Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marino B. Arnao | -- | 1457 | 2022-12-06 08:20:51 | | | |
| 2 | Camila Xu | Meta information modification | 1457 | 2022-12-06 08:56:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Arnao, M.B.; Hernández-Ruiz, J.; Cano, A. Melatonin in Nitrogen Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/38104 (accessed on 12 January 2026).
Arnao MB, Hernández-Ruiz J, Cano A. Melatonin in Nitrogen Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/38104. Accessed January 12, 2026.
Arnao, Marino B., Josefa Hernández-Ruiz, Antonio Cano. "Melatonin in Nitrogen Metabolism" Encyclopedia, https://encyclopedia.pub/entry/38104 (accessed January 12, 2026).
Arnao, M.B., Hernández-Ruiz, J., & Cano, A. (2022, December 06). Melatonin in Nitrogen Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/38104
Arnao, Marino B., et al. "Melatonin in Nitrogen Metabolism." Encyclopedia. Web. 06 December, 2022.
Copy Citation
Melatonin (N-acetyl-5-methoxytryptamine) is a biogenic amine discovered in 1958 in the pineal gland of cow, and later in humans.
low nitrogen use
melatonin
nitrogen uptake
nitrogen metabolism
1. Introduction
Nitrogen is the main element that limits plant productivity in crops, with nitrate being the main nitrogen form for plants. The massive use of nitrates and other nitrogen compounds, such as ammonium, has led to serious problems in agricultural soils, such as the high salinity and contamination of aquifers with nitrogen [1][2][3][4]. Excess nitrogen in soil usually has negative consequences on plant physiology, such as a lower photosynthetic rate, osmotic stress, nitrogen metabolism disorders, and the excessive appearance of ROS (reactive oxygen species) and RNS (reactive nitrogen species). Furthermore, alterations in the assimilation of other elements such as Ca and Mg cause a lower response in defense against pathogens [5]. Either directly or indirectly, excess nitrates increase ammonium levels in the soil, affecting the overall growth of the plant, both in aerial and root systems [6][7][8][9]. Therefore, overuse of nitrogen can result in decreased crop productivity. Therefore, producing more whilst using less nitrogen, the so-called Nitrogen Use Efficiency (NUE), is a recommended practice [10][11][12]. Currently, the use of “smart fertilizers”, such as coated fertilizers, and the application of plant-growth-promoting rhizobacteria (PGPR) to the soil has ostensibly improved the nutrition of nitrogen in plants [13][14][15]. On the other hand, the deficit of nitrogen in soils usually entails great limitations in plant growth and serious nitrogen and carbon metabolic dysfunctions, reducing the photosynthesis and biosynthesis of amino acids and proteins [16][17].
Melatonin (N-acetyl-5-methoxytryptamine) is a biogenic amine discovered in 1958 in the pineal gland of cow [18], and later in humans [19][20]. Its properties as a hormone which regulates light/dark cycles and other endogenous rhythms in mammals have been extensively studied. Remarkably, melatonin was identified in plants in 1995 simultaneously by three groups of researchers [21][22][23]. Although initially there were many doubts about the presence of melatonin in plant tissues, today it is one of the most prolific and exciting areas of study within plant physiology [24]. In plants, melatonin has a multi-regulatory role, behaving as a “plant master regulator”, stimulating processes such as seed germination, photosynthesis, growth, and rooting, whilst inhibiting leaf senescence, and regulating fruit ripening and senescence. In addition, melatonin considerably increases tolerance to biotic (bacteria, fungi, and viruses) agents and to abiotic stressors, such as water deficit, extreme temperatures, salinity, contaminants, etc. These aspects are of great interest in its application in crops [25][26][27][28][29][30][31].
In plant metabolism, melatonin regulates many primary metabolism pathways, mainly in carbohydrates (starch and sucrose) [32], lipids, and nitrogen compound routes, and also in secondary metabolism pathways (phenolics, flavonoids, and terpenoids) [24]. The broad functions and regulatory capacities of melatonin in plant and animal cells have been studied and their analogies and differences have been compared [33][34].
