology; Germana, M., Lambardi, M., Eds.; Humana Press: Totowa, NJ, USA, 2016; Volume 1359, pp. 371–386.
- Ishii, Y.; Takamura, T.; Goi, M.; Tanaka, M. Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep. 1998, 17, 446–450.
- Chen, J.T.; Chang, W.C. Direct somatic embryogenesis and plant regeneration from leaf explants of Phalaenopsis amabilis. Biol. Plant. 2006, 50, 169–173.
- Gow, W.P.; Chen, J.T.; Chang, W.C. Enhancement of direct somatic embryogenesis and plantlet growth from leaf explants of Phalaenopsis by adjusting culture period and explant length. Acta Physiol. Plant. 2010, 32, 621–627.
- Gow, W.P.; Chen, J.T.; Chang, W.C. Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis orchids. Acta Physiol. Plant. 2008, 30, 507–512.
- Gow, W.P.; Chen, J.T.; Chang, W.C. Effects of genotype, light regime, explant position and orientation on direct somatic embryogenesis from leaf explants of Phalaenopsis orchids. Acta Physiol. Plant. 2009, 31, 363–369.
- Niknejad, A.; Kadir, M.A.; Kadzimin, S.B. In vitro plant regeneration from protocorms-like bodies (PLBs) and callus of Phalaenopsis gigantea (Epidendroideae: Orchidaceae). Afr. J. Biotechnol. 2011, 10, 11808–11816.
- Feng, J.H.; Chen, J.T. A novel in vitro protocol for inducing direct somatic embryogenesis in Phalaenopsis aphrodite without taking explants. Sci. World J. 2014, 7, 263642.
- Hong, P.I.; Chen, J.T.; Chang, W.C. Promotion of direct somatic embryogenesis of Oncidium by adjusting carbon sources. Biol. Plant. 2008, 52, 597–600.
- Chen, J.T.; Chang, C.; Chang, W.C. Direct somatic embryogenesis on leaf explants of Oncidium Gower Ramsey and subsequent plant regeneration. Plant Cell Rep. 1999, 19, 143–149.
- Chen, J.T.; Chang, W.C. Effects of tissue culture conditions and explant characteristics on direct somatic embryogenesis in Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult. 2002, 69, 41–44.
- Su, Y.J.; Chen, J.T.; Chang, W.C. Efficient and repetitive production of leaf-derived somatic embryos of Oncidium. Biol. Plant. 2006, 50, 107–110.
- Hong, P.I.; Chen, J.T.; Chang, W.C. Effects of salicylic and acetylsalicylic acid on direct somatic embryogenesis in Oncidium. J. Plant Biochem. Biotechnol. 2008, 17, 149–153.
- Shen, H.J.; Chen, J.T.; Chung, H.H.; Chang, W.C. Plant regeneration via direct somatic embryogenesis from leaf explants of Tolumnia Louise Elmore ‘Elsa’. Bot. Stud. 2018, 59, 4.
- Chung, H.H.; Chen, J.T.; Chang, W.C. Cytokinins induce direct somatic embryogenesis of Dendrobium Chiengmai Pink and subsequent plant regeneration. In Vitro Cell. Dev. Biol. Plant 2005, 41, 765–769.
- Chung, H.H.; Chen, J.T.; Chang, W.C. Plant regeneration through direct somatic embryogenesis from leaf explants of Dendrobium. Biol. Plant. 2007, 51, 346–350.
- Asghar, S.; Ahmad, T.; Hafiz, I.A.; Yaseen, M. In vitro propagation of orchid (Dendrobium nobile) var. Emma White. Afr. J. Biotechnol. 2011, 10, 3097–3103.
- Parthibhan, S.; Rao, M.V.; Teixeira da Silva, J.A.; Kumar, T.S. Somatic embryogenesis from stem thin cell layers of Dendrobium aqueum. Biol. Plant. 2018, 62, 439–450.
