Ornamentals come in a variety of shapes, sizes, and colors to suit a wide range of climates, landscapes, and gardening needs. Compared to demand, a shortage of plant materials and diversity force the search for solutions for their constant acquisition and improvement to increase their commercial value, respectively. In vitro cultures are a suitable solution to meet expectations using callus culture, somatic embryogenesis, protoplast culture, and the organogenesis of protocorm-like bodies; many of these techniques are commercially practiced. Factors such as culture media, explants, carbohydrates, plant growth regulators, and light are associated with the success of in vitro propagation. Techniques, especially embryo rescue and somatic hybridization, are widely used to improve ornamentals. The development of synthetic seed allows season-independent seed production and preservation in the long term. Despite the advantages of propagation and the improvement of ornamentals, many barriers still need to be resolved. In contrast to propagation and crop developmental studies, there is also a high scope for molecular studies, especially epigenetic changes caused by plant tissue culture of ornamentals.
1. Callus Culture
In the early 20th century, callus formation and its ability to generate independent life were first noticed
[1][2][65,66]. The callus is a mass of loosely packed parenchymatous cells with various degrees of differentiation, which is raised from the in vitro proliferating cells of plant tissue in response to biotic and abiotic stimuli. It is similar to non-differentiated meristematic cells but different from differentiated plant cells. Depending on the accumulated compounds, calli may be pale brown, creamish yellow, greenish, or colorless. The callus is cytologically diverse in shape and type of cells and is genetically heterogeneous. Under the influence of selected phytohormones, a certain pool of parenchymal callus cells is dedifferentiated and has dividing activity. Calli lack chloroplasts for photosynthesis and have a small vacuole, and their culture can generate new plants. Callus can be inducted from any plant part, such as seeds, leaves, stem, root, flowers, etc.; successful callus induction depends on plant species, explant used for the callus inductions, culture media, PGR supplements in culture media, and growth conditions
[3][67]. Two major PGR groups, auxin and cytokinin, are largely used for callus induction
[3][67]. Some plant species induce callus in day–night conditions, while some need entirely night conditions. Callus induction gives an idea of the potentiality of in vitro regeneration of any plant species, while it can also be a good source of materials for other in vitro culture techniques and can be used for long-term preservation
[3][67]. Callus has been used for the successful plant regeneration and genetic modification of different ornamental plant species
[4][68].
2. Protoplast Culture
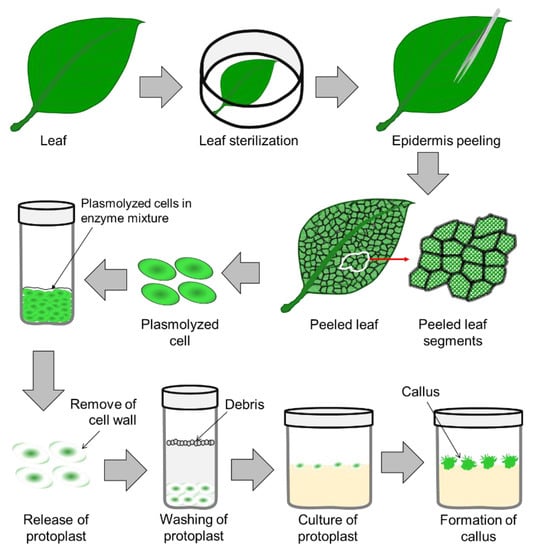
Protoplast culture is used for plantlet regeneration (process illustrated in
Figure 12), and protoplast fusion is used for crop improvement, which is known as somatic hybridization
[5] (details in Section 4.2) [69]. The nature of the explant tissue and the thickness of the cell wall play an important role in high-efficiency protoplast isolation, which is a critical stage in the process of seedling regeneration or somatic hybridization. However, protoplasts were successfully isolated and cultured in different ornamentals, such as
Dendrobium [6][70], lily
[7][71], rose
[8][72], chrysanthemum
[9][73], petunia
[10][74], carnation
[11][75], coneflower
[12][76], geraniums
[13][14][77,78], Persian silk tree
[15][79], etc. Pre-plasmolyzing the explant tissue with osmotic stabilizers, such as mannitol and sorbitol, before enzyme treatment is effective for protoplast isolation in most plant tissue
[16][80].