2. Melatonin Biosynthesis
In plants, melatonin is synthetized from chorismic acid, which is generated from shikimic acid (a condensation product of phosphoenolpyruvate from glycolysis and erythrose 4-phosphate from the pentose phosphate pathway (Figure 1)). Chorismic acid, a precursor to aromatic amino acids (phenylalanine, tyrosine, and tryptophan), is transformed through the anthranilate/indole pathway to tryptophan [35]. Tryptophan is the origin of the melatonin biosynthesis pathway in both animal and plant cells [33][36][37]. In animals, tryptophan is converted to 5-hydroxytryptophan by TPH, an enzyme that apparently has not been identified in plants. Tryptophan is mainly transformed into tryptamine by TDC present in cytoplasm of plant cells, and then up to serotonin (5-hydroxytryptamine) by T5H (in endoplasmic reticulum) (Figure 1). The transformation of serotonin into melatonin is produced in the chloroplast or cytoplasm depending on the enzyme involved. Thus, serotonin can first be acetylated by SNAT to N-acetylserotonin (in the chloroplast) and subsequently hydroxylated to melatonin (in the cytoplasm) by ASMT/COMT. Under conditions of stress or excess serotonin, 5-methoxytryptamine is formed preferentially by the action of ASMT/COMT [38], and finally melatonin is generated by SNAT (in the chloroplast) [39][40][41]. Melatonin is usually hydroxylated at different positions on the indole ring, with 2-hydroxymelatonin being the major catabolite in plants, showing interesting regulatory properties [42][43][44].
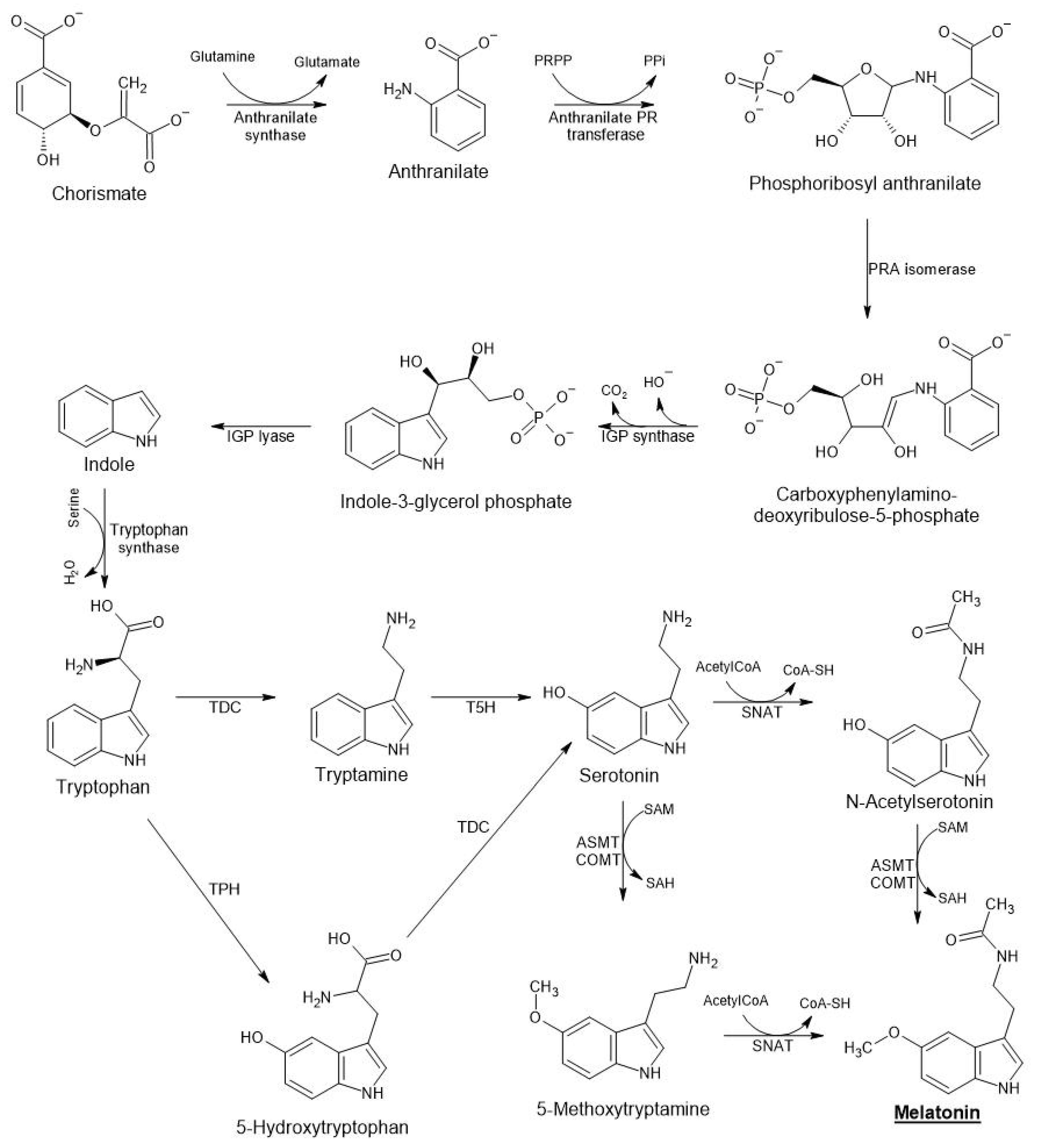
Figure 1. Pathways of melatonin biosynthesis in plants.