- Islam, S.S.; Bhattacharjee, B. Plant regeneration through somatic embryogenesis from leaf and root explants of Rhynchostylis retusa (L.) Blume. Appl. Biol. Res. 2015, 17, 158–165.
- Wu, K.L.; Zeng, S.J.; Teixeira da Silva, J.A.; Chen, Z.L.; Zhang, J.X.; Yang, Y.S.; Duan, J. Efficient regeneration of Renanthera Tom Thumb ‘Qilin’ from leaf explants. Sci. Hortic. 2012, 135, 194–201.
- Hong, P.I.; Chen, J.T.; Chang, W.C. Plant regeneration via protocormlike body formation and shoot multiplication from seed-derived callus of a maudiae type slipper orchid. Acta Physiol. Plant. 2008, 30, 755–759.
- Long, B.; Niemiera, A.X.; Cheng, Z.Y.; Long, C.L. In vitro propagation of four threatened Paphiopedilum species (Orchidaceae). Plant Cell Tissue Organ Cult. 2010, 101, 151–162.
- Cheruvathur, M.K.; Abraham, J.; Mani, B.; Thomas, T.D. Adventitious shoot induction from cultured internodal explants of Malaxis acuminata D. Don, a valuable terrestrial medicinal orchid. Plant Cell Tissue Organ Cult. 2010, 101, 163–170.
- Mahendran, G.; Bai, V.N. Direct somatic embryogenesis of Malaxis densiflora (A. Rich.) Kuntze. J. Genet. Eng. Biotechnol. 2016, 14, 77–81.
- Moradi, S.; Daylami, S.D.; Arab, M.; Vahdati, K. Direct somatic embryogenesis in Epipactis veratrifolia, a temperate terrestrial orchid. J. Hortic. Sci. Biotechnol. 2017, 92, 88–97.
- Manokari, M.; Priyadharshini, S.; Shekhawat, M.S. Direct somatic embryogenesis using leaf explants and short term storage of synseeds in Spathoglottis plicata Blume. Plant Cell Tissue Organ Cult. 2021, 145, 321–331.
- Bhadra, S.K.; Hossain, M.M. In vitro germination and micropropagation of Geodorum densiflorum (Lam.) Schltr., an endangered orchid species. Plant Tissue Cult. 2003, 13, 165–171.
- Sherif, N.A.; Benjamin, J.H.F.; Kumar, T.S.; Rao, M.V. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult. 2018, 132, 303–316.
- Zeng, S.J.; Chen, Z.L.; Wu, K.L.; Bai, C.K.; Zhang, J.X.; Teixeira da Silva, J.A.; Duan, J. Asymbiotic seed germination, induction of calli and protocorm-like bodies, and in vitro seedling development of the rare and endangered Nothodoritis zhejiangensis Chinese orchid. HortScience 2011, 46, 460–465.
- Azadi, P.; Kermani, M.J.; Samiei, L. Somatic embryogenesis in Rosa hybrida. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Jain, S., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; Volume II, pp. 161–170.
- Pati, P.K.; Sharma, M.; Sood, A.; Ahuja, P.S. Direct shoot regeneration from leaf explants of Rosa damascena Mill. Vitr. Cell Dev. Biol. Plant 2004, 40, 192–195.
- Tanaka, K.; Kanno, Y.; Kudo, S.; Suzuki, M. Somatic embryogenesis and plant regeneration in chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura). Plant Cell Rep. 2000, 19, 946–953.
- Teixeira da Silva, J.A.; Lema-Rumińska, J.; Tymoszuk, A.; Kulpa, D. Regeneration from chrysanthemum flowers: A review. Acta Physiol. Plant. 2015, 37, 67–77.
- Khosravi, S.; Azghandi, A.V.; Hadad, R.; Mojtahedi, N. In vitro micrpropagation of Lilium longiflorum. J. Agric. Res. Seed Plant 2007, 23, 159–168.