Figure 12.
A detailed scheme of protoplast isolation and establishment of an in vitro protoplast culture.
Sugar concentration is another important factor for high-yield protoplasts, and the effective sugar concentration ranges from 0.3 to 0.8 M in ornamentals
[5][10][12][17][18][19][20][69,74,76,81,82,83,84]. Factors such as the concentration of enzyme, digestion period, pH of the enzyme solution, temperature, and agitation during incubation are also important for protoplast isolation in ornamentals
[5][9][10][15][19][21][22][69,73,74,79,83,85,86]. In orchids, the first protoplasts were isolated in 1978
[23][24][87,88], while few studies reported colony formation
[25][26][27][28][29][89,90,91,92,93]. After successful protoplast isolation, there are some challenges to plantlet regeneration from an isolated protoplast. Types of culture medium, culture medium components, strength of the culture medium, carbon sources, pH of the culture medium, supplements of the culture medium, PGRs, and culture conditions have been proven to be vital factors for plantlet generations from protoplasts
[5][69]. Considering these factors and despite these limitations, plantlets have been generated successfully in several ornamental plant species
[5][9][10][14][30][31][69,73,74,78,94,95].
3. Somatic Embryogenesis
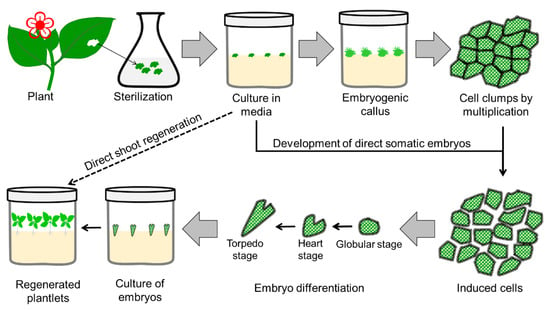
An alternative to root and shoot regeneration from the callus, regeneration of the whole plant from the plant cell throughout embryo formation, was identified in 1958
[32][33][96,97]. The development of an embryo or plant from the vegetative/somatic cell is known as somatic embryogenesis
[34][98]. The procedure for somatic embryogenesis is illustrated in
Figure 23. Somatic embryogenesis is considered more efficient than other propagation techniques. which guarantees variability. It produces identical genotypes differing from zygotic embryos, which guarantees variability. The bipolar structure of a somatic embryo consists of apical (known as plumule) and basal meristem regions (known as radicles), which are responsible for shoot and root formation, respectively
[35][99]. Cytological and histological studies have confirmed that PLBs
(deta
ils in Section 3.4) are also somatic embryos
[35][99]. Morphogenesis or regeneration of PLBs can be initiated by direct or indirect embryogenesis. Organogenesis of PLB avoiding the callus phase is known as direct embryogenesis, and PLB generated from the callus (an intermediate phase) is known as indirect embryogenesis
[35][99].
Figure 23.
Diagrammatic presentation of the steps involved in somatic embryogenesis for mass propagation in plants.
In somatic embryogenesis, the morphogenic response varies on factors like explants, PGRs, hormones, concentrations of PGRs or hormones, light, etc.