3. Role of Melatonin in Nitrogen Metabolism
The role of melatonin on nitrogen metabolism has been studied under conditions of nitrogen excess and deficiency. Table 1 summarizes several representative examples of the effect of melatonin on nitrogen metabolism in different plant species and conditions. Under nitrogen-excess conditions, melatonin treatments induced a decrease in endogenous nitrogen levels in the form of nitrate and ammonium. In cucumber plants grown with excess nitrate, 100 µM melatonin increased nitrogen tolerance and growth, reorganizing NPK balance and lowering nitrogen damage by reducing nitrate and ammonium levels in seedlings. Furthermore, cucumber melatonin-treated seedlings increased enzyme activities, and nitrate reductase (NR), glutamine synthase (GS), glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH) gene expression, reducing the negative effect of excess nitrate [45]. Also in nitrate excess, melatonin, in co-action with nitric oxide (NO), reversed the excess nitrogen inhibition of root growth, and also up/down-regulated several genes of IAA and ABA metabolism, including melatonin biosynthesis genes [46]. Previously Zhao et al. (2012), also investigating cucumber, demonstrated for the first time, that melatonin increased high-temperature tolerance, regulating nitrate and ammonium levels and nitrogen-related enzymes [47]. The role that melatonin plays in increasing nitrogen-excess tolerance was also studied in alfalfa plants (Table 1). In this case, melatonin increased nitrogen-excess tolerance through the up-regulation of NR, GS, GOGAT, and GDH enzymes. Furthermore, it reduced the total nitrogen levels (nitrate and ammonium content) and increased the biomass, length, width of leaves, and energy levels (P and ATP) and decreased the Na, K, and Ca mineral contents [48]. Similar results were obtained in soybean plants treated with melatonin in nitrogen-excess [49].
Table 1. Effects on nitrogen metabolism by melatonin in different species.
| Plant Species | Nitrogen Nutrition/Stress | Melatonin Treatment (µM) | Observed Effects | Reference |
|---|---|---|---|---|
| Cucumber | Normal High temperature |
100 | ↑ temperature tolerance, NR, GS, GOGAT, GDH, nitrate, ammonium restrained | [47] |
| Nitrate: N-excess | 100 | ↑ tolerance, growth, NPK balance, Ca, NR, GS, GOGAT, GDH ↓ damage, nitrate, ammonium |
[45] | |
| Nitrate: N-excess | 2 | ↑ tolerance N excess, co-action with NO, lateral roots, root length, Ca, Mg, Fe, melatonin, NO, IAA, ABA, transcription levels of several genes of N metabolism, IAA, ABA and melatonin | [46] | |
| Apple | Normal (urea) Drought stress |
100 | ↑ drought tolerance, growth, photosynthesis, stomatal open, chls, RWC, NR, NiR, GS, GOGAT, N uptake genes (AMTs, NRTs), N, P, K, Ca, Mg, Cu, Zn, and B levels | [50] |
| Alfalfa | Nitrate: N-excess | 100 | ↑ tolerance N excess, shoot height, leaves (length, width, area), P, ATP, biomass, amino acids, energy charge, upregulates NR, GS, GOGAT, GDH ↓ total N, nitrate, ammonium, Na, K, Ca |
[48] |
| Wheat | Nitrate and ammonium: N-low | 1 | ↑ N and nitrate, N absorption, N metabolism, NR, GS, growth, yield, in shoots and roots | [51] |
| Maize | Normal | 100 | ↑ nitrite, nitrate, NR, NiR, GS, GOGAT, GDH ↓ ammonium |
[52] |
| Soybean | Normal Salt/drought stress |
50–100 | ↑ stress tolerance, growth, seed yield and fatty acid; up-regulates cell division, photosynthesis, carbohydrate, fatty acid, and ASC genes | [53] |
| Normal | 100 | ↑ number and size of nodules, fresh shoot biomass in 3 varieties | [54] | |
| Nitrate and ammonium: N-excess, N-normal and N-low | 100 | ↑ tolerance N-excess, N content in N-low, stem diameter, leaf area, nodule number, ATP, biomass in three-N conditions, antioxidant enzymes at N-excess, N-related genes | [49] | |