- Bakhshaie, M.; Babalar, M.; Mirmasoumi, M.; Khalighi, A. Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tissue Organ Cult. 2010, 102, 229–235.
- Zhang, J.; Gai, M.; Li, X.; Li, T.; Sun, H. Somatic embryogenesis and direct as well as indirect organogenesis in Lilium pumilum DC. Fisch., an endangered ornamental and medicinal plant. Biosci. Biotechnol. Biochem. 2016, 80, 1898–1906.
- Fu, L.; Zhu, Y.; Li, M.; Wang, C.; Sun, H. Autopolyploid induction via somatic embryogenesis in Lilium distichum Nakai and Lilium cernuum Komar. Plant Cell Tissue Organ Cult. 2019, 139, 237–248.
- Priyadharshini, S.; Manokari, M.; Shekhawat, M.S. In vitro conservation strategies for the critically endangered Malabar river lily (Crinum malabaricum Lekhak & Yadav) using somatic embryogenesis and synthetic seed production. S. Afr. J. Bot. 2020, 135, 172–180.
- Yan, R.; Sun, Y.; Sun, H. Current status and future perspectives of somatic embryogenesis in Lilium. Plant Cell Tissue Organ Cult. 2020, 143, 229–240.
- de Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191.
- Gaber, M.K.; Barakat, A.A. Micropropagation and somatic embryogenesis induction of Gardenia jasminoides plants. Alex. Sci. Exch. J. 2019, 40, 190–202.
- Yumbla-Orbes, M.; da Cruz, A.C.F.; Pinheiro, M.V.M.; Rocha, D.I.; Batista, D.S.; Koehler, A.D.; Barbosa, J.G.; Otoni, W.C. Somatic embryogenesis and de novo shoot organogenesis can be alternatively induced by reactivating pericycle cells in Lisianthus (Eustoma grandiflorum (Raf.) Shinners) root explants. Vitr. Cell Dev. Biol. Plant 2017, 53, 209–218.
- Yumbla-Orbes, M.; Rocha, D.I.; de Matos, E.M.; Koehler, A.D.; Pinheiro, M.V.M.; Batista, D.S.; Freitas, D.M.S.; da Cruz, A.C.; Barbosa, J.G.; Viccini, L.F.; et al. Somatic embryogenesis induced from vascular tissues in leaf explants of Lisianthus (Eustoma grandiflorum (Raf.) Shinn) generates true-to-type diploid plants. Vegetos 2020, 33, 135–144.
- Nhut, D.T.; Tuan, N.S.; Ngoc, H.M.; Uyen, P.N.; Don, N.T.; Mai, N.T.; Teixeira da Silva, J.A. Somatic embryogenesis induction from in vitro leaf cultures of Lisianthus (Eustoma grandiflorum (Raf.) Shinn.). Propag. Ornam. Plants 2006, 6, 121–127.
- Ruffoni, B.; Bassolino, L. Somatic embryogenesis in Lisianthus (Eustoma russellianum Griseb.). In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology Series; Maria, A.G., Maurizio, L., Eds.; Humana Press: Totowa, NJ, USA, 2016; Volume 1359, Chapter 17; pp. 359–370.
- Iantcheva, A. Somatic embryogenesis and genetic transformation of carnation (Dianthus caryophyllus L.). In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 7; pp. 107–120.
- Vieitez, A.M.; Barciela, J. Somatic embryogenesis and plant regeneration from embryonic tissues of Camellia japonica L. Plant Cell Tissue Organ Cult. 1990, 21, 267–274.
- Ponsamuel, J.; Samson, N.P.; Ganeshan, P.S.; Sathyaprakash, V.; Abraham, G.C. Somatic embryogenesis and plant regeneration from the immature cotyledonary tissues of cultivated tea (Camellia sinensis (L).O. Kuntze). Plant Cell Rep. 1996, 16, 210–214.