[35][36][37][38][99,100,101,102]. Plantlet regeneration by somatic embryogenesis has been reported in many genera of orchids; for example—
Cymbidium [39][40][41][42][43][44][103,104,105,106,107,108],
Phalaenopsis [44][45][46][47][48][49][50][51][108,109,110,111,112,113,114,115],
Oncidium [52][53][54][55][56][57][28,116,117,118,119,120],
Dendrobium [58][59][60][61][121,122,123,124],
Rhynchostylis [62][125],
Renanthera [63][126],
Paphiopedilum [64][65][127,128],
Malaxis [66][67][129,130],
Epipactis veratrifolia [68][131],
Spathoglottis plicata [69][132],
Geodorum densiflorum [70][133],
Anoectochilus elatus [71][134], and
Nothodoritis zhejiangensis [72][135]. In addition to orchids, it has also been reported in diverse ornamentals, such as rose
[73][136],
Rosa × damascena [74][137], chrysanthemum
[75][76][138,139], lilies
[77][78][79][80][81][82][83][140,141,142,143,144,145,146], jasmine
[84][147], lisianthus
[85][86][87][88][148,149,150,151], carnation
[89][152],
Camellia [90][91][92][93][94][153,154,155,156,157],
Cineraria [95][158], coneflower
[96][97][159,160],
Crocus [98][99][100][161,162,163],
Clematis [101][102][103][164,165,166]; Sawara cypress
[104][167], cyclamen
[105][168], bellflower
[106][169], passion flowers
[107][170], perennial daisy and false daisy
[108][109][171,172]; tulip
[110][173], periwinkle
[111][174], peony
[112][113][175,176], anthurium
[114][115][116][117][118][177,178,179,180,181], gentian
[119][120][121][122][182,183,184,185],
Exacum trinervium [123][186], gloriosa
[124][125][187,188], amaryllis
[126][189], phlox
[127][190],
Centaurium erythraea [128][191],
Lachenalia viridiflora [129][192], pine
[130][131][132][133][193,194,195,196], Japanese black pine
[134][197], agave
[135][136][137][138][198,199,200,201], and hosta
[139][202].
4. Protocorm-like Body
In
Cymbidium orchid, protocorm-like bodies (PLBs) were noticed for the first time during the shoot-tip culture by Morel (1960)
[140][203]. Protocorms are small spherical tuber-like structures formed in a germinating seed; protocorm-like structures with similar characteristics generated from somatic cells in tissue culture techniques are known as PLBs
[141][142][204,205]. PLBs are induced directly from explants and/or indirectly from calluses
[143][206], and the formation, regeneration, and proliferation of PLBs are among the most efficient techniques of micropropagation, especially for clonal propagation of orchids
[144][207]. Meristemoids in callus cells initiate polarized growth, and continuous cell division causes the shoot pole (for shoot initiation) and the base pole (for root initiation) of a protocorm-like body (PLB)
[64][141][145][127,204,208]. The induction of PLBs has several advantages over typical shoot and plantlet regeneration, such as a higher rate of multiplications, long-term preservation, easy differentiation into shoots, generations of secondary PLBs, etc. The success of efficient PLB induction, regeneration, and proliferation depends on multiple factors. Culture media ingredients, such as carbohydrate sources, plant growth regulators, elicitors, etc., are also crucial for efficient PLB organogenesis and regeneration
[142][205]. Growth retardants also stimulate PLB regeneration in orchids through the inhibition of GA biosynthesis
[146][26]. Setting up the optimum temperature in the growth chamber is also necessary for PLB proliferation, and a higher or lower temperature compared to the optimum causes stress in PLB regeneration in orchids
[147][209]. Light quality is another crucial factor for PLB organogenesis and regeneration for photosynthetic and phototropic responses, and many studies have suggested the efficiency of LEDs over traditional fluorescent light, suggesting the advantages of monochromatic light for PLB organogenesis and regeneration
[142] (Supplementary Table S4) [205]. However, different factors can work synergistically for better PLB organogenesis and regeneration compared with their independent applications. However, all these external factors are highly species-specific
[142](Supplementary Table S5) [205].
ThWe
researchers hhave also reported the manipulation of culture media and growth conditions for PLB regeneration in
Dendrobium [147][148][149][150][151][152][153][30,209,210,211,212,213,214] and
Phalaenopsis [146][154][155][156][26,215,216,217].
ThWe
researchers found that culture media manipulation and light quality are highly species-specific in orchid PLB proliferation.
Besides these techniques, seed culture, meristem culture, anther culture, embryo culture, ovule culture, cell suspension culture, and direct shoot organogenesis are also practiced for in vitro plantlet generation in ornamentals.