| Nitrate and ammonium: N-low | 100 | ↑ nodule number, total N fixed, tolerance to N deficiency, upregulating genes: NR2, NiR, GS1β, GOGAT, AAP6a, promoting enzyme activity: NR, GS, GOGAT, GDH, amino acids, protein, total N, chls, seed yield | [55] | |
| Normal Drought stress |
100 | ↑ N, NR, NiR, NRT, GS, GOGAT, GDH, protein, proline, ureides, N transport, growth, biomass | [56] | |
| Normal Drought stress |
100 | ↑ stress tolerance, growth, seed yield, amino acids, photosynthesis, antioxidants, regulates C/N ratio, and plant hormone levels | [57] |
↑ Increased content or action; ↓ Decreased content or action.
In nitrogen-normal conditions, melatonin-treated maize seedlings were found to increase nitrogen content (nitrate and nitrite) and decrease ammonium content with respect to the control plants. Furthermore, NR, NiR GS, GOGAT, and GDH activities and gene expression were up-regulated in melatonin treatments [52]. Unexpectedly, melatonin is also able to favorably regulate nitrogen levels under deficit conditions. Winter wheat grown in a nitrogen-deficit medium was capable of increasing nitrogen uptake and nitrate contents in melatonin-treated seedlings, increasing shoot and root growth, as well as yield, and possibly improving nitrogen metabolism [51]. Also in soybean plants, the role of melatonin in nitrogen-low conditions has been studied. In nitrate and ammonium deficiency, melatonin improved plant tolerance, increasing the total number of nodules and fixed nitrogen, and up-regulating several nitrogen-related gene expressions (see Table 1), with the result of an increase in the levels of amino acids, proteins, chlorophyll, and also an increase in seed yield [49][55].
Moreover, in different stress conditions, such as drought stress, melatonin improved stress tolerance and nitrogen uptake. It also improved the contents of amino acid, protein, proline, and ureides, whilst up-regulating NR, NiR, NRT, GS, GOGAT, and GDH gene expression, consequently improving the growth and total biomass of soybean plants [56]. The authors suggested that melatonin regulated the assimilation, metabolism and transport of nitrogen, thereby maintaining the carbon/nitrogen balance [48][50][58][59].
References
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of Biological Sewage Sludge: Fertilizer Nitrogen Recovery as the Solution to Fertilizer Crisis. J. Environ. Manag. 2022, 326, 116602.
- Mayor, Á.; Beltran, E.; Cortina, J.L.; Valderrama, C. Nitrogen Flow Analysis in Spain: Perspectives to Increase Sustainability. Sci. Total Environ. 2022, 858, 160117.
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of Human Modification of the Global Nitrogen Cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116.
- Schmidt, R.; Mieulet, D.; Hubberten, H.-M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.M.; et al. SALT-RESPONSIVE ERF1 Regulates Reactive Oxygen Species–Dependent Signaling during the Initial Response to Salt Stress in Rice. Plant Cell 2013, 25, 2115–2131.
- Barraclough, P.B.; Howarth, J.R.; Jones, J.; Lopez-Bellido, R.; Parmar, S.; Shepherd, C.E.; Hawkesford, M.J. Nitrogen Efficiency of Wheat: Genotypic and Environmental Variation and Prospects for Improvement. Eur. J. Agron. 2010, 33, 1–11.
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends Plant Sci. 2017, 22, 163–174.
- Huarancca Reyes, T.; Scartazza, A.; Pompeiano, A.; Ciurli, A.; Lu, Y.; Guglielminetti, L.; Yamaguchi, J. Nitrate Reductase Modulation in Response to Changes in C/N Balance and Nitrogen Source in Arabidopsis. Plant Cell Physiol. 2018, 59, 1248–1254.