- Lü, J.; Chen, R.; Zhang, M.; Teixeira da Silva, J.A.; Ma, G. Plant regeneration via somatic embryogenesis and shoot organogenesis from immature cotyledons of Camellia nitidissima. J. Plant Physiol. 2013, 170, 1202–1211.
- San José, M.C.; Couselo, J.L.; Martínez, M.T.; Mansilla, P.; Corredoira, E. Somatic embryogenesis in Camellia japonica L.: Challenges and future prospects. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 6; pp. 91–105.
- Gladfelter, H.J.; Johnston, J.; Wilde, H.D.; Markle, S.A. Somatic embryogenesis and cryopreservation of Stewartia species. Plant Cell Tissue Organ Cult. 2021, 144, 211–221.
- Sivanesan, I.; Jeong, B.R. Optimizing factors affecting somatic embryogenesis in Cineraria. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 4; pp. 55–65.
- Choffe, K.L.; Victor, J.M.; Muruch, S.J.; Saxena, P.K. In vitro regeneration of Echinacea purpurea L.: Direct somatic embryogenesis and indirect shoot organogenesis in petiole culture. Vitr. Cell Dev. Biol. Plant 2000, 36, 30–36.
- Dehestani-Ardakani, M.; Hejazi, M.; Aliabad, K.K. Indirect somatic embryogenesis of purple coneflower (Echinacea purpurea (L.) Moench): A medicinal-ornamental plant: Evaluation of antioxidant enzymes activity and histological study. Mol. Biol. Rep. 2020, 47, 6621–6633.
- Sivanesan, I.; Son, M.S.; Jana, S.; Jeong, B.R. Secondary somatic embryogenesis in Crocus vernus (L.) Hill. Propag. Ornam. Plants 2012, 12, 163–170.
- Mitrofanova, I.; Ivanova, N.; Kuzmina, T.; Mitrofanova, O.; Zubkova, N. In vitro regeneration of clematis plants in the Nikita Botanical Garden via somatic embryogenesis and organogenesis. Front. Plant Sci. 2021, 12, 541171.
- Verma, S.K.; Das, A.K.; Cingoz, G.S.; Uslu, E.; Gurel, E. Influence of nutrient media on callus induction, somatic embryogenesis and plant regeneration in selected Turkish crocus species. Biotechnol. Rep. 2016, 10, 66–74.
- Sevindik, B.; Mendi, Y.Y. Somatic embryogenesis in Crocus sativus L. In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology Series; Germana, M.A., Lambardi, K., Eds.; Humana Press: Totowa, NJ, USA, 2016; Chapter 16; pp. 351–357.
- Mandegaran, Z.; Sieber, V.K. Somatic embryogenesis in Clematis integrifolia × C. viticella. Plant Cell Tissue Organ Cult. 2000, 62, 163–165.
- Mitrofanova, I.V.; Galaev, A.V.; Sivolap, Y.M. Investigation of molecular-genetic heterogeneity of clematis plants (Clematis L.) obtained by organogenesis and somatic embryogenesis in vitro. Tsitol. Genet. 2003, 37, 12–26.
- Hosoi, Y.; Maruyama, T.E. Somatic embryogenesis in Sawara cypress (Chamaecyparis pisifera Sieb. et Zucc.). In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 6; pp. 41–53.
- Tagipur, M.E.; Seker, G.; Teixeira da Silva, J.A.; Mendi, Y.Y. Somatic embryogenesis, cryopreservation, and in vitro mutagenesis in Cyclamen. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 10; pp. 155–167.
- Sivanesan, I.; Lim, M.Y.; Jeong, B.R. Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. var. rubriflora Makino. Plant Cell Tissue Organ Cult. 2011, 107, 365–369.
- Pipino, L.; Braglia, L.; Giovannini, A.; Fascella, G.; Mercuri, A. In vitro regeneration of Passiflora species with ornamental value. Propag. Ornam. Plants 2008, 8, 47–49.
- Correa, C.M.; de Oliveira, G.N.; Astariata, L.V.; Santarem, E.R. Plant regeneration through somatic embryogenesis of yacon . Braz. Arch. Biol. Technol. 2009, 52, 549–554.
- Salma, U.; Kundu, S.; Ali, M.N.; Mandal, N. Somatic embryogenesis-mediated plant regeneration of Eclipta alba (L.) Hassk. and its conservation through synthetic seed technology. Acta Physiol. Plant. 2019, 41, 103.
- Podwyszyńska, M.; Marasek-Ciolakowska, A. Micropropagation of tulip via somatic embryogenesis. Agronomy 2020, 10, 1857.
- Mujib, A.; Ali, M.; Isah, T.; Dipti, T. Somatic embryo mediated mass production of Catharanthus roseus in culture vessel (bioreactor)—A comparative study. Saudi J. Biol. Sci. 2014, 21, 442–449.
- Jana, S.; Sivanesan, I.; Lim, M.Y.; Jeong, B.R. In vitro zygotic embryo germination and somatic embryogenesis through cotyledonary explants of Paeonia lactiflora Pall. Kor. Soc. Floricult. Sci. 2013, 21, 17–22.
- Du, Y.; Cheng, F.; Zhong, Y. Induction of direct somatic embryogenesis and shoot organogenesis and histological study in tree peony (Paeonia sect. Moutan). Plant Cell Tissue Organ Cult. 2020, 141, 557–570.
- Kuehnle, A.R.; Chen, F.C.; Sugii, N. Somatic embryogenesis and plant regeneration in Anthurium andraeanum hybrids. Plant Cell Rep. 1992, 11, 438–442.
- Pinheiro, M.V.M.; Martins, F.B.; da Cruz, A.C.F.; de Carvalho, A.C.P.P.; Ventrella, M.C.; Otoni, W.C. Somatic embryogenesis in anthurium (Anthurium andraeanum cv. Eidibel) as affected by different explants. Acta Sci. Agron. 2014, 36, 87–98.
- Teixeira da Silva, J.A.; Dobránszki, J.; Winarto, B.; Zeng, S. Anthurium in vitro: A review. Sci. Hortic. 2015, 186, 266–298.
- Bhattacharya, C.; Dam, A.; Karmakar, J.; Bandyopadhyay, T.K. Direct somatic embryogenesis and genetic homogeneity assessment of regenerated plants of Anthurium andraeanum Linden cv. Fantasia. Vitr. Cell Dev. Biol. Plant 2016, 52, 512–519.
- Wang, G.; Xu, C.; Yan, S.; Xu, B. An efficient somatic embryo liquid culture system for potential use in large-scale and synchronic production of Anthurium andraeanum seedlings. Front. Plant Sci. 2019, 10, 29.
- Fiuk, A.; Rybczyński, J.J. Morphogenic capability of Gentiana kurroo Royle seedling and leaf explants. Acta Physiol. Plant. 2008, 30, 157–166.
- Fiuk, A.; Rybczyński, J.J. The effect of several factors on somatic embryogenesis and plant regeneration in protoplast cultures of Gentiana kurroo (Royle). Plant Cell Tissue Organ Cult. 2007, 91, 263–271.
- Wu, H.J.; Wang, X.X.; Li, Y.; Zhang, D.G.; Zhang, B.W.; Xin, Y. Propagation of Gentiana macrophylla (Pall) from hairy root explants via indirect somatic embryogenesis and gentiopicroside content in obtained plants. Acta Physiol. Plant. 2011, 33, 2229–2237.
- Vinterhalter, B.; Mitić, N.; Vinterhalter, D.; Uzelac, B.; Krstić-Milošević, D. Somatic embryogenesis and in vitro shoot propagation of Gentiana utriculosa. Biologia 2016, 71, 139–148.