- Yin, Y.; Ying, H.; Zheng, H.; Zhang, Q.; Xue, Y.; Cui, Z. Estimation of NPK Requirements for Rice Production in Diverse Chinese Environments under Optimal Fertilization Rates. Agric. For. Meteorol. 2019, 279, 107756.
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can Less Yield More? Is Reducing Nutrient Input into the Environment Compatible with Maintaining Crop Production? Trends Plant Sci. 2004, 9, 597–605.
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108.
- Tantray, A.Y.; Hazzazi, Y.; Ahmad, A. Physiological, Agronomical, and Proteomic Studies Reveal Crucial Players in Rice Nitrogen Use Efficiency under Low Nitrogen Supply. Int. J. Mol. Sci. 2022, 23, 6410.
- Kong, Z.; Liu, H. Modification of Rhizosphere Microbial Communities: A Possible Mechanism of Plant Growth Promoting Rhizobacteria Enhancing Plant Growth and Fitness. Front. Plant Sci. 2022, 13, 920813.
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022, 13, 916488.
- Zhang, H.; Sun, X.; Dai, M. Improving Crop Drought Resistance with Plant Growth Regulators and Rhizobacteria: Mechanisms, Applications, and Perspectives. Plant Comm. 2022, 3, 100228.
- Hao, Q.; Shang, W.; Zhang, C.; Chen, H.; Chen, L.; Yuan, S.; Chen, S.; Zhang, X.; Zhou, X. Identification and Comparative Analysis of CBS Domain-Containing Proteins in Soybean (Glycine max) and the Primary Function of GmCBS21 in Enhanced Tolerance to Low Nitrogen Stress. Int. J. Mol. Sci. 2016, 17, 620.
- Liu, D.; Li, M.; Liu, Y.; Shi, L. Integration of the Metabolome and Transcriptome Reveals the Resistance Mechanism to Low Nitrogen in Wild Soybean Seedling Roots. Environ. Exp. Bot. 2020, 175, 104043.
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, a Pineal Factor that Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587.
- Lerner, A.B.; Case, J.D.; Heinzelmann, R.V. Structure of Melatonin. J. Am. Chem. Soc. 1959, 81, 6084–6085.
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821.
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31.
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634.
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm Res. 1995, 26, 406–409.
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, 73, 5779–5800.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Plant Biostimulant in Crops and during Post-Harvest: A New Approach Is Needed. J. Sci. Food Agric. 2021, 101, 5297–5304.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2022, 22, 413–429.
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An Overview of the Importance and Mediating Functions of Melatonin against Environmental Stresses. Physiol. Plant. 2021, 172, 820–846.
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of Melatonin during Postharvest of Horticultural Crops. Plant Cell Physiol. 2021, pcab175.
- Giraldo-Acosta, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a Possible Natural Safener in Crops. Plants 2022, 11, 890.
- Hernández-Ruiz, J.; Ruiz-Cano, D.; Giraldo-Acosta, M.; Cano, A.; Arnao, M. Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules 2022, 27, 1523.
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249.
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996.
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of Melatonin and Salicylic Acid in Biotic/Abiotic Plant Stress Responses. Agronomy 2018, 8, 33.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plants. In Amino Acids in Higher Plants; D’ Mello, J., Ed.; CAB Intern.: Boston, MA, USA, 2015; pp. 390–435. ISBN 978-1-78064-263-5.
- Tan, D.X.; Reiter, R.J. An Evolutionary View of Melatonin Synthesis and Metabolism Related to Its Biological Functions in Plants. J. Exp. Bot. 2020, 71, 4677–4689.
- Menhas, S.; Yang, X.; Hayat, K.; Aftab, T.; Bundschuh, J.; Arnao, M.B.; Zhou, Y.; Zhou, P. Exogenous Melatonin Enhances Cd Tolerance and Phytoremediation Efficiency by Ameliorating Cd-Induced Stress in Oilseed Crops: A Review. J. Plant Growth Regul. 2022, 41, 922–935.
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Latorre-Jimenez, M.A.; Reiter, R.J. On the Significance of an Alternate Pathway of Melatonin Synthesis via 5-Methoxytryptamine: Comparisons across Species. J. Pineal Res. 2016, 61, 27–40.
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437.
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391.
- Lee, H.Y.; Back, K. 2-Hydroxymelatonin, Rather Than Melatonin, Is Responsible for RBOH-Dependent Reactive Oxygen Species Production Leading to Premature Senescence in Plants. Antioxidants 2021, 10, 1728.
- Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Predominance of 2-Hydroxymelatonin over Melatonin in Plants. J. Pineal Res. 2015, 59, 448–454.
- Byeon, Y.; Back, K. Molecular Cloning of Melatonin 2-Hydroxylase Responsible for 2-Hydroxymelatonin Production in Rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351.
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of Melatonin on Seedling Growth, Mineral Nutrition, and Nitrogen Metabolism in Cucumber under Nitrate Stress. J. Pineal Res. 2017, 62, e12403.
- Zhang, Y.; Liu, A.; Hao, Y.; Su, W.; Sun, G.; Song, S.; Liu, H.; Chen, R. Nitric Oxide Is Essential for Melatonin to Enhance Nitrate Tolerance of Cucumber Seedlings. Molecules 2022, 27, 5806.
- Zhao, N.; Sun, Y.; Wang, D.Y.; Zheng, J.X. Effects of Exogenous Melatonin on Nitrogen Metabolism in Cucumber Seedlings under High Temperature Stress. Zhiwu Shengli Xuebao/Plant Physiol. J. 2012, 48, 557–564.
- Chen, Z.; Cao, X.; Niu, J. Effects of Melatonin on Morphological Characteristics, Mineral Nutrition, Nitrogen Metabolism, and Energy Status in Alfalfa under High-Nitrate Stress. Front. Plant Sci. 2021, 12, 694179.
- Wang, H.; Ren, C.; Cao, L.; Jin, X.; Wang, M.; Zhang, M.; Zhao, Q.; Li, H.; Zhang, Y.; Yu, G. The Mechanisms Underlying Melatonin Improved Soybean Seedling Growth at Different Nitrogen Levels. Funct. Plant Biol. 2021, 48, 1225–1240.
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-Term Exogenous Application of Melatonin Improves Nutrient Uptake Fluxes in Apple Plants under Moderate Drought Stress. Environ. Exp. Bot. 2018, 155, 650–661.
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin Promotes Plant Growth by Increasing Nitrogen Uptake and Assimilation under Nitrogen Deficient Condition in Winter Wheat. Plant Physiol. Biochem. 2019, 139, 342–349.
- Erdal, S. Melatonin Promotes Plant Growth by Maintaining Integration and Coordination between Carbon and Nitrogen Metabolisms. Plant Cell Rep. 2019, 38, 1001–1012.
- Wei, W.; Li, Q.; Chu, Y.-N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin Enhances Plant Growth and Abiotic Stress Tolerance in Soybean Plants. J. Exp. Bot. 2015, 66, 695–707.
- Ren, S.; Jiang, G.-L.; Rutto, L. Melatonin Priming Enhances Symbiotic Nitrogen Fixation in Soybean, Glycine max L. J. Biotech Res. 2019, 10, 136–144.
- Wang, H.; Ren, C.; Cao, L.; Zhao, Q.; Jin, X.; Wang, M.; Zhang, M.; Yu, G.; Zhang, Y. Exogenous Melatonin Modulates Physiological Response to Nitrogen and Improves Yield in Nitrogen-Deficient Soybean (Glycine max L. Merr.). Front. Plant Sci. 2022, 13, 865758.
- Cao, L.; Qin, B.; Gong, Z.; Zhang, Y. Melatonin Improves Nitrogen Metabolism during Grain Filling under Drought Stress. Physiol. Mol. Biol. Plants 2022, 28, 1477–1488.
- Zou, J.; Yu, H.; Yu, Q.; Jin, X.; Cao, L.; Wang, M.; Wang, M.; Ren, C.; Zhang, Y. Physiological and UPLC-MS/MS Widely Targeted Metabolites Mechanisms of Alleviation of Drought Stress-Induced Soybean Growth Inhibition by Melatonin. Ind. Crops Prod. 2021, 163, 113323.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48.
- Ren, J.; Yang, X.; Zhang, N.; Feng, L.; Ma, C.; Wang, Y.; Yang, Z.; Zhao, J. Melatonin Alleviates Aluminum-Induced Growth Inhibition by Modulating Carbon and Nitrogen Metabolism, and Reestablishing Redox Homeostasis in Zea mays L. J. Hazard. Mater. 2022, 423, 127159.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
692
Revisions:
2 times
(View History)
Update Date:
13 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