- da Silva, V.; Eeswara, J.P. Induction of somatic embryogenesis from leaf explants of Exacum trinervium (L.) Druce (Binara). J. Natl. Sci. Found. Sri Lanka 2022, 50, 27–33.
- Mahendran, D.; Kavi Kishor, P.B.; Geetha, N.; Venkatachalam, P. Phycomolecule-coated silver nanoparticles and seaweed extracts induced high-frequency somatic embryogenesis and plant regeneration from Gloriosa superba L. J. Appl. Phycol. 2018, 30, 1425–1436.
- Balamurugan, V.; Amal, T.C.; Karthika, P.; Selvakumar, S.; Vasanth, K. Somatic embryogenesis and plant regeneration in Gloriosa superba L.: An endangered medicinal plant. In In Vitro Plant Breeding Towards Novel Agronomic Traits; Kumar, M., Muthusamy, A., Kumar, V., Bhalla-Sarin, N., Eds.; Springer: Singapore, 2019; Chapter 2; pp. 27–42.
- Ren, Z.; Lv, X.; Zhang, D.; Xia, Y. Efficient somatic embryogenesis and bulblet regeneration of the endangered bulbous flower Griffinia liboniana. Plant Cell Tissue Organ Cult. 2018, 135, 523–533.
- Vejsadová, H.; Matiska, P.; Obert, B.; Ürgeová, E.; Preťová, A. Somatic embryogenesis in Phlox paniculata—Histological analysis. Biologia 2016, 71, 763–768.
- Simonović, A.D.; Trifunović-Momčilov, M.; Filipović, B.K.; Marković, M.P.; Bogdanović, M.D.; Subotić, A.R. Somatic embryogenesis in Centaurium erythraea Rafn—Current status and perspectives: A review. Plants 2021, 10, 70.
- Kumar, V.; Moyo, M.; Van Staden, J. Enhancing plant regeneration of Lachenalia viridiflora, a critically endangered ornamental geophyte with high floricultural potential. Sci. Hortic. 2016, 211, 263–268.
- von Aderkas, P.; Label, P.; Lelu, M.A. Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol. 2002, 22, 431–434.
- Nunes, S.; Marum, L.; Farinha, N.; Pereira, V.T.; Almeida, T.; Sousa, D.; Mano, N.; Figueiredo, J.; Dias, M.C.; Santos, C. Somatic embryogenesis of hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis and ploidy assessment of somatic plants. Plant Cell Tissue Organ Cult. 2018, 132, 71–84.
- Abrahamsson, M.; Clapham, D.; Arnold, S. Somatic embryogenesis in Scots pine (L.). In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants, Forestry Sciences; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 84, pp. 123–133.
- Aalifar, M.; Arab, M.; Aliniaeifard, S.; Dianati, S.; Mehrjerdi, M.Z.; Limpens, E.; Serek, M. Embryogenesis efficiency and genetic stability of Dianthus caryophyllus embryos in response to different light spectra and plant growth regulators. Plant Cell Tissue Organ Cult. 2019, 139, 479–492.
- Maruyama, T.E.; Hosoi, Y. Progress in somatic embryogenesis of Japanese pines. Front. Plant Sci. 2019, 10, 31.
- Rodríguez-Garay, B.; Gutiérrez-Mora, A.; Acosta-Duefias, B. Somatic embryogenesis of Agave victoria-reginae Moore. Plant Cell Tissue Organ Cult. 1996, 46, 85–87.
- Tejavathi, D.H.; Rajanna, M.D.; Sowmya, R.; Gayathramma, K. Induction of somatic embryos from cultures of Agave vera-cruz Mill. Vitr. Cell Dev. Biol. Plant 2007, 43, 423–428.
- Portillo, L.; Santacruz-Ruvalcaba, F.; Gutiérrez-Mora, A.; Rodríguez-Garay, B. Somatic embryogenesis in Agave tequilana Weber cultivar azul. Vitr. Cell Dev. Biol. Plant 2007, 43, 569–575.
- Reyes-Diaz, J.I.; Arzate-Fernández, A.M.; Pina-Escutia, J.L.; Vázquez-García, L.M. Media culture factors affecting somatic embryogenesis in Agave angustifolia Haw. Ind. Crops Prod. 2017, 108, 81–85.
- Kim, D.H.; Sivanesan, I. Somatic embryogenesis in Hosta minor (Baker) Nakai. Propag. Ornam. Plants 2017, 19, 24–29.
- Morel, G.M. Producing virus-free cymbidiums. Amer. Orchid Soc. Bull. 1960, 29, 495–497.
- Lee, Y.I.; Hsu, S.T.; Yeung, E.C. Orchid protocorm-like bodies are somatic embryos. Am. J. Bot. 2013, 100, 2121–2213.
- Cardoso, J.C.; Zanello, C.A.; Chen, J.T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985.
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hortic. 2009, 122, 507–520.
- Yam, T.W.; Arditti, J. History of orchid propagation: A mirror of the history of biotechnology. Plant Biotechnol. Rep. 2009, 3, 1–56.
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33.
- Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs combined with CHO sources and CCC priming PLB regeneration of Phalaenopsis. Horticulturae 2019, 5, 34.
- Habiba, S.U.; Shimasaki, K.; Hasan, K.M.; Mehraj, H.; Alam, M.M.; Sharma, S.; Ahasan, M.M. Very low and high temperature act as stress factor on organogenesis in protocorm-like bodies (PLBs) of Dendrobium kingianum. World Appl. Sci. J. 2016, 34, 278–282.
- Capellades, M.; Lemeur, R.; Debergh, P. Effects of sucrose on starch accumulation and rate of photosynthesis in Rosa cultured in vitro. Plant Cell Tissue Organ Cult. 1991, 25, 21–26.
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Alam, M.M. Effect of 6-benzylaminopurine (BA) and hyaluronic acid (HA) under white light emitting diode (LED) on organogenesis in protocorm-like bodies (PLBs) of Dendrobium kingianum. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 605–609.
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Kamal, M.M.; Alam, M.M. 5-aminolevulinic acid regulates growth and development of protocorm-like bodies (PLBs) in Dendrobium kingianum cultured in vitro. Middle East J. Sci. Res. 2014, 22, 279–283.
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Uddin, A.F.M.J. Effect of two bio polysaccharides on organogenesis of PLBs in Dendrobium kingianum cultured in vitro. Acta Hortic. 2017, 1167, 127–132.
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Uddin, A.F.M.J. Effect of ethylene precursor 1-aminocyclopropane-1-carboxylic acid and ethylene inhibitor, silver thiosulfateon organogenesis of PLBs in Dendrobium kingianum cultured in vitro. Acta Hortic. 2017, 1167, 133–138.
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M. Effects of ethrel on organogenesis of protocorm-like bodies in Dendrobium kingianum in vitro. Plant Tissue Cult. Biotech. 2018, 28, 141–146.
- Sultana, K.S.; Hasan, K.M.; Hasan, K.M.; Sultana, S.; Mehraj, H.; Ahasan, M.; Shimasaki, K.; Habiba, S.U. Effect of two elicitors on organogenesis in protocorm-like-bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. World Appl. Sci. J. 2015, 33, 1528–1532.
- Sultana, K.S.; Hasan, K.M.; Hasan, K.M.; Sultana, S.; Mehraj, H.; Ahasan, M.; Shimasaki, K.; Habiba, S.U. Effect of hyaluronic acid (HA) on organogenesis in protocorm-like bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1721–1724.
- Mehraj, H.; Shimasaki, K. In vitro PLBs organogenesis of Phalaenopsis using different concentrations of HA9 and HA12 combination. J. Biosci. Agric. Res. 2017, 12, 1036–1040.